Abstract
Context: Although obesity has been, in general, associated with glomerular hyperfiltration, visceral adiposity has been suggested to be associated with reduced glomerular filtration.
Objective: The aim of the study was to evaluate the differential effects of obesity and body fat distribution on glomerular filtration.
Design and Setting: We conducted a cross-sectional study of the Japanese-American community in Seattle, Washington.
Participants: We studied a representative sample of second-generation Japanese-American men and women with normal glucose tolerance (n = 124) and impaired glucose metabolism (impaired fasting glucose and/or impaired glucose tolerance) (n = 144) residing in King County, Washington.
Main Outcome Measures: Glomerular filtration rate was estimated by 24-h urinary creatinine clearance, body size by body mass index (BMI), and intra-abdominal fat (IAF), sc fat (SCF), and lean thigh areas by CT scan.
Results: Creatinine clearance was positively correlated with BMI (r = 0.429; P < 0.001), fasting glucose (r = 0.198; P = 0.001), and insulin levels (r = 0.125; P = 0.042), as well as IAF (r = 0.239; P < 0.001), SCF (r = 0.281; P < 0.001), and lean thigh (r = 0.353; P < 0.001) areas. The association between creatinine clearance and BMI remained significant after adjustments for IAF, SCF areas, and fasting insulin levels (r = 0.337; P < 0.001); whereas IAF and SCF areas were not independently associated with creatinine clearance after adjusting for BMI. Creatinine clearance increased with increasing BMI after adjusting for fasting insulin, fasting glucose, IAF and SCF areas in subjects with normal glucose tolerance (r = 0.432; P < 0.001) and impaired glucose metabolism (r = 0.471; P < 0.001).
Conclusions: BMI rather than body fat distribution is an independent determinant of creatinine clearance in nondiabetic subjects. Lean body mass, rather than adiposity, may explain this association.
Body mass index, rather than body fat distribution, is an independent determinant of creatinine clearance, suggesting a role for lean rather than fat mass on this relationship.
Chronic kidney disease (CKD) is a significant public health problem in the United States, affecting 20 million people (11% of the U.S. adult population) (1). It not only results in renal failure and related complications but also has been associated with increased cardiovascular morbidity and mortality (1).
Renal function can be affected by many factors (2). It declines with age, and measurement of glomerular filtration rate (GFR) differs by gender (1). Long-standing hypertension and diabetes are major causes of decreased GFR (1), whereas early-stage hypertension and decompensated diabetes cause hyperfiltration (2,3). In addition, recent studies have suggested an association between insulin resistance or impaired glucose tolerance (IGT) and CKD (4,5,6). Additionally, obesity has been shown to be a strong predictor of CKD (7,8,9), even after adjustments for classical risk factors such as hypertension and diabetes (10).
Obesity, in particular visceral obesity, is associated with insulin resistance, the metabolic syndrome, and type 2 diabetes (11,12). In nondiabetic subjects, a high waist-to-hip ratio, considered a marker of central obesity, has been associated with a lower creatinine clearance (13). However, waist-to-hip ratio as an anthropometric measure is not the ideal proxy for intraabdominal fat (IAF) quantification (14). This is especially true in the Asian population, which characteristically presents with a lower body mass index (BMI) but a greater proportion of fat area comprised of IAF than do other populations (15,16). For example, Japanese-Americans with equivalent waist circumference measures can have marked differences in IAF quantified by computed tomography (CT) scan (17). Furthermore, IAF area, but not other measures of body fat, predicts the future development of glucose intolerance and hypertension in Japanese-Americans (18,19). Similarly, others have suggested that BMI, which by definition includes both fat and fat-free mass, estimates relative body weight but does not necessarily estimate body fat very well (20).
Based on these differences, examining Japanese-Americans provides a unique opportunity to determine the role of central adiposity on renal function. Therefore, we determined in a cross-sectional study whether the association between obesity and GFR, estimated with creatinine clearance, is related to body fat distribution.
Subjects and Methods
Subjects
This is a cross-sectional study of second generation (Nisei) Japanese-American men and women (ages 34–76 yr) participating in the Japanese-American Community Diabetes Study. All participants were of 100% Japanese ancestry. They were recruited between 1983 and 1985 using a comprehensive mailing list and telephone directory that included 95.5% of the 15,875 Japanese-Americans residing in households in King County, Washington, and were representative of the Japanese-American community in age distribution, residential distribution, and parental immigration pattern. The inclusion and exclusion criteria for the Japanese-American Community Diabetes Study have been previously published (21).
For this particular analysis, subjects were excluded if they were being treated with medications known to affect glucose tolerance (e.g. glucocorticoids) or had a history of kidney disease (e.g. chronic glomerulonephritis). Of the 420 individuals who met these criteria, a total of 406 had abdominal CT scan fat measures available, of whom 19 were excluded because of an inadequate urinary collection. Of the remaining 387 subjects, 119 had diabetes and were excluded. Thus, data from 268 nondiabetic subjects were used for this analysis. The 19 subjects excluded due to inadequate 24-h urinary collection were slightly younger (60.8 ± 8.4 vs. 61.9 ± 5.8; P = 0.009), but otherwise were not different than the 268 subjects used for the final analyses. The study was approved by the University of Washington Institutional Review Board, and all participants provided written informed consent.
Study procedures and assays
Standing height (in centimeters) and weight (in kilograms) were measured in shoeless subjects wearing light clothing and were used to calculate BMI as weight (kilograms)/height2 (meters). While the participant was in a recumbent position, blood pressure was measured in the right arm three times by auscultation, and the average of the last two measurements was used for the estimation of mean arterial blood pressure (MAP), defined as diastolic blood pressure plus one third of the difference between the systolic and diastolic pressure. Hypertension was diagnosed if the average systolic blood pressure was 140 mm Hg or greater, the average diastolic blood pressure was 90 mm Hg or greater, or the participant was taking antihypertensive medications.
A standard 75-g oral glucose tolerance test was performed in the morning after a 10-h overnight fast. Blood samples for measurements of glucose were drawn before and 120 min after glucose ingestion. A baseline blood sample was also drawn for creatinine and insulin measurements.
Participants were instructed on how to collect a 24-h urine sample, and they recorded the collection start and end times, with the end time being the morning of the clinical visit. The urine was kept cold (4 C) until brought to the clinical visit. Subjects avoided intense physical activity during the collection period, and urinary collection was postponed in case of fever, urinary tract infection, or menstruation. Subjects were considered as having adhered to the collection procedure if their 24-h creatinine excretion was 14 to 26 mg/kg (124 to 230 μmol/kg) per 24 h for men or 11 to 20 mg/kg (97 to 177 μmol/kg) per 24 h for women (22).
Plasma glucose was assayed by an automated glucose oxidase method and plasma insulin by RIA as previously described (23). Insulin resistance was assessed using plasma insulin levels and the homeostasis model assessment (HOMA) [fasting insulin (pmol) × fasting glucose (mmol)/22.5] (24). Serum and urinary creatinine levels were measured by the automated picric acid method.
Body fat distribution and lean body mass
Abdominal fat distribution was quantified using CT scans. A single 10-mm slice of the abdomen at the umbilicus level was used to measure cross-sectional abdominal sc fat (SCF) and IAF areas (measured in cm2), using the transversalis fascia to demarcate the boundary between these two (17). This measurement has a high correlation with directly ascertained total visceral fat volume measured by CT scan or magnetic resonance imaging (25,26,27).
A 10-mm cross-sectional CT scan slice of the right midthigh was used, and the estimated adipose tissue area was subtracted from the total midthigh area. This difference represents the lean thigh area and was used as an estimation of lean body mass.
Classification of glucose tolerance
Using fasting and 2-h plasma glucose concentrations, subjects were categorized based on the World Health Organization/International Diabetes Federation Report on Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia as having normal glucose tolerance (NGT) [fasting plasma glucose (FPG) <110 mg/dl (6.1 mmol/liter) and 2-h plasma glucose <140 mg/dl (7.8 mmol/liter)], impaired fasting glucose (IFG) [FPG of 110–125 mg/dl (6.1–6.9 mmol/liter) and 2-h plasma glucose <140 mg/dl (7.8 mmol/liter)], or IGT [FPG <110 mg/dl (6.1 mmol/liter) and 2-h plasma glucose of 140–199 mg/dl (7.8–11.0 mmol/liter)] (28). Subjects with IFG and/or IGT were considered to have impaired glucose metabolism (IGM) and comprised 11 individuals with isolated IFG, 111 with isolated IGT, and 22 with both IFG and IGT.
Creatinine clearance estimation
Creatinine clearance was estimated with the 24-h urine collection (ml/min) using the following formula: urinary creatinine (mg/dl) × urinary volume (ml)/plasma creatinine (mg/dl) × time (min). Creatinine clearance was expressed in absolute values (ml/min) and not indexed per 1.73 m2 of body surface area because indexing of GFR by body surface area has been shown to result in an unacceptable underestimation of the real GFR and would be considered inappropriate in searching for a relationship between renal function and body size (29,30).
Data analysis and statistical methods
Statistical analysis was performed using STATA version 9.1 (STATACorp, College Station, TX). Data are presented as mean ± sd unless otherwise specified. To compare demographic, clinical, and laboratory data, the Fisher exact test and the independent-samples t test were used as appropriate. Insulin and all fat area measurements were log-transformed to achieve a normal distribution before analysis for comparison between groups.
Correlations were performed with the Pearson’s correlation test. Multiple linear regression was performed to assess the relationship between BMI, body fat distribution, and creatinine clearance while adjusting for potential confounders. Logarithmic transformation was performed for the dependent variable, namely creatinine clearance, to satisfy the normality assumptions for linear regression. Regression models using creatinine clearance adjusted for body surface area as the dependent variable and incorporating BMI as an independent variable would have weight and height incorporated into both dependent and independent variables, resulting in a spurious association. To test for this, we performed a univariate analysis using these two variables and found that BMI was associated with creatinine clearance adjusted for a body surface area of 1.73 m2, confirming our presumption. Multicolinearity was assessed by using the variance inflation factor, which when it exceeds 10 is regarded as indicating serious multicolinearity, whereas values greater than 4.0 may be a cause for concern (31). Effect modification was assessed with standard methods involving testing the significance of first-order interaction terms in regression models.
A two-sided P value <0.05 was considered significant.
Results
Subject characteristics
The study group comprised 268 subjects, of whom 145 (54.1%) were males and 123 (45.9%) were females. Table 1 lists the characteristics of the subjects subdivided by glucose tolerance. Among these individuals, 46.3% had NGT, and 53.7% had IGM. Although these two groups did not differ by age, gender distribution, systolic and diastolic blood pressure, fasting insulin levels, HOMA of insulin resistance (HOMA-IR), creatinine clearance, abdominal SCF area or lean thigh area, the prevalence of hypertension was higher in subjects with IGM, and MAP also tended to be greater in this group. BMI, waist circumference, and IAF area were greater in subjects with IGM than in subjects with NGT, and by definition, subjects with IGM had higher fasting and 2-h plasma glucose levels than subjects with NGT. A total of 131 (48.9%) participants had a creatinine clearance of at least 90 ml/min (≥1.5 ml/sec), 123 (45.9%) had a 60–90 ml/min (1.0–1.5 ml/sec) clearance, and 14 (5.2%) had a 30–60 ml/min (0.5–1.0 ml/sec) clearance. No subjects were taking angiotensin-converting enzyme inhibitors, Ninety-one were using diuretics, and 74 were taking adrenergic blockers. Creatinine clearance did not differ between subjects with or without hypertension (88.3 ± 21.9 vs. 92.4 ± 18.4; P = 0.095) or those taking or not taking adrenergic blockers (88.9 ± 21.5 vs. 90.9 ± 19.8; P = 0.476), but it was lower in those taking diuretics (85.4 ± 19.0 vs. 93.0 ± 20.5; P = 0.004).
Table 1.
Demographic, clinical, and laboratory characteristics in subjects with NGT and IGM
| NGT | IGM | P | |
|---|---|---|---|
| n | 124 | 144 | |
| Age (yr) | 61.6 ± 5.6 | 62.2 ± 5.9 | 0.412 |
| Female sex | 62 (50.0) | 61 (42.4) | 0.130 |
| Arterial hypertension | 51 (41.1) | 80 (55.6) | 0.013 |
| Diastolic blood pressure (mm Hg) | 77.4 ± 0.9 | 79.4 ± 0.8 | 0.088 |
| Systolic blood pressure (mm Hg) | 133.1 ± 1.7 | 137.6 ± 1.6 | 0.058 |
| MAP (mm Hg) | 96.0 ± 12.5 | 98.8 ± 11.2 | 0.051 |
| BMI (kg/m2) | 23.9 ± 2.9 | 24.8 ± 3.4 | 0.019 |
| Waist (cm) | 86.6 ± 7.0 | 88.7 ± 8.2 | 0.029 |
| FPG (mg/dl) | 93.5 ± 7.9 | 100.2 ± 10.5 | <0.001 |
| 2-h plasma glucose (mg/dl) | 114.9 ± 18.6 | 161.1 ± 18.6 | <0.001 |
| Fasting insulin (μU/ml) | 12.5 ± 5.6 | 12.5 ± 5.3 | 0.698 |
| HOMA-IR (pmol · mmol) | 2.9 ± 1.4 | 3.1 ± 1.4 | 0.214 |
| Creatinine clearance (ml/min) | 88.9 ± 17.9 | 91.6 ± 22.1 | 0.370 |
| IAF area (cm2) | 93.8 ± 46.4 | 108.7 ± 51.6 | 0.034 |
| Abdominal SCF area (cm2) | 160.8 ± 74.4 | 164.9 ± 77.4 | 0.662 |
| Lean thigh area (cm2) | 117.0 ± 29.9 | 119.6 ± 32.1 | 0.502 |
Data are expressed as mean ± sd or number (%). FPG and 2-h plasma glucose in mg/dl may be converted to mmol/liter by dividing by 18. Fasting insulin in μU/ml may be converted in pmol/liter by multiplying by 7.175. Creatinine clearance in ml/min may be converted in ml/sec by multiplying by 0.0167.
Relationship of blood pressure, metabolic variables, obesity, fat distribution, and lean body mass with creatinine clearance
FPG, fasting insulin, and HOMA-IR adjusted for age and gender were positively correlated with creatinine clearance (Table 2; all subjects). No significant correlations were observed between MAP, 2-h plasma glucose, and creatinine clearance (Table 2; all subjects).
Table 2.
Associations of creatinine clearance with BMI, body fat measures, lean thigh area, and factors involved in renal homeostasis in all subjects and subdivided based on glucose tolerance
| Independent variable | All Subjects (n = 268)
|
NGT (n = 124)
|
IGM (n = 144)
|
|||
|---|---|---|---|---|---|---|
| Partial r | P | Partial r | P | Partial r | P | |
| BMI | 0.429 | <0.001 | 0.359 | <0.001 | 0.480 | <0.001 |
| IAF area | 0.239 | <0.001 | 0.236 | 0.009 | 0.245 | 0.003 |
| Abdominal SCF area | 0.281 | <0.001 | 0.221 | 0.015 | 0.324 | <0.001 |
| Lean thigh area | 0.353 | <0.001 | 0.332 | <0.001 | 0.361 | <0.001 |
| MAP | −0.028 | 0.649 | 0.001 | 0.995 | −0.060 | 0.475 |
| FPG | 0.198 | 0.001 | 0.163 | 0.073 | 0.233 | 0.005 |
| 2-h plasma glucose | 0.006 | 0.925 | −0.088 | 0.334 | −0.039 | 0.647 |
| Fasting insulin | 0.125 | 0.042 | 0.135 | 0.139 | 0.122 | 0.148 |
| HOMA-IR | 0.156 | 0.011 | 0.133 | 0.145 | 0.173 | 0.039 |
All models were adjusted for age and gender.
To examine the relationship between renal function, obesity, and body fat distribution, we determined the partial correlation between these factors and creatinine clearance adjusted for age and gender (Table 2, all subjects, and Fig. 1). BMI, IAF, and abdominal SCF areas were positively correlated with creatinine clearance. A similar positive correlation was found when height was substituted for BMI (r = 0.274; P < 0.001). Adjustment for the presence of hypertension did not change these results.
Figure 1.
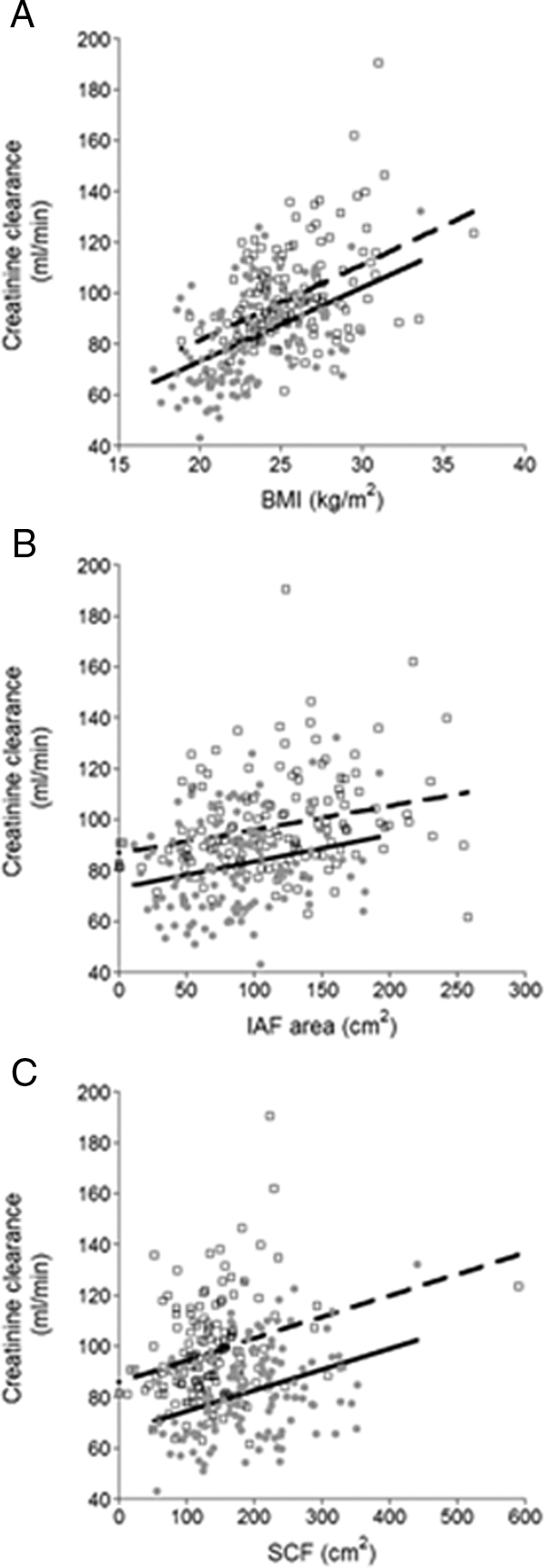
Relationship between creatinine clearance and BMI (A), IAF (B), and abdominal SCF (C) in 145 men (broken line) and 123 women (solid line).
To examine the relationship between lean body mass and renal function, we determined the relationship between lean thigh area, estimated by CT scan, and creatinine clearance. Lean thigh area was associated with creatinine clearance, after adjustment for age and sex (Table 2).
Impact of gender on the relationship between metabolic parameters and body fat distribution with creatinine clearance
In males, BMI (r = 0.370; P < 0.001), FPG (r = 0.166; P = 0.047), IAF (r = 0.220; P = 0.008), and abdominal SCF (r = 0.178; P = 0.032) areas were significantly associated with creatinine clearance. In females, BMI (r = 0.488; P < 0.001), FPG (r = 0.234; P = 0.010), fasting insulin (r = 0.217; P = 0.016), IAF (r = 0.277; P = 0.002), and abdominal SCF (r = 0.367; P < 0.001) areas were significantly associated with creatinine clearance.
Multivariate analysis: adjustments for factors significantly associated with creatinine clearance
We performed multiple linear regression analysis to adjust for the potential confounders, age and gender (Table 3). First we investigated whether BMI and IAF were independent determinants of creatinine clearance. BMI was significantly associated with creatinine clearance after adjusting for IAF area (Table 3, model 1; all subjects). However, IAF area was not associated with creatinine clearance independent of BMI. In addition, the association between BMI and creatinine clearance remained significant when adjusting for abdominal SCF area (Table 3, model 2; all subjects) or both IAF and abdominal SCF areas (Table 3, model 3; all subjects). Moreover, BMI remained significantly associated with creatinine clearance even after adjusting for fasting insulin in addition to IAF and abdominal SCF areas (Table 3, model 4; all subjects). Furthermore, adjustments for FPG did not change this relationship (r = 0.329; P < 0.001). The association remained significant after replacing fasting insulin with HOMA-IR in the model (r = 0.325; P < 0.001). Because some clinical and laboratory characteristics differed by gender, we performed the same analyses stratified by gender and found that BMI remained significantly associated with creatinine clearance (P < 0.01) in all four models. The variance inflation factor was less than 4 in all models tested, verifying that colinearity was unlikely in these analyses.
Table 3.
Multiple linear regression analyses of the association of creatinine clearance with BMI, measures of body fat distribution, and fasting insulin in all subjects, and subdivided based on glucose tolerance
| Independent variable | All Subjects (n = 268)
|
NGT (n = 124)
|
IGM (n = 144)
|
|||
|---|---|---|---|---|---|---|
| Partial r value | P value | Partial r value | P value | Partial r value | P value | |
| Model 1 | ||||||
| BMI | 0.371 | <0.001 | 0.278 | 0.002 | 0.430 | 0.001 |
| IAF area | −0.059 | 0.342 | −0.012 | 0.888 | −0.071 | 0.402 |
| Model 2 | ||||||
| BMI | 0.343 | <0.001 | 0.299 | 0.001 | 0.377 | <0.001 |
| Abdominal SCF area | −0.067 | 0.274 | −0.078 | 0.397 | −0.056 | 0.509 |
| Model 3 | ||||||
| BMI | 0.331 | <0.001 | 0.271 | 0.003 | 0.368 | <0.001 |
| IAF area | −0.053 | 0.389 | 0.002 | 0.984 | −0.070 | 0.411 |
| Abdominal SCF area | −0.063 | 0.309 | −0.077 | 0.405 | −0.055 | 0.522 |
| Model 4 | ||||||
| BMI | 0.337 | <0.001 | 0.273 | 0.003 | 0.375 | <0.001 |
| IAF area | −0.041 | 0.514 | 0.007 | 0.938 | −0.054 | 0.527 |
| Abdominal SCF area | −0.063 | 0.313 | −0.074 | 0.427 | −0.058 | 0.502 |
| Fasting insulin | −0.066 | 0.285 | −0.041 | 0.658 | −0.074 | 0.386 |
All models were adjusted for age and gender.
Impact of hypertension on the relationship between obesity and body fat distribution with creatinine clearance
In subjects without hypertension, using the full model (Table 3, model 4), BMI was again independently associated with creatinine clearance (r = 0.271; P = 0.002), whereas IAF area (r = −0.034; P = 0.697), abdominal SCF area (r = −0.030; P = 0.731), and fasting insulin (r= 0.015, P = 0.862) were not associated with creatinine clearance. In subjects with hypertension, BMI was also associated with creatinine clearance (r = 0.357; P < 0.001), and again IAF area (r = 0.024; P = 0.791), abdominal SCF area (r = −0.098; P = 0.274), and fasting insulin (r = −0.118; P = 0.187) were not. Additionally, the association between BMI and creatinine clearance remained even when analyzing subjects with hypertension not taking antihypertensive medications (r = 0.537; P < 0.001), whereas again IAF area (r = −0.057; P = 0.725), abdominal SCF area (r = −0.273; P = 0.088), and fasting insulin (r = −0.065; P = 0.688) did not. We also performed analyses excluding subjects who were on antihypertensive medications. In normotensive subjects and those who were not taking antihypertensive medications (n = 160), BMI was associated with creatinine clearance (r = 0.352; P < 0.001), whereas IAF area (r = 0.028; P = 0.728), SCF area (r = −0.126; P = 0.117), and insulin (r = −0.036; P = 0.661) were not. Finally, when hypertension was added as an interaction term to BMI in the model, the relationship between BMI and creatinine clearance did not vary by the presence or absence of hypertension (P = 0.146 for interaction).
Impact of glucose tolerance status on the relationship between obesity and body fat distribution with creatinine clearance
We performed the same analyses comparing subjects with and without IGM (Table 2). In NGT subjects (Table 2), greater creatinine clearance was associated with increasing BMI, IAF, and abdominal SCF areas, even after adjustments in different models for age and gender. In subjects with IGM (Table 2), greater creatinine clearance was associated in different models with BMI, IAF area, abdominal SCF area, and FPG.
Using the same models as shown in Table 3, creatinine clearance was independently associated with BMI, adjusting for IAF area, abdominal SCF area, and fasting insulin in subjects with and without IGM (Table 3, models 1–4; NGT and IGM). Furthermore, adjustments for FPG did not change this relationship (P < 0.001; NGT and IGM). When replacing fasting insulin with HOMA-IR in this model BMI continued to be associated with creatinine clearance in NGT (r = 0.261; P = 0.004) and IGM (r = 0.356; P < 0.001) subjects.
Discussion
In the present study, we assessed whether BMI and/or body fat distribution in Japanese-Americans might mediate the previously described association between obesity and creatinine clearance (32,33,34). Our data indicate that in Japanese-Americans without diabetes, higher BMI is associated with greater creatinine clearance independent of body fat distribution, age, gender, fasting insulin, and HOMA-IR—the latter two as surrogate markers of insulin sensitivity. Although we found a positive relationship between abdominal visceral fat and creatinine clearance, this association was not independent of BMI. We also found that SCF area was positively related with creatinine clearance but was not independent of BMI. The relationship between body fat distribution and creatinine clearance did not differ between males and females in simple and multiple regression analyses, suggesting that gender was not a major modifier of the independent relationship between BMI and creatinine clearance. We used lean thigh area as a surrogate estimate of lean body mass and found that it was associated with creatinine clearance. These results support the association between creatinine clearance and lean body mass as reflected by the thigh measurement and overall as reflected by BMI after adjustment for adipose depots.
Several epidemiological studies have suggested that the association between BMI and creatinine clearance is related to obesity and the amount of body fat or body fat distribution, the latter using measurements of waist and hip circumferences (7,8,10,13,33). Our study is unique because we performed analyses using CT scan-derived measures to estimate the contribution of abdominal fat distribution to this relationship. CT scan is a useful method for estimating IAF and SCF areas (35). Although used as measures of central adiposity, waist circumference and the waist-to-hip ratio are less useful measures of visceral adiposity (14,27) and could explain, in part, the differences between our findings and those reported by Pinto-Sietsma et al. (13).
Several studies have shown an association of obesity with glomerular hyperfiltration (32,34,36), CKD (7,9,33,37), and end-stage renal disease (8,10). BMI was used to estimate and classify obesity in these studies. The mechanism that leads obesity to be a determinant of renal function is not well understood (9). Because nephron number does not increase with weight gain in adults, excessive weight gain raises renal plasma flow and increases single nephron perfusion, resulting in increased glomerular intracapillary pressure, hyperfiltration, and the triggering of the process that results in subsequent loss of GFR over time (7,9).
Other factors such as hypertension and insulin resistance have been implicated as predictors of renal function (1,4,5). Studies have shown obesity to be a risk factor for hypertension (38). Obese subjects also have increased sympathetic and renin-angiotensin system activity that leads to an increment of blood pressure accelerating the progressive deterioration of renal function over time in a kidney with an already increased single nephron GFR (9,32,38). However, we found no relationship of creatinine clearance with blood pressure. Insulin resistance has also been associated with decreased GFR and microalbuminuria in some studies, although none of these assessed body fat distribution by CT scan (4,5). In contrast, we found that insulin sensitivity, as determined by fasting insulin and HOMA-IR, was not associated with creatinine clearance after adjustments for BMI, IAF, and abdominal SCF areas.
Although fasting, but not 2-h plasma glucose, was associated with creatinine clearance in the entire sample, it was strongly associated with creatinine clearance in subjects with IGM, but not in those with NGT, corroborating a previous finding of others that suggests a possible association between mild hyperglycemia (FPG ≥110 mg/dl) and alterations in renal homeostasis (4).
There are potential limitations to our study. First, we were not able to directly test whether increased muscle mass is responsible for our findings. However, we were able to use lean thigh area as a surrogate of lean body mass. Second, the cross-sectional study design makes it difficult to infer causality between obesity and changes in renal function. Thus, although showing that relative weight is positively correlated with creatinine clearance in the healthy state, our results do not address which elements of body size are related to the development of impaired kidney function over time. Third, GFR was estimated with 24-h creatinine clearance and not directly using inulin or radioisotope methods, which are considered the best measures of renal function (1). Clearly, the use of these exogenous markers to estimate GFR is impractical in clinical practice (2); however, in subjects with a normal GFR, creatinine clearance calculated from a 24-h urine provides estimates that are very similar to those obtained with insulin or radioisotopes (39,40). Additionally, and in contrast to the Cockcroft-Gault and Modification of Diet in Renal Disease (MDRD) formulas, creatinine clearance does not include adjustments for weight and body surface area, thus avoiding double corrections in the regression models in which BMI is included. Lastly, our findings were obtained in a single ethnic group and may not be generalizable to other ethnic groups.
In conclusion, we have demonstrated that BMI is related to renal function estimated by creatinine clearance, and this relationship is independent of body fat distribution. Along with our observation of a relationship between creatinine clearance and lean tissue area in the thigh, this finding suggests that this association may be related to lean body mass rather than adiposity.
Footnotes
This work was supported by the Department of Veterans Affairs and National Institutes of Health Grants DK-55460, DK-02860, DK-02654, DK-35816, and RR-37. F.G. was supported by a scholarship from Coordination of Higher Education and Graduate Training (CAPES) in Brazil and by the International Scholarship Program of The Endocrine Society.
Disclosure Summary: The authors have nothing to declare.
First Published Online July 7, 2009
Abbreviations: BMI, Body mass index; CKD, chronic kidney disease; CT, computed tomography; FPG, fasting plasma glucose; GFR, glomerular filtration rate; HOMA, homeostasis model assessment; HOMA-IR, HOMA of insulin resistance; IAF, intraabdominal fat; IFG, impaired fasting glucose; IGM, impaired glucose metabolism; IGT, impaired glucose tolerance; MAP, mean arterial blood pressure; NGT, normal glucose tolerance; SCF, sc fat.
References
- Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G 2003 National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139:137–147 [DOI] [PubMed] [Google Scholar]
- Stevens LA, Coresh J, Greene T, Levey AS 2006 Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med 354:2473–2483 [DOI] [PubMed] [Google Scholar]
- Silveiro SP, Friedman R, Gross JL 1993 Glomerular hyperfiltration in NIDDM patients without overt proteinuria. Diabetes Care 16:115–119 [DOI] [PubMed] [Google Scholar]
- Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J 2004 The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med 140:167–174 [DOI] [PubMed] [Google Scholar]
- Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, Whelton PK, He J 2003 Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J Am Soc Nephrol 14:469–477 [DOI] [PubMed] [Google Scholar]
- Singleton JR, Smith AG, Russell JW, Feldman EL 2003 Microvascular complications of impaired glucose tolerance. Diabetes 52:2867–2873 [DOI] [PubMed] [Google Scholar]
- Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D 2005 Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis 46:587–594 [DOI] [PubMed] [Google Scholar]
- Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S 2004 Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int 65:1870–1876 [DOI] [PubMed] [Google Scholar]
- Hall JE, Henegar JR, Dwyer TM, Liu J, Da Silva AA, Kuo JJ, Tallam L 2004 Is obesity a major cause of chronic kidney disease? Adv Ren Replace Ther 11:41–54 [DOI] [PubMed] [Google Scholar]
- Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS 2006 Body mass index and risk for end-stage renal disease. Ann Intern Med 144:21–28 [DOI] [PubMed] [Google Scholar]
- Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L 2000 Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 23:465–471 [DOI] [PubMed] [Google Scholar]
- Tong J, Boyko EJ, Utzschneider KM, McNeely MJ, Hayashi T, Carr DB, Wallace TM, Zraika S, Gerchman F, Leonetti DL, Fujimoto WY, Kahn SE 2007 Intra-abdominal fat accumulation predicts the development of the metabolic syndrome in non-diabetic Japanese-Americans. Diabetologia 50:1156–1160 [DOI] [PubMed] [Google Scholar]
- Pinto-Sietsma SJ, Navis G, Janssen WM, de Zeeuw D, Gans RO, de Jong PE 2003 A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis 41:733–741 [DOI] [PubMed] [Google Scholar]
- Clasey JL, Bouchard C, Teates CD, Riblett JE, Thorner MO, Hartman ML, Weltman A 1999 The use of anthropometric and dual-energy x-ray absorptiometry (DXA) measures to estimate total abdominal and abdominal visceral fat in men and women. Obes Res 7:256–264 [DOI] [PubMed] [Google Scholar]
- Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson Jr RN 1994 Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr 60:23–28 [DOI] [PubMed] [Google Scholar]
- McNeely MJ, Boyko EJ, Shofer JB, Newell-Morris L, Leonetti DL, Fujimoto WY 2001 Standard definitions of overweight and central adiposity for determining diabetes risk in Japanese Americans. Am J Clin Nutr 74:101–107 [DOI] [PubMed] [Google Scholar]
- Shuman WP, Morris LL, Leonetti DL, Wahl PW, Moceri VM, Moss AA, Fujimoto WY 1986 Abnormal body fat distribution detected by computed tomography in diabetic men. Invest Radiol 21:483–487 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, Fujimoto WY 2003 Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes Care 26:650–655 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, Fujimoto WY 2004 Visceral adiposity is an independent predictor of incident hypertension in Japanese Americans. Ann Intern Med 140:992–1000 [DOI] [PubMed] [Google Scholar]
- Benn RT 1971 Some mathematical properties of weight-for-height indices used as measures of adiposity. Br J Prev Soc Med 25:42–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto WY, Leonetti DL, Kinyoun JL, Newell-Morris L, Shuman WP, Stolov WC, Wahl PW 1987 Prevalence of diabetes mellitus and impaired glucose tolerance among second-generation Japanese-American men. Diabetes 36:721–729 [DOI] [PubMed] [Google Scholar]
- Painter PC, Smith JL 2001 Reference information for the clinical laboratory. In: Burtis CA, Ashwood ER, eds. Tietz fundamentals of clinical chemistry. 5th ed. Philadelphia: WB Saunders; 955–1027 [Google Scholar]
- Boyko EJ, Leonetti DL, Bergstrom RW, Newell-Morris L, Fujimoto WY 1996 Low insulin secretion and high fasting insulin and C-peptide levels predict increased visceral adiposity. Five-year follow-up among initially nondiabetic Japanese-American men. Diabetes 45:1010–1015 [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC 1985 Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419 [DOI] [PubMed] [Google Scholar]
- Han TS, Kelly IE, Walsh K, Greene RM, Lean ME 1997 Relationship between volumes and areas from single transverse scans of intra-abdominal fat measured by magnetic resonance imaging. Int J Obes Relat Metab Disord 21:1161–1166 [DOI] [PubMed] [Google Scholar]
- Han TS, McNeill G, Seidell JC, Lean ME 1997 Predicting intra-abdominal fatness from anthropometric measures: the influence of stature. Int J Obes Relat Metab Disord 21:587–593 [DOI] [PubMed] [Google Scholar]
- Schoen RE, Thaete FL, Sankey SS, Weissfeld JL, Kuller LH 1998 Sagittal diameter in comparison with single slice CT as a predictor of total visceral adipose tissue volume. Int J Obes Relat Metab Disord 22:338–342 [DOI] [PubMed] [Google Scholar]
- 2006 WHO and International Diabetes Federation (IDF) Technical Advisory Group: Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation. Available from www.idf.org/webdata/docs/WHO_IDF_definition_diagnosis_of_diabetes.pdf. Accessed on 12 April, 2009 [Google Scholar]
- Delanaye P, Radermecker RP, Rorive M, Depas G, Krzesinski JM 2005 Indexing glomerular filtration rate for body surface area in obese patients is misleading: concept and example. Nephrol Dial Transplant 20:2024–2028 [DOI] [PubMed] [Google Scholar]
- Turner ST, Reilly SL 1995 Fallacy of indexing renal and systemic hemodynamic measurements for body surface area. Am J Physiol 268:R978–R988 [DOI] [PubMed] [Google Scholar]
- Glantz S, Slinder B 1990 Primer of applied regression and analysis of variance. New York: McGraw-Hill; 181–238 [Google Scholar]
- Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U 2000 Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol 278:F817–F822 [DOI] [PubMed] [Google Scholar]
- Chertow GM, Hsu CY, Johansen KL 2006 The enlarging body of evidence: obesity and chronic kidney disease. J Am Soc Nephrol 17:1501–1502 [DOI] [PubMed] [Google Scholar]
- Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y 2003 The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 14:1480–1486 [DOI] [PubMed] [Google Scholar]
- Shen W, Wang Z, Punyanita M, Lei J, Sinav A, Kral JG, Imielinska C, Ross R, Heymsfield SB 2003 Adipose tissue quantification by imaging methods: a proposed classification. Obes Res 11:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszewski M, Charchar FJ, Maric C, McClure J, Crawford L, Grzeszczak W, Sattar N, Zukowska-Szczechowska E, Dominiczak AF 2007 Glomerular hyperfiltration: a new marker of metabolic risk. Kidney Int 71:816–821 [DOI] [PubMed] [Google Scholar]
- Kurella M, Lo JC, Chertow GM 2005 Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol 16:2134–2140 [DOI] [PubMed] [Google Scholar]
- Hall JE, Brands MW, Henegar JR 1999 Mechanisms of hypertension and kidney disease in obesity. Ann NY Acad Sci 892:91–107 [DOI] [PubMed] [Google Scholar]
- Coresh J, Toto RD, Kirk KA, Whelton PK, Massry S, Jones C, Agodoa L, Van Lente F 1998 Creatinine clearance as a measure of GFR in screenees for the African-American Study of Kidney Disease and Hypertension pilot study. Am J Kidney Dis 32:32–42 [DOI] [PubMed] [Google Scholar]
- Levey AS, Greene T, Schluchter MD, Cleary PA, Teschan PE, Lorenz RA, Molitch ME, Mitch WE, Siebert C, Hall PM, Steffes MW 1993 Glomerular filtration rate measurements in clinical trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group. J Am Soc Nephrol 4:1159–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]


