Abstract
Context: Recent reports suggest a higher prevalence (1–5%) of Cushing’s syndrome in certain patient populations with features of the disorder (e.g., diabetes), but the prevalence in the overweight and obese population is not known.
Objective: The aim of the study was to evaluate the diagnostic performance of screening tests for Cushing’s syndrome in overweight and obese subjects with at least two other features of the disorder.
Design and Setting: We conducted a cross-sectional prospective study.
Subjects and Methods: A total of 369 subjects (73% female) completed two or three tests: a 24-h urine cortisol, and/or late-night salivary cortisol, and/or 1 mg dexamethasone suppression test (DST). If any result was abnormal [based on laboratory reference range or cortisol after DST ≥1.8 μg/dl (50 nmol/liter)], tests were repeated and/or a dexamethasone-CRH test was performed. Subjects with abnormal DST results and a low dexamethasone level were asked to repeat the test with 2 mg of dexamethasone.
Results: In addition to obesity, subjects had a mean of five to six features of Cushing’s syndrome. None was found to have Cushing’s syndrome. Test specificities to exclude Cushing’s syndrome for subjects who completed three tests were: urine cortisol, 96% [95% confidence interval (CI), 93–98%]; DST, 90% (95% CI, 87–93%); salivary cortisol, 84% by RIA (95% CI, 79–89%) and 92% by liquid chromatography-tandem mass spectrometry (95% CI, 88–95%). The combined specificity (both tests normal) for all combinations of two tests was 84 to 90%, with overlapping CIs.
Conclusion: These data do not support widespread screening of overweight and obese subjects for Cushing’s syndrome; test results for such patients may be falsely abnormal.
Data from this study does not support widespread screening of overweight and obese subjects for Cushing’s syndrome.
Cushing’s syndrome is a rare disorder characterized by clinical signs and symptoms that reflect chronic exposure to hypercortisolism. However, many of its features (weight gain, hypertension, glucose intolerance, depression, irregular menses, and hirsutism) are common in the general population, raising the question of who should be screened. Several studies report a prevalence of Cushing’s syndrome of 1–5% in patients with uncontrolled diabetes and/or hypertension (1,2,3,4). However, there are few studies of the prevalence of Cushing’s syndrome or the performance of screening tests in an overweight and obese population (5). It is important to evaluate the rate of false-positive results on screening tests in light of recent Endocrine Society guidelines regarding case detection (6). To address some of these issues, this study determined the prevalence of true and false-positive Cushing’s syndrome screening test results in a population of overweight and obese subjects with at least two other clinical features of Cushing’s syndrome.
Subjects and Methods
The Institutional Review Board of the National Institute of Child Health and Human Development approved the study protocol. All subjects provided written informed consent.
Evaluation at The George Washington University Weight Management Program (GWUWMP)
From October 2003 to March 2008, individuals aged 18–75 presenting for weight loss treatment at GWUWMP indicated on a questionnaire the presence of signs or symptoms of Cushing’s syndrome (Table 1). Overweight or obese subjects with at least two additional features of Cushing’s syndrome and willingness to return for follow-up were invited to participate. Exclusion criteria included: 1) weight more than 350 pounds (159 kg), the limit for radiology examination tables; 2) serum creatinine above 2.6 mg/dl; 3) pregnancy; 4) serious medical conditions that might alter pituitary-adrenal function; and 5) recent or anticipated use of oral or injected glucocorticoids, black licorice, chewing tobacco, phenytoin, barbiturates, loperamide, or opiates.
Table 1.
Percentage of subjects with positive responses to items on the inclusion criteria questionnaire
| % | % | ||
|---|---|---|---|
| Lethargy/depression | 56 | Facial plethora | 22 |
| Irritability | 50 | Abnormal fat distribution | 21 |
| Decreased concentration | 46 | Balding (females) | 21 |
| Impaired short-term memory | 44 | Ecchymoses | 21 |
| Hypertension | 42 | Acne | 21 |
| Decreased libido | 41 | Change in appetite | 20 |
| Edema | 41 | Weakness | 18 |
| Headache | 29 | Striae | 14 |
| Glucose intolerance | 27 | Thin skin | 14 |
| Hirsutism (females) | 25 | Recurrent infections | 11 |
| Irregular menses (females ≤ 40 yr old; n = 68) | 24 | Osteopenia | 9 |
Data represent subjects with valid inclusion criteria who had results from at least two screening tests. For each criterion, the number of evaluable subjects ranged from 359–363 (except 299 for headache) of 369 women and men.
After enrollment, GWUWMP staff reviewed a history, performed a physical examination, and recorded the presence or absence of generalized obesity, truncal obesity, dorsocervical fat, temporal fat, supraclavicular fat, moon facies, ruddy/red face, stretch marks, edema, thin skin, poorly healed skin, impaired memory, mood lability, proximal muscle weakness (strength quantified as 1–5; 5 is maximum), acne, and hirsutism. Weight, height, and systolic and diastolic blood pressure measurements were noted. Fasting blood sugar was measured in known diabetics; all others underwent an oral glucose tolerance test.
Initially, subjects completed the Psychiatric Diagnostic Screening Questionnaire; subsequently, the Patient Health Questionnaire was substituted and was completed by most subjects.
Subjects underwent screening tests for Cushing’s syndrome at GWUWMP, including a 1 mg overnight dexamethasone suppression test (DST) with measurement of serum cortisol and, in subjects with an abnormal response, measurement of dexamethasone levels [both by liquid chromatography-tandem mass spectrometry (LC-MS/MS); assay sensitivities, 1 μg/dl (27.6 nmol/liter) and 5 ng/dl (0.13 nmol/liter), respectively, at Esoterix Laboratories, Calabasas Hills, CA], measurement of 24-h urine free cortisol (UFC) excretion [LC-MS/MS; assay sensitivity, 0.07 μg/dl (1.9 nmol/liter) Mayo Laboratories, Rochester, MN)] (7,8) (with creatinine measurement), and measurement of bedtime salivary cortisol by RIA (Esoterix Laboratories) or LC-MS/MS (Mayo Laboratories) (9). Initially saliva samples were analyzed only by RIA. Subsequently split samples were also assayed by LC-MS/MS. Because LC-MS/MS had a lower rate of false-positive results, it was used alone for the remainder of the study (9).
Screening test results were considered abnormal if they exceeded the laboratory’s established normal range: UFC, above 45 μg/24 h (124 nmol/24 h); salivary cortisol, above 170 ng/dl (4.7 nmol/liter) by RIA or above 100 ng/dl (2.8 nmol/liter) by LC-MS/MS; or if the DST cortisol was at least 1.8 μg/dl (49.6 nmol/liter) (10). Subjects with dexamethasone levels below 140 ng/dl (3.6 nmol/liter) were asked to repeat the test with 2 mg dexamethasone. UFC results were considered invalid if the creatinine was not 15–25 mg/kg body weight/24 h.
Evaluation at the National Institutes of Health (NIH)
Subjects with any abnormal screening result were asked to undergo evaluation at the NIH Clinical Center. At least two phone and letter reminders were sent to encourage follow-up visits.
At the NIH, each subject was asked to submit two bedtime saliva samples (LC-MS/MS; some with split samples for RIA) and two additional 24-h urine samples for cortisol measurement by Nichols Advantage immunochemiluminescence metric assay (ICMA; Department of Laboratory Medicine, NIH), early in the study [normal range, ≤77 μg/24 h (213 nmol/24 h)] or later LC-MS/MS by Mayo Laboratories. UFC and salivary cortisol by LC-MS/MS results were available immediately and were used for clinical decisions. The saliva specimens for RIA were batched and assayed later. Subjects underwent a dexamethasone-CRH (Dex-CRH) test if their initial DST result was abnormal despite an appropriate serum dexamethasone level, and/or if any repeat UFC or salivary cortisol result was abnormal (11).
Subjects with an abnormal Dex-CRH result [15 min post-dex cortisol ≥1.4 μg/dl (38 nmol/liter)] and suboptimal dexamethasone levels [<500 ng/dl (<12.7 nmol/liter)] underwent repeat testing if possible, using a doubled dexamethasone dose (1 mg every 6 h). Cortisol binding globulin (CBG) levels (RIA; Esoterix Laboratories) were obtained.
Subjects with any abnormal screening test result were later contacted again at the end of the study and asked whether they had received any endocrine diagnosis since the initial testing.
Analysis
Subject demographics and clinical characteristics were characterized by simple descriptive statistics and frequency distributions. Data are reported as mean ± sd.
Specificity [and 95% confidence intervals (CI)] for each test was calculated for subjects who completed two or three tests (UFC + DST, DST + salivary cortisol LC-MS/MS, or UFC + salivary cortisol LC-MS/MS), both including and excluding subjects without follow-up. DST specificity was also calculated after excluding women taking estrogen. Categorical data were compared by χ2 and Fisher’s exact tests, and continuous data were compared using nonparametric (Wilcoxon rank sum) tests. To compare specificities of unpaired and paired data, Fisher’s exact test and McNemar’s test were used, respectively. If comparisons consisted of both paired and unpaired data, a generalization of Fisher’s exact test, a composite exact test, was used to compare overall false-positive rates (9,12). Specificity of follow-up UFC results (ICMA vs. LC-MS/MS) was calculated by assigning a subject as “normal” if all UFC results were within the reference range or “abnormal” if any result was above the range. A P value less than or equal to 0.05 was considered statistically significant, unless a multiple comparison adjustment was applied. Data were analyzed using SAS system software version 9.1 (SAS Institute, Cary, NC).
Repeat test results were considered abnormal according to aforementioned laboratory reference ranges. Post-CRH 15 min cortisol levels of at least 1.4 μg/dl (38 nmol/liter) were considered abnormal if dexamethasone levels were at least 500 ng/dl (12.7 nmol/liter) (11).
Multiple unexplained abnormal results over time were required for a diagnosis of Cushing’s syndrome.
To create a composite score, the physical examination features of dorsocervical fat, supraclavicular fat, moon face, pink or purple stretch marks, ruddy face, proximal muscle weakness, thin skin, and history of eccymoses and menstrual change (for those women ≤40 yr) were assigned “1” point if present and summed. This composite score was compared between the groups with and without any screening abnormality.
Subjects taking a glucose-lowering medication for glycemic control, or having a fasting blood glucose of at least 126 mg/dl (7.0 mmol/liter), or having 2-h post-glucose of at least 200 mg/dl (11.1 mmol/liter) were considered to have diabetes mellitus (13). Subjects were considered to have hypertension if systolic blood pressure was at least 140 mm Hg and/or diastolic blood pressure was at least 90 mm Hg (14) or if they were taking medication(s) to treat hypertension.
Results
Demographics
Of 471 subjects enrolled, 70 (15%) did not return for any screening test, and six completed one screening test. Twenty-six subjects had insufficient inclusion criteria. The remaining 369 subjects form the basis of this report: 73% were female, 83% were white, and 67% had at least a bachelor’s degree. Mean age was 48 ± 12 yr for women and 50 ± 12 yr for men. Mean weight and body mass index (BMI) were 102 ± 20 kg and 38 ± 7 kg/m2 in women and 128 ± 29 kg and 41 ± 9 kg/m2 in men. Fourteen subjects weighing more than 350 pounds (159 kg) were studied. The BMI distribution was: 25–29.9 kg/m2, 11%; 30–34.9 kg/m2, 26%; 35–39.9 kg/m2, 25%; and above 40 kg/m2, 38%.
Clinical features
Table 1 lists the percentage of positive responses to the screening questionnaire for enrollment. On average, women answered “yes” to six questions (range, 2–14), and men answered “yes” to five questions (range, 2–12).
The most common physical examination findings were edema (206 of 352; 59%), and striae (145 of 352; 41%). Twelve of 88 subjects (14%) with available data had purple stretch marks of at least 1 cm.
Mean composite scores in subjects with data available for all nine variables (eight in men) were: all females (239 of 267), 1.6 ± 1.5 (max score, 6.0; 77% scored ≤2.0); females age 40 or younger (62 of 68), 1.6 ±1.4 (max score, 5.0; 82% scored ≤2.0); and men (89 of 101), 1.0 ± 1.0 (max score, 4.0; 90% ≤2.0). Composite scores were similar between subjects with or without a screening test abnormality.
Seventy-two of 362 evaluable subjects had diabetes. A total of 170 of 356 evaluable subjects had hypertension: blood pressure was normal in 96 taking medication but abnormal in 33 on medication, 39 not on medication, and two without medication data.
Fifty-five of 320 (17%) subjects who completed questionnaires reported depression symptoms. Thirty-four (11%) reported signs of alcohol abuse.
Initial screening test results
Data were available from three screening tests (i.e. UFC, DST, and at least one saliva sample) in 326 subjects. The others had results for two tests (11 UFC and DST, four UFC and salivary cortisol RIA, seven UFC and salivary cortisol LC-MS/MS, five DST and salivary cortisol LC-MS/MS, and 16 DST and salivary cortisol RIA).
Of subjects who completed three screening tests, 28 had an abnormal result after 1 mg dexamethasone despite an adequate dexamethasone level (three of 43 in the two-test group) (Fig. 1A and Table 2). Three subjects did not have dexamethasone levels available. Two subjects had an initially abnormal response with a low dexamethasone level; each had normal cortisol and adequate dexamethasone levels after the 2 mg overnight DST. They had additional evaluation only if other results were abnormal. Fourteen of 49 women taking estrogen and who completed at least two tests had an abnormal DST result. Eleven women had normal results on follow-up testing; three did not follow up.
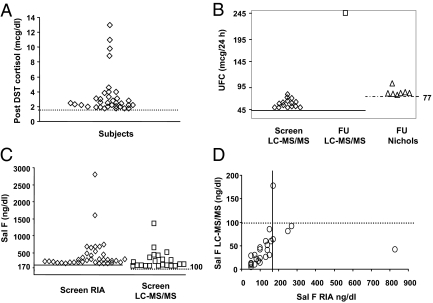
Figure 1.
Abnormal test values for subjects with two or more completed tests. A, Abnormal post 1 mg DST cortisol levels at the initial screening visit. Dotted line represents 1.8 μg/dl (49.6 nmol/liter) diagnostic cutoff level. When available, the dexamethasone levels were all appropriate. Two subjects taking oral contraceptives had cortisol levels of 4.0 and 8.5 μg/dl (110 and 234 nmol/liter); cortisol binding globulin levels were not measured. B, Abnormal UFC values at screening visit (screen) and follow-up (FU). Solid line, Upper normal limit LC-MS/MS assay, 45 μg/24 h (124 nmol/24 h); dashed line, upper normal limit Nichols assay, 77 μg/24 h (213 nmol/24 h). C, Abnormal screening salivary cortisol (Sal F) values by RIA and LC-MS/MS at the screening visit. Solid line, Upper normal limit RIA, 170 ng/dl (4.7 nmol/liter); dashed line, upper normal limit LC-MS/MS, 100 ng/dl (2.8 nmol/liter). D, Follow-up salivary cortisol results in 25 of 58 subjects with RIA and LC-MS/MS split samples. Solid vertical line, upper normal limit RIA, 170 ng/dl (4.7 nmol/liter); dashed line, upper normal limit LC-MS/MS, 100 ng/dl (2.8 nmol/liter).
Table 2.
Combination of abnormal screening test results for UFC, DST, and salivary cortisol (Sal F) by RIA and/or LC-MS/MS
| Subjects with normal DST | n | Subjects with abnormal DST | n | |
|---|---|---|---|---|
| Abnormalities among 326 subjects with data from at least 3 tests | UFC | 6 | DST | 20 |
| Sal F RIA | 18 | DST + UFC | 3 | |
| Sal F LC-MS/MS | 11 | DST + Sal F RIA | 5 | |
| UFC + Sal F RIA | 1 | DST + Sal F LC-MS/MS | 0 | |
| UFC + Sal F LC-MS/MS | 1 | DST + Sal F RIA & LC-MS/MS | 1 | |
| Sal F RIA and LC-MS/MS | 6 | DST + UFC + Sal F RIA | 1 | |
| DST + UFC + Sal F LC-MS/MS | 0 | |||
| DST + UFC + Sal F RIA & LC-MS/MS | 1 | |||
| Total | 43 | 31 | ||
| Abnormalities among 43 subjects with data from only 2 screening tests | ||||
| Sal F RIA | 3 | DST | 3 | |
| Sal F LC-MS/MS | 2 | |||
| UFC + Sal F RIA | 2 | |||
| Total | 7 | 3 | ||
| Total no. of abnormalities in both groups: 84 |
Fifteen subjects had an abnormal UFC result (Fig. 1B). Except for one result of 101 μg/24 h (normal ≤45 μg/24 h), all values were less than twice normal. The salivary cortisol concentration was abnormal in 38 of 232 subjects by RIA and in 22 of 269 subjects by the LC-MS/MS technique (Fig. 1C). Among 143 subjects who provided split saliva samples, LC-MS/MS had a significantly greater specificity than RIA (92 vs. 85%; P = 0.01). Excluding subjects with abnormal screening results and no follow-up, the specificities were 94 vs. 87% (P = 0.01).
In these subjects, the specificity of salivary cortisol by RIA was 84% (CI, 79–89%); salivary cortisol by LC-MS/MS, 92% (CI, 88–95%); UFC, 96% (CI, 93–98%); and DST, 90% (CI, 87–93%). Excluding those with no follow-up, specificities were: 87% (CI, 81–91%), 94% (CI, 90–97%), 97% (CI, 95–99%), and 92% (CI, 89–95%), respectively. DST specificity excluding women on estrogen was unchanged.
All sets of specificity data were similar for those with only two test results (data not shown). The combined specificity in all subjects (both results normal) for UFC and salivary cortisol by LC-MS/MS was 90% (CI, 86–93%); UFC and DST was 88% (CI, 84–91%); and DST and salivary cortisol by LC-MS/MS was 84% (CI, 79–88%).
Due to laboratory error, no urine creatinine was available in the first 96 subjects; additional evaluation was recommended in eight with an abnormal UFC result. UFC results in nine subjects were excluded based on creatinine or volume results.
Follow-up in patients with abnormal screening results
Fifty-eight of 84 subjects with at least one abnormal result (Table 2) underwent additional testing. The interval between the GWUWMP visit and the last test at NIH was 167–230 d. Twenty-five of the 26 subjects who did not return had only one screening test abnormality: four UFC, seven salivary cortisol RIA, six salivary cortisol LC-MS/MS, one salivary cortisol by RIA and LC-MS/MS, and seven DST. One subject had abnormal DST and UFC results.
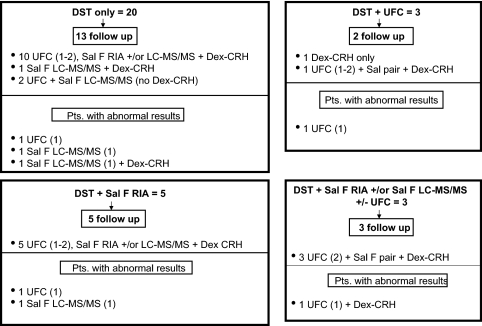
Figures 2 and 3 show the subsequent evaluation of initial DST abnormalities in 31 subjects with three tests and three with two tests. The four subjects with the highest post-DST cortisol [8.5–13 μg/dl (235–359 nmol/liter)] had normal UFC and salivary cortisol on repeat testing; three had a normal response to Dex-CRH testing, and the fourth had an abnormal result due to oral contraceptive pill use (described below). This was the likely cause of her initial abnormal DST response.
Figure 2.
Distribution of 1 mg DST abnormalities in subjects with three completed screening tests. Separate boxes indicate the types of initial abnormalities at the top, followed by the number of subjects who underwent follow-up testing. The combinations of tests performed and abnormal results are preceded by the number of subjects. The numbers in parentheses indicate the number of completed or abnormal samples. Sal F RIA, Salivary cortisol by RIA; Sal F LC-MS/MS, salivary cortisol by LC-MS/MS; Sal F pair, salivary cortisol by RIA and LC-MS/MS.
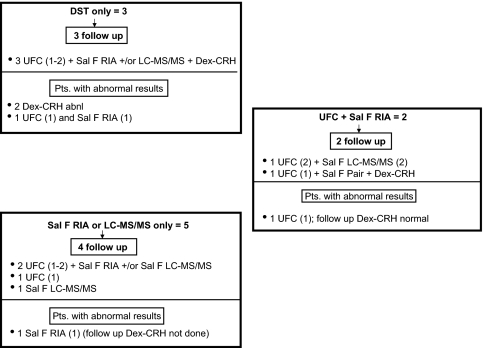
Figure 3.
Distribution of 1 mg DST, UFC, and salivary cortisol abnormalities in subjects with two completed screening tests. Separate boxes indicate the types of initial abnormalities at the top, followed by the number of subjects who underwent follow-up testing. The combinations of tests performed and abnormal results are preceded by the number of subjects. The numbers in parentheses indicate the number of completed or abnormal samples. Sal F RIA, Salivary cortisol by RIA; Sal F LC-MS/MS, salivary cortisol by LC-MS/MS; Sal F pair, salivary cortisol by RIA and LC-MS/MS.
Figures 3 and 4 show the evaluation of 50 subjects with an initially normal response to dexamethasone but other abnormal results (43 subjects with three tests and seven with two tests). One woman with a salivary cortisol LC-MS/MS value of 1360 ng/dl (37.5 nmol/liter) reportedly had a nasal steroid injection that may have contained hydrocortisone 2–3 d before collection. Repeat testing was normal.
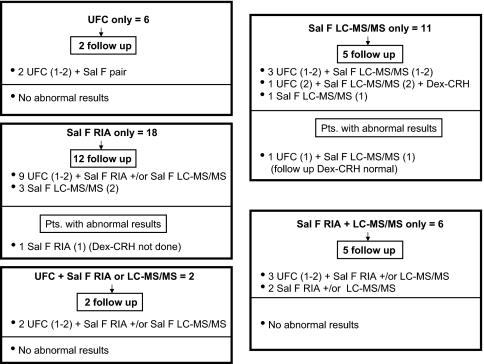
Figure 4.
Distribution of urine and salivary cortisol abnormalities in subjects with three completed screening tests and normal dexamethasone results. Separate boxes indicate the types of initial abnormalities at the top, followed by the number of subjects who underwent follow-up testing. The combinations of tests performed and abnormal results are preceded by the number of subjects. The numbers in parentheses indicate the number of completed or abnormal samples. Sal F RIA, Salivary cortisol by RIA; Sal F LC-MS/MS, salivary cortisol by LC-MS/MS; Sal F pair, salivary cortisol by RIA and LC-MS/MS.
Two subjects had extremely high screening salivary cortisol RIA results of 1600 ng/dl (44 nmol/liter) and 2800 ng/dl (77 nmol/liter). The first did not return for evaluation; however, a simultaneous LC-MS/MS salivary cortisol level of 39 ng/dl, UFC result of 7.6 μg/24 h, and post-dexamethasone cortisol result of 1.0 μg/dl were all normal. The other subsequently had normal results for one paired saliva and two urine samples.
In follow-up, 26 of 58 subjects provided saliva for RIA and LC-MS/MS evaluation (Fig. 1D; one pair insufficient quantity), whereas 29 (two with no specimen) had samples analyzed by LC-MS/MS only. Four salivary cortisol LC-MS/MS results were abnormal; one with a simultaneous RIA was normal. All four subjects underwent Dex-CRH testing (see below).
Three of the batched salivary cortisol RIA results were abnormal, each with a normal paired LC-MS/MS result. One subject had already undergone Dex-CRH testing (normal) because of a follow-up UFC of 83 μg/24 h (ICMA). The other two who had normal UFC and salivary cortisol by LC-MS/MS were considered not to have Cushing’s syndrome and did not have further testing.
Seven of the 58 subjects had eight UFC abnormalities in follow-up. The seven abnormal results by ICMA were less than 1.5-fold normal. The one abnormal result by LC-MS/MS was 2.5-fold normal and corresponded to an abnormal salivary cortisol level that was 1.5-fold normal. Repeat urine and salivary cortisol levels were normal in this subject, as was her Dex-CRH test. The specificity of follow-up UFC results by ICMA was 21 of 27, 78% (CI, 58–91%) and by LC-MS/MS was 21 of 22, 95% (CI, 77–100%).
Four Dex-CRH results were considered to be falsely abnormal; all others were normal. One subject with a 15-min post-CRH cortisol value of 2.1 μg/dl (58 nmol/liter) had a low dexamethasone level [281 ng/dl (7.2 nmol/liter)]. After a doubled dexamethasone dose every 6 h, the dexamethasone level was appropriate and cortisol was below 1.0 μg/dl (<27.6 nmol/liter). This subject had four normal repeat UFC results and one abnormal out of two repeat salivary cortisol results [LC-MS/MS; 177 ng/dl (4.8 nmol/liter)]. Two women taking oral contraceptives had borderline abnormal responses to Dex-CRH [cortisol, 1.4 μg/dl (38.0 nmol/liter) and 1.9 μg/dl (52.4 nmol/liter)]. One had a low dexamethasone level [247 ng/dl (6.3 nmol/liter)]; a CBG level was not measured. The other had an elevated CBG level [5.8 mg/dl (1100 nmol/liter); reference 2.3–3.9 mg/dl (437–741 nmol/liter)]. Each had normal repeat UFC and saliva testing and did not wish to discontinue the medication, so neither repeated the Dex-CRH test.
A fourth subject (screening UFC normal, DST abnormal) had a 15-min post-CRH cortisol value of 2.5 μg/dl (69.0 nmol/liter) and a low dexamethasone level [368 ng/dl (9.4 nmol/liter)]. Repeat testing with a doubled dexamethasone dose gave an appropriate dexamethasone level but a post-CRH cortisol value of 2.0 μg/dl (55.2 nmol/liter); CBG was normal. A detailed history was unrevealing. Based on clinical features and two normal subsequent UFC results (insufficient saliva collections), clinically significant hypercortisolism seemed unlikely. At 6, 18, and 30 months after her screening visit, she did not report diabetes, hypertension, or new symptoms of Cushing’s syndrome and had improvement in her initial symptoms.
The groups with or without any test abnormality were not statistically different in mean weight, age, gender, composite symptom scores, or frequency of diabetes, hypertension (Table 3), depression, or alcohol abuse. The BMI groups had similar rates of abnormal screening tests.
Table 3.
Characteristics and presence of comorbidities in subjects with and without test abnormalities after at least two screening tests (urine cortisol, 1 mg dexamethasone suppression, and/or salivary cortisol by RIA or LC-MS/MS)
| No. with diabetes/total | No. with HTN/total | Weight (kg)a | BMI (kg/m2)a | |
|---|---|---|---|---|
| No abnormality | 57/213 | 125/274 | 110 ± 25 | 39 ± 8 |
| ≥1 abnormality | 15/60 | 45/82 | 109 ± 26 | 39 ± 8 |
| Pvalue | 0.87 | 0.16 | 0.84 | 0.90 |
Diabetes was diagnosed by fasting blood sugar, oral glucose tolerance test, or medication use. HTN, Hypertension by medication use or physical examination.
Mean ± sd.
Sixty-three (including 16 of 26 who had not completed any follow-up) of the 84 subjects with any screening test abnormality responded to the late evaluation contact at a median of 3.2 yr (range, 1 to 5 yr) after the initial screening. None reported any further symptoms, evaluation, or recommendation for evaluation of Cushing’s syndrome.
Some subjects reported subsequent endocrine evaluations unrelated to Cushing’s syndrome. New diagnoses included polycystic ovarian syndrome (n = 4), hypothyroidism (n = 1), vitamin D deficiency (n = 2), thyroid nodule (n = 1), sleep apnea (n = 1), and type II diabetes mellitus (n = 1). A pituitary adenoma was found incidentally in one woman. One subject with an abnormal salivary cortisol result by RIA and normal UFC and 1 mg DST result at screening was later found to have an adrenal incidentaloma. She reported that hormonal evaluation was negative. She also reported a 139-pound weight loss.
Discussion
Using tests and criteria recommended by the recent Endocrine Society guidelines for screening for Cushing’s syndrome (6), we found no subject with the disorder among 369 people who were overweight or obese with a mean of five to six signs or symptoms of Cushing’s syndrome. The guidelines suggest that Cushing’s syndrome should not be diagnosed unless two tests are abnormal. When applying that strategy to this group of patients, the combined specificities for double combinations of UFC, DST, and/or salivary cortisol by LC-MS/MS ranged from 84 to 90% (88–94% excluding those with no follow-up). The specificity of UFC, DST, and salivary cortisol by LC-MS/MS together was 78% (CI, 73–83%) [or 84% (CI, 80–89%) excluding those with no follow-up].
About one fourth of the subjects had at least one abnormal result; yet, mean composite scores among subjects with and without test abnormalities were not significantly different. This highlights the difficulty in determining whom to screen and supports the fact that these screening tests were falsely positive. These results support The Endocrine Society’s suggestion to screen only those patients with unusual or multiple and progressive features of Cushing’s syndrome that increase the pretest probability of the condition (6).
Until recently there have been few data on the performance of diagnostic tests in large populations of patients suspected of having Cushing’s syndrome. Pecori Giraldi et al. (5) retrospectively studied 4104 patients in whom Cushing’s syndrome was excluded. Although UFC was measured in 3461 patients, fewer also had DST (n = 235), midnight serum cortisol (n = 335), or both (n = 88). The specificities for DST [80.2% (95% CI, 77.5–82.9%)] and UFC [89.6% (95% CI, 88.9–90.3%)] were lower than those seen in this study. However, dexamethasone levels were not measured, and the assay for UFC was antibody-based, which may have introduced bias against better results. Despite these differences, both studies underscore the high chance for falsely abnormal results when conservative criteria are used to judge response.
In the current study, screening UFC by LC-MS/MS had a specificity of 96% (CI, 93–98%). All but one abnormal result were less than 2-fold normal, in keeping with previous studies suggesting that patients suspected but not proven to have Cushing’s syndrome have values in this range (5,11). Historically, the reported specificity of UFC ranged from 73–97% in various lean, obese, and chronically ill patients (15,16) and was 90% in the Pecori-Giraldi study (5). Most previous studies used antibody-based assays to measure cortisol. Differences in results may reflect differences in study populations, compliance, and/or assays.
As we previously reported, LC-MS/MS performs better than RIA for measurement of salivary cortisol (7). The reduced performance of RIA in this study contrasts with earlier reports using diagnostic thresholds determined retrospectively with data collected within the study itself (16,17). This suggests that salivary cortisol by RIA may have high specificity in efficacy trials, but does not perform as well in a real world setting using laboratory-provided normative data (9). Additionally, the “normal” range may increase with age and comorbidities, so that use of a single reference range may lead to misdiagnosis (18).
Limitations of the study include possible verification bias caused by lack of a gold standard test to diagnose Cushing’s syndrome, the remote possibility of cyclic Cushing’s syndrome (19), and the lack of biochemical follow-up of nearly 30% of subjects with an abnormal screening result. To address this, we contacted over 60% of subjects without follow-up; none reported a new diagnosis of or evaluation for Cushing’s syndrome. In nearly all subjects, the screening abnormality was mild, and only one without follow-up had two abnormal test results. Thus, it is unlikely that these subjects had Cushing’s syndrome. However, the reader must consider these limitations when interpreting the specificities reported here.
The strengths of the current study include its ability to determine prospectively the prevalence of Cushing’s syndrome in a population enriched with symptoms consistent with the disorder and the ability to compare all three tests recommended for screening of Cushing’s syndrome.
How might an endocrinologist incorporate these results into the evaluation of an obese patient who might have Cushing’s syndrome? First, it is important to note that many features of Cushing’s syndrome and overweight and obesity overlap. This must be a factor in determining a patient’s pretest probability for the disease and the decision of whether to test, and how to interpret test results, as suggested by Elamin et al. (20). Monitoring for worsening symptoms over time and then screening, if appropriate, may be more useful than screening for Cushing’s syndrome at a first visit. Using their Fagan nomogram, if the pretest probability of Cushing’s syndrome is 5%, the posttest probability that a patient has the disorder is about 20–50% for an abnormal urine cortisol result, and about 1–2% for a normal result. Additionally, the testing strategy should be individualized. For example, the use of medications affecting CBG or dexamethasone metabolism may mitigate against the dexamethasone test. In this study, there was a 27% false-positive rate in women taking estrogen; dexamethasone levels unmasked fast metabolism in others. The choice of assay may influence the results, and further study is needed to examine this possibility. Finally, diagnostic criteria are not immutable: a borderline abnormal result is more likely to be falsely positive than an extremely elevated result.
In conclusion, broad screening programs for Cushing’s syndrome in an obese population would likely lead to unnecessary testing, false-positive results, and anxiety. We caution against a literal interpretation of the recent guidelines and suggest that clinical judgment, taking into account the number and progression of symptoms, remains essential for the diagnosis of Cushing’s syndrome.
Acknowledgments
The authors thank Ms. Elizabeth Saverino and The Eunice Kennedy Shriver National Institute of Child Health and Development Clinical Trials Database team for their help in the collection and organization of the data.
Footnotes
This research was supported (in part) by the Intramural Research Program of the National Institutes of Health, The Eunice Kennedy Shriver National Institute of Child Health and Development.
Disclosure Summary: The authors have nothing to declare.
First Published Online July 14, 2009
Abbreviations: BMI, Body mass index; CBG, cortisol binding globulin; CI, confidence interval; Dex-CRH, dexamethasone-CRH (test); DST, dexamethasone suppression test; ICMA, immunochemiluminescence metric assay; LC-MS/MS, liquid chromatography-tandem mass spectrometry; UFC, urine free cortisol.
References
- Catargi B, Rigalleau V, Poussin A, Ronci-Chaix N, Bex V, Vergnot V, Gin H, Roger P, Tabarin A 2003 Occult Cushing’s syndrome in type-2 diabetes. J Clin Endocrinol Metab 88:5808–5813 [DOI] [PubMed] [Google Scholar]
- Reimondo G, Pia A, Allasino B, Tassone F, Bovio S, Borretta G, Angeli A, Terzolo M 2007 Screening of Cushing’s syndrome in adult patients with newly diagnosed diabetes mellitus. Clin Endocrinol (Oxf) 67:225–229 [DOI] [PubMed] [Google Scholar]
- Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T 2004 Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res 27:193–202 [DOI] [PubMed] [Google Scholar]
- Newell-Price J, Bertagna X, Grossman AB, Nieman LK 2006 Cushing’s syndrome. Lancet 367:1605–1617 [DOI] [PubMed] [Google Scholar]
- Pecori Giraldi F, Ambrogio AG, De Martin M, Fatti LM, Scacchi M, Cavagnini F 2007 Specificity of first-line tests for the diagnosis of Cushing’s syndrome: assessment in a large series. J Clin Endocrinol Metab 92:4123–4129 [DOI] [PubMed] [Google Scholar]
- Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM 2008 The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 93:1526–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RL, Machacek D, Singh RJ 2002 Validation of a high-throughput method for urinary cortisol and cortisone. Clin Chem 48:1511–1519 [PubMed] [Google Scholar]
- Turpeinen U, Stenman UH 2003 Determination of urinary free cortisol by liquid chromatography-tandem mass spectrometry. Scand J Clin Lab Invest 63:143–150 [PubMed] [Google Scholar]
- Baid SK, Sinaii N, Wade M, Rubino D, Nieman LK 2007 Radioimmunoassay and tandem mass spectrometry measurement of bedtime salivary cortisol levels: a comparison of assays to establish hypercortisolism. J Clin Endocrinol Metab 92:3102–3107 [DOI] [PubMed] [Google Scholar]
- Wood PJ, Barth JH, Freedman DB, Perry L, Sheridan B 1997 Evidence for the low dose dexamethasone suppression test to screen for Cushing’s syndrome—recommendations for a protocol for biochemistry laboratories. Ann Clin Biochem 34:222–229 [DOI] [PubMed] [Google Scholar]
- Yanovski JA, Cutler Jr GB, Chrousos GP, Nieman LK 1998 The dexamethasone-suppressed corticotropin-releasing hormone stimulation test differentiates mild Cushing’s disease from normal physiology. J Clin Endocrinol Metab 83:348–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agresti A 1992 A survey of exact inference for contingency tables. Statist Sci 7:131–177 [Google Scholar]
- 2003 Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 26(Suppl 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo Jr JL, Jones DW, Materson BJ, Oparil S, Wright Jr JT, Roccella EJ 2003 Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42:1206–1252 [DOI] [PubMed] [Google Scholar]
- Crapo L 1979 Cushing’s syndrome: a review of diagnostic tests. Metabolism 28:955–977 [DOI] [PubMed] [Google Scholar]
- Viardot A, Huber P, Puder JJ, Zulewski H, Keller U, Müller B 2005 Reproducibility of nighttime salivary cortisol and its use in the diagnosis of hypercortisolism compared with urinary free cortisol and overnight dexamethasone suppression test. J Clin Endocrinol Metab 90:5730–5736 [DOI] [PubMed] [Google Scholar]
- Yaneva M, Mosnier-Pudar H, Dugué MA, Grabar S, Fulla Y, Bertagna X 2004 Midnight salivary cortisol for the initial diagnosis of Cushing’s syndrome of various causes. J Clin Endocrinol Metab 89:3345–3351 [DOI] [PubMed] [Google Scholar]
- Liu H, Bravata DM, Cabaccan J, Raff H, Ryzen E 2005 Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin Endocrinol (Oxf) 63:642–649 [DOI] [PubMed] [Google Scholar]
- Meinardi JR, Wolffenbuttel BH, Dullaart RP 2007 Cyclic Cushing’s syndrome: a clinical challenge. Eur J Endocrinol 157:245–254 [DOI] [PubMed] [Google Scholar]
- Elamin MB, Murad MH, Mullan R, Erickson D, Harris K, Nadeem S, Ennis R, Erwin PJ, Montori VM 2008 Accuracy of diagnostic tests for Cushing’s syndrome: a systematic review and metaanalyses. J Clin Endocrinol Metab 93:1553–1562 [DOI] [PubMed] [Google Scholar]