Abstract
Background: Testosterone (T) production declines and LH pulses become smaller and more frequent in middle-aged men. The mechanisms underlying these changes are not known.
Rationale: Small frequent LH pulses in middle-aged men could reflect impaired feedback by systemic T.
Hypothesis: Middle age disrupts negative feedback by T on selected facets of LH secretion.
Subjects and Setting: Healthy men were studied at an academic medical center.
Methods: The protocol comprised blockade of gonadal steroidogenesis and graded transdermal addback of T doses of 0, 2.5, 5, or 7.5 mg/d designed to span the castrate to physiological range of T concentrations in each of 23 healthy men ages 19–71 yr (interquartile range, 28–53 yr). We quantified 12-h basal and pulsatile LH secretion (92 time series) using a mathematically justified deconvolution method.
Results: Stepwise T supplementation from the hypogonadal through the eugonadal range repressed mean (12-h) LH concentrations (P = 0.001). By regression analysis, age attenuated the capabilities of increasing T concentrations to 1) increase LH secretory-burst mass (P < 0.0001); and 2) decrease LH secretory-burst frequency (P = 0.025). Age did not alter T’s feedback on basal LH secretion, interpulse regularity, the waveform of LH secretory bursts, or the slow half-life of LH.
Conclusion: Middle age impairs both the positive and negative actions of systemic T on pulsatile LH secretion in healthy men, thus potentially explaining earlier inconsistencies in feedback studies based upon single-sample mean LH concentrations. Longitudinal studies will be required to elucidate the precise age dependence of inferred dual feedback failure.
Aging attenuates the positive action of testosterone on the mass of LH secreted per burst, and also diminishes the negative-feedback effect of testosterone on LH secretory-burst frequency; specialized models are required to ascertain such complex changes.
Cross-sectional and longitudinal studies show progressive androgen depletion in middle-aged and older men (1,2). The fall in unbound testosterone (T) concentrations from young adulthood to later decades of life in healthy community-dwelling men is about 30–50%. Epidemiological analyses correlate hypoandrogenemia with insulin resistance, dyslipidemia, sarcopenia, osteopenia, reduced physical stamina, sexual dysfunction, depressive mood, and cognitive impairment. Conversely, androgen-supplementation trials suggest that T administration may reverse certain features of physical frailty in older men (3). Whereas the precise cause of declining T production is not known, available data suggest that mechanisms are likely to be multifactorial.
Clinical investigations of the bases of aging-associated T deficiency have suggested at least four regulatory deficits; viz., increased LH pulse frequency, reduced LH pulse size, disorderly release of LH and T, and impaired Leydig-cell responsiveness to human chorionic gonadotropin and LH (4,5,6). In experimental paradigms and mathematical feedback models, reduced T concentrations in healthy young men evoke frequent, small, and irregular LH pulses, thereby mimicking aging-related patterns of LH secretion (7). Thus, lower T concentrations in middle-aged and older men may either be responsible for or contribute to altered LH pulsatility. Alternatively, aging may impair negative feedback on GnRH-LH secretion by any given T concentration due to decreased feedback sensitivity per se. It is not known which of these hypotheses, if either, is correct.
Feedback studies using exogenous androgens have reported normal, accentuated, and diminished inhibition of mean LH concentrations in older compared with young men (8,9,10,11,12). Conversely, investigations of endogenous T-dependent suppression of LH secretion have inferred primarily reduced negative feedback in aging individuals (13), consistent with analytical predictions of an ensemble model of GnRH-LH-T interactions (7). Although the reasons for these differences are not known, an evaluable hypothesis is that the dose dependence of T feedback changes in middle age and thereafter. Exploring this postulate is particularly important because prior studies have used only single exogenous T doses of 7 to 35 mg daily (14,15), a potent anabolic steroid, fluoxymesterone (16), or pharmacological amounts of 5α-dihydrotestosterone (5α-DHT) (17).
The present investigation implements a combined strategy of short-term inhibition of Leydig-cell steroidogenesis and graded transdermal repletion of randomly ordered T doses (0, 2.5, 5, and 7.5 mg T daily) to enforce four strata of mean T-negative feedback in healthy men of middle age and older. The objective was to assess how age (mean, 44 yr) alters concentration-dependent feedback of T on LH secretory-burst size (mass), number (frequency), and shape (waveform); basal LH secretion; and LH half-life.
Subjects and Methods
Subjects
Twenty-three men ages 19 to 71 yr (mean, 44 yr; with two to six men per decade) enrolled in the study after providing written voluntary informed consent approved by Mayo Clinic Institutional Review Board. All men were of Caucasian origin, and baseline characteristics are provided in Supplemental Table 1 (published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Volunteers were healthy community-dwelling men within 20% of ideal body weight and with no history of infertility, systemic disease, acute weight change (exceeding 2 kg in the preceding 6 wk), T therapy, or psychoactive drug use. None had undertaken recent transmeridian travel (within 10 d) or consumed alcohol, caffeine, or systemic medications within five biological half-lives. Outpatient screening was unremarkable in relation to medical history (particularly libido and erectile function), physical examination (including testis size), and fasting morning (0800 h) biochemical tests of renal, hepatic, hematological, endocrine, and metabolic function (plasma glucose and serum T4, prolactin, IGF-I, LH, FSH, T, and estradiol). Volunteers were recruited by public advertisement, and 20% of men screened did not participate further. One half of those excluded were obese.
Intervention
Participants were admitted to the Mayo Clinical Research Unit (CRU) for four separate overnight inpatient blood-sampling studies spaced at least 10 d apart. The cholesterol side-chain cleavage (CYP11A) inhibitor, ketoconazole (1000 mg initial loading dose, then 400 mg four times each day), together with glucocorticoid replacement (dexamethasone 0.75 mg, twice each day), was begun orally on an outpatient basis and continued for 48 h (18,19). Concomitant T addback consisted of nightly (2200 h) application of transdermal gel containing any one of 0 (placebo), 2.5, 5, or 7.5 g T (Androgel, impregnated with 1% steroid, of which about 10% is absorbed; Solvay Pharmaceuticals, Inc., Marietta, GA). Thus, the doses are referred to as 0, 2.5, 5.0, or 7.5 mg T. Gel was applied on 3 consecutive nights in a randomized, double-blind study, i.e. independent random permutations of the order of the four T doses. The ketoconazole regimen reduces total T concentrations to less than 100 ng/dl (19). Twice the above dexamethasone dose exerts no detectable effect on 24-h mean, pulsatile, or GnRH-stimulated LH release in normal men (20).
Blood-sampling protocol
Subjects were admitted to the CRU on the third night of the outpatient regimen. Ketoconazole and dexamethasone were continued, and transdermal T gel was applied at 2000 h. Blood samples (1.0 ml) were withdrawn every 10 min, beginning at 2200 h, for a total of 12 h through a forearm iv catheter. At 1000 h, a single submaximal iv dose of GnRH (100 ng/kg) was administered to test pituitary responsiveness, and 10-min blood sampling was continued for a further 2 h. Blood was allowed to clot at room temperature, and sera were frozen at −20 C for later assay of serum LH and T concentrations.
Assays
LH and T concentrations were measured in duplicate by automated immunochemiluminometry (ACS Corning, Bayer, Tarrytown, NY). All samples from a single volunteer were measured in one assay. Concentration-dependent intraassay coefficients of variation (CVs) averaged 5.5, 4.7, 3.5, and 3.8%, and interassay CVs averaged 6.5, 5.2, 3.7, and 4.7% at LH concentrations of 1.3, 4.4, 18, and 38 IU/liter, respectively. Procedural sensitivity was 0.02 IU/liter using the World Health Organization Second International Reference Preparation 80/552 as standard. Median intra- and interassay CV were 6.8 and 8.3%, respectively, and the sensitivity was 18 ng/dl for T. T measurements obtained by immunochemiluminometry correlate strongly (r2 = 0.98) with those determined by gas chromatography-mass spectrometry (21).
Serum SHBG and albumin were measured by Immulite 2000 (Diagnostic Products Corporation, Los Angeles, CA) (22) and Roche/Hitachi 912 (Roche Diagnostics, Basal, Switzerland), respectively (23). Inter- and intraassay CVs were all less than 6.0% at SHBG concentrations ranging from 5.4 to 96 nmol/liter. Inter- and intraassay CVs were all less than 2.0% at albumin concentrations from 2.5 and 4.6 g/dl.
Calculation of free and bioavailable (bio) T concentrations
Free and bio T concentrations were calculated in each 10-min serum sample from measured total T concentrations, albumin, and SHBG (24) using an equation system that was adapted from Sodergard et al. (25). The association constants were estimated empirically for T-SHBG and T-albumin as 1.78 × 109 m−1 and 1.80 × 104 m−1, respectively, by optimizing the correlation between calculated and directly measured bio and free T concentrations (see supplemental data in Ref. 24).
Deconvolution procedure
LH concentration time series were analyzed using a recently developed automated deconvolution method, which was mathematically justified (see Appendix, published as supplemental data) and empirically validated using hypothalamo-pituitary sampling and simulated pulsatile time series (13,26). The 12-h pre-GnRH and 2-h post-GnRH time series were deconvolved together, and the mass of LH secreted in pulses was analyzed separately. The Matlab-implemented algorithm first detrends the data and normalizes concentrations to the unit interval (0, 1). Second, the program creates multiple successively decremental potential pulse-time sets, each containing one fewer burst, by a selective smoothing process (via a nonlinear diffusion equation). Third, a maximum-likelihood estimation procedure calculates all secretion and elimination parameters simultaneously, conditional on each of the candidate pulse-time sets (see Appendix). Deconvolution parameters comprise basal secretion (β0), two half-lives (α1, α2), secretory-burst mass (η0, η1), random effects on burst mass (ςA), procedural/measurement error (ςε), and a three-parameter flexible γ-secretory-burst waveform (β1, β2, β3). The fast half-life of LH was represented as 18 min and constituted 37% of the decay amplitude, and the slow half-life was an estimated variable (27). Statistical model selection was performed to distinguish among the independently framed fits of the individual candidate pulse-time sets using the Akaike information criterion (28). Observed interpulse intervals were described by a two-parameter Weibull process (more general than a Poisson process). The parameters (and units) are frequency (number of bursts per 24 h, λ of Weibull distribution), regularity of interpulse intervals (unitless γ of Weibull), slow-phase half-life (minutes), basal and pulsatile secretion rates (concentration units per session), mass secreted per burst (concentration units), and waveform mode (time delay to maximal secretion after objectively estimated burst onset, in minutes). Minimal pulses were required to exhibit an absolute 0.2 IU/liter increment and a relative 6.5% increase in concentrations (see Appendix). Analytical assumptions were evaluated by three in vivo paradigms, one computer-based model, and mathematical proof of parameter asymptotics (26,29,30,31).
Statistics
Primary analysis examined the sensitivity of LH secretion measures to inhibition by T concentrations (T sensitivity). T sensitivity was calculated in each individual by linear regression of primary parameters of LH secretion on log mean T concentrations in the four separate T echelons. The resultant 23 slopes were then regressed on age to examine the influence of age on T sensitivity. Significance of the relationship between T sensitivity and age (linear relationship of 23 individual slopes on age) was assessed using Pearson’s correlation coefficient. The independence of the age effect from body mass index (BMI) was evaluated through Pearson’s partial correlation coefficients (32).
To confirm the above analyses using an alternate statistical framework, two-way repeated-measures ANOVA of differenced data and age was performed secondarily. Within-individual differences in LH secretory parameters were calculated by differencing results from the zero T-dose intervention in each subject. The ANOVA model assumed a symmetrical variance-covariance matrix. This permitted assessment of three T dose-difference levels (2.5, 5.0, and 7.5 mg, each differenced from the zero-T dose), age, and their interaction. In the absence of a significant two-way ANOVA interaction term, differenced data were reanalyzed for main effects by one-way ANOVA. Post hoc comparison of T dose-dependent differences between least-square means was made with Bonferroni adjustment at experiment-wise P < 0.05.
As a final confirmatory analytical strategy, linear regression analysis was applied to evaluate the relationship between differenced LH-secretion values and age at each T stratum. Statistical significance for each of the three regressions was assessed by Pearson correlation coefficient and defined at multiple-comparison protected P < 0.0167.
Our motivation in performing multiple complementary analyses is to show coherent and consistent findings despite differing assumptions of the various underlying statistical models. All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC) and included Proc Mixed, Reg and Corr. Normality of data as well as order and order-by-treatment interactions were evaluated as required. An outlier was defined if the Studentized residual was more than 3 sd values from the mean regression line. Only one outlier was so identified. Conclusions were not altered by retaining or removing the single outlier. Data are presented as the mean ± sem.
Results
The transdermal T-addback regimen yielded four distinct strata of total T concentrations (Supplemental Table 2; P < 0.001 by repeated-measures one-way ANOVA). By post hoc comparisons, T concentrations differed by transdermal T dose (P < 0.001), except for 2.5 and 5.0 mg T doses (Supplemental Table 2). Mean T concentrations for the cohort (n = 23) spanned the near-castrate (30 ng/liter), low (280 ng/dl), and physiological (420 and 640 ng/dl) ranges for healthy young men (normal morning reference limits, 365–870 ng/dl). Age had no effect on experimentally imposed T concentrations (P > 0.30 by linear regression on age at each T dose level; P = 0.89 by ANOVA). Similar contrasts were observed for free and bio T (Supplemental Table 2).
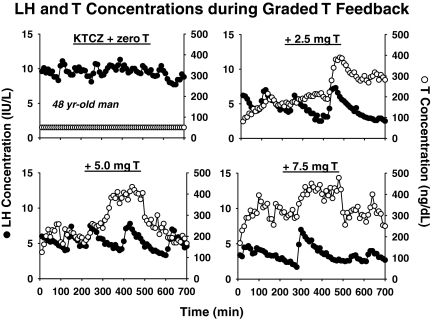
Figure 1 illustrates four 12-h LH and T profiles in one 48-yr-old subject. T replacement reduced 12-h mean LH concentrations dose-dependently in the cohort as a whole (P < 0.001 by repeated-measures ANOVA). Higher T strata progressively decreased mean LH concentrations, LH pulse frequency, basal LH secretion, and pulsatile LH secretion, but did not alter secretory burst mass or interpulse regularity (Supplemental Table 3). Age did not modulate T’s suppression of mean LH concentrations (P > 0.20 by linear regression on age at each dose level; P = 0.37 by repeated-measures ANOVA) (Supplemental Table 2).
Figure 1.
Illustrative LH (•) and T (○) concentration profiles in a 48-yr-old man administered ketoconazole (KTCZ) to block steroidogenesis, and then given four individual doses of T transdermally (zero, 2.5, 5.0, and 7.5 mg daily) on separate randomly ordered occasions. Blood was sampled every 10 min for 12 h.
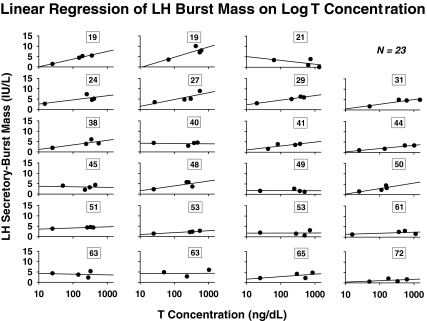
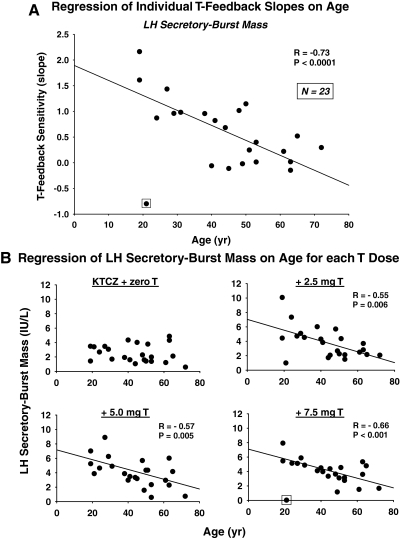
Linear regression of LH secretory-burst mass on log mean T concentrations in each of 23 subjects yielded 23 slopes, each defined as a T-feedback sensitivity (Fig. 2). Eighteen positive slopes were observed in the 23 subjects (P = 0.011, exact binomial test), indicating that higher T concentrations increase, and lower T concentrations decrease, LH secretory- burst mass. Older subjects exhibited lower absolute regression slopes, in some cases approaching zero, as shown in Fig. 3A. This plot depicts the negative linear regression of all 23 T-feedback sensitivities (slopes) on age (R = −0.48; P = 0.020, which becomes R = −0.73; P < 10−3 after excluding a single outlier). Partial Pearson correlation coefficients for the age effect (after removing the effect of BMI) remained significant (R = −0.47; P = 0.026, which became R = −0.72; P = 0.0002 after excluding a single outlier). T-feedback sensitivities (slopes) determined from calculated free T or bio T yielded analogous correlations, R = −0.49, P = 0.018 for each. This became R = −0.48, P = 0.024 after removing the effect of BMI. The negative association of LH secretory-burst mass sensitivity to suppression by T with age was confirmed by repeated-measures ANOVA (P = 0.01), and separately at each nonzero-T dose level by Pearson’s correlation (Fig. 3B). Adjustment for BMI did not alter the significance of these findings (data not shown). There were no effects of age on the sensitivity of basal or total pulsatile LH secretion to (total, free, or bio-) T feedback (P > 0.10).
Figure 2.
Regression of LH secretory-burst mass on log total T concentrations in 23 men of indicated ages (numbers in boxes) yielding 23 T-feedback sensitivities (slopes). Deconvolution estimates of LH secretory-burst mass and mean total T concentrations were determined from 12-h profiles sampled every 10 min (Fig. 1). Slopes were regressed subsequently on age in Fig. 3A.
Figure 3.
A, Age reduces stimulatory T feedback onto LH secretory-burst mass. Data points represent individual-subject slope values from Fig. 2. B, Regression of individual LH secretory-burst mass values (averaged over 12 h of sampling in each subject) on age by each T stratum. R and P values reflect Pearson’s correlation coefficient. A single outlier (Studentized residual P < 0.001) is marked by a box.
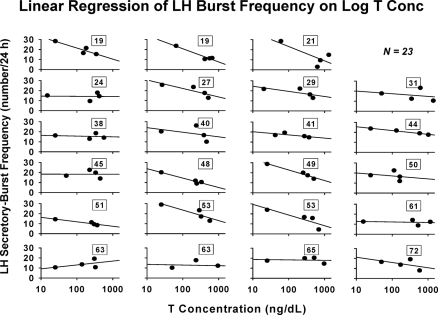
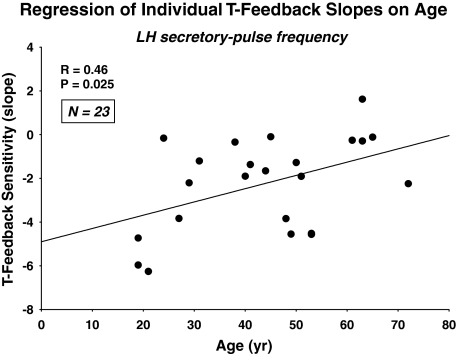
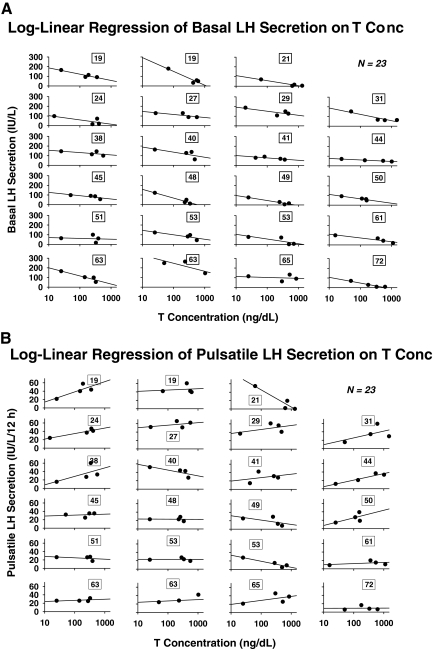
The question arises whether the age-related decrease in the sensitivity of LH-secretory burst mass to T concentrations is accompanied by similar or opposite changes in the feedback sensitivity of LH secretory-burst frequency to T. By ANOVA, age increased the number of LH secretory bursts observed in 12-h sampling session (P = 0.034) or per 24 h as extrapolated by the Weibull frequency estimator, λ (P = 0.015). Log-linear regression of LH burst frequency on mean T concentrations yielded negative slopes in 22 of 23 subjects (P < 0.001, exact binomial test) (Fig. 4). Free and bio T-feedback sensitivities (slopes) yielded analogous correlations of R = −0.45, P = 0.030; and R = −0.44, P = 0.034, respectively. Thus, higher total, free, and bio-T exposure decreases LH pulse frequency. Further analysis disclosed that age significantly attenuates T’s suppression of LH secretory-burst frequency, estimated by the Weibull parameter, λ (R = 0.46, P = 0.025) (Fig. 5). Adjustment for BMI yielded a comparable partial Pearson correlation coefficient of R = 0.44, P = 0.036. In contradistinction, the Weibull pulse-regularity estimator, γ, LH secretory-burst mode (waveform shape), basal LH secretion, and the slow LH half-life were not influenced by age or T concentration (P > 0.12). However, basal LH secretion was suppressed by higher T concentrations in 23 of 23 subjects (P < 0.001) (Fig. 6A). This was not true for pulsatile LH secretion for which seven of 23 slopes on T concentration were negative (Fig. 6B). The last finding reflects the opposite effects of T and age on LH secretory-burst size and number.
Figure 4.
Regression of LH secretory-burst frequency on log total T concentrations in 23 individual men. LH pulse frequency (number of pulses per 24 h) was estimated as λ of a Weibull renewal process (see Subjects and Methods).
Figure 5.
Age decreases the sensitivity of LH-pulse frequency to T feedback. Sensitivity was defined as the slope of the regression of LH pulse-frequency on total T concentrations in each volunteer (Fig. 4). Data are presented as described in Fig. 3A.
Figure 6.
Log-linear regression of basal (A) and total pulsatile (summed mass of LH secretory bursts) (B) LH secretion on 12-h mean T concentration in 23 men of the indicated ages (numbers in boxes).
Age progressively attenuated increments in LH-secretory burst mass compared with zero-T addback at all active T doses (2.5, 5.0, and 7.5 mg) and reduced decrements in LH-secretory burst frequency otherwise observed at the two lower (2.5 and 5.0 mg) T doses (0.027 > P > 0.015). Correlation coefficients for incremental mass and frequency regressions on age at the two lower T doses were approximately equal in absolute magnitude (R = 0.46 to R = 0.50), but opposite in sign. Adjustment for BMI did not alter the significance of any of these relationships (data not shown). There was also a strongly negative relationship between age and LH secretory-burst mass at the 7.5 mg T dose (with one outlier excluded, R = −0.66, P < 0.001). Age did not affect frequency inhibition by the maximal T dose, suggesting age-comparable maximal inhibition (efficacy of T).
Analogous analyses of the 2-h post-GnRH time series did not disclose any significant T-dose effect or age relationships (data not shown). Specifically, there were no significant age associations with T feedback sensitivity of GnRH-stimulated LH secretory burst mass.
Discussion
The present investigation used an experimental protocol of androgen depletion with graded transdermal T replacement to test the hypothesis that middle age modifies T’s concentration-dependent feedback regulation of selected facets of pulsatile and basal LH secretion in healthy men. The experimental strategy allowed within-subject estimates of T-feedback sensitivity (slope term) across four echelons of T concentrations. For the cohort as a whole, escalating T exposure decreased mean LH concentrations by repressing LH secretory-burst frequency and basal (nonpulsatile) LH secretion significantly, while amplifying the mass of LH secreted per burst (each P ≤ 0.011). Regression analysis revealed strong age-related decreases in the feedback sensitivity of 1) LH secretory-burst mass, and 2) LH secretory-burst frequency to T. Age did not modify T’s inhibition of basal LH secretion, thus defining selectively the age effect.
An unanticipated outcome was that experimentally controlled, graded increases in T concentrations repress total pulsatile and basal LH secretion (and thus mean LH concentrations) comparably in middle-aged and young men (interquartile range, 28–53 h). This result was explicable on the grounds that middle age attenuated two countervailing effects of T feedback. In particular, middle age or factors associated with it diminished the T-associated decrease in LH secretory-burst frequency and the T-associated increase in LH secretory-burst mass. Investigations in animal models and clinical settings are consistent with a reciprocal relationship between LH pulse frequency and LH pulse size (33,34). The remarkable finding here is that middle age blunts both the fall in frequency and the rise in mass of LH secretory bursts otherwise evoked by physiological T replenishment compared with a T-deficiency state. Furthermore, the anatomical basis of the age-associated modulation of these feedback effects of T is presumably hypothalamic because age did not influence exogenous GnRH effects.
The precise molecular bases for age-related attenuation of T’s feedback on LH secretory-burst frequency (negative effect) and size (positive effect) in healthy men are not known. LH pulse frequency is principally determined by hypothalamic GnRH pulse frequency (35). In contrast, GnRH pulse frequency is regulated by numerous neuronal and extraneuronal signals (34). Moreover, multiple factors influence LH pulse amplitude, such as GnRH outflow, gonadotrope function, nutritional status (36), diurnal rhythmicity (7), SHBG concentrations (37), visceral adiposity, T metabolism (38), chronic T depletion, and in principle, hypothalamo-pituitary activity of aromatase and/or 5 α-reductase enzymes and local expression of androgen and/or estradiol receptors (13,39). Studies in the male rodent report less hypothalamic expression and release of GnRH, normal pituitary GnRH action, and in some analyses, fewer androgen and estrogen receptors in aged than young animals (40,41,42). Fewer sex-steroid receptors and/or lesser aromatase or 5 α-reductase activity could explain the present finding of age-related escape of GnRH/LH pulse frequency from the inhibitory effects of T. In the human and the monkey, physiological T concentrations have little direct effect on pituitary GnRH action (43), whereas estrogen is clearly inhibitory. Thus, in principle, failure of stepwise-increasing T concentrations to augment the mass of LH secreted per burst in older compared with young men could reflect greater inhibitory feedback by estradiol derived from T in aging individuals. Alternatively, impaired suppression of LH pulse frequency may be a primary alteration in older men because rapid GnRH/LH pulsatility is expected to diminish LH secretory-burst mass (34).
Transdermal T delivery suppressed 12-h mean LH concentrations in a dose-dependent but age-independent fashion in the 23 men studied here. Earlier studies reported that pharmacological and high-physiological amounts of T or 5α-DHT can decrease LH concentrations to a greater (8,9,10) or lesser (11,12) extent in older men than in young men. Possible limitations of previous studies include evaluation of negative feedback after administration of a single T or DHT dose, typically pharmacological in amount; comparison of feedback effects at two age extrema only; variability in subject-selection criteria; and analysis of single-sample or mean LH concentrations. These issues were addressed in the present study by evaluating T concentration-dependent feedback effects over a more than 20-fold range of T concentrations (30 to 640 ng/dl); regressing feedback responses on age in young to middle-aged individuals; including only healthy unmedicated subjects; and implementing a mathematically justified deconvolution procedure to quantify discrete facets of pulsatile and basal LH secretion (13,26).
Caveats include the need to extend the generality of these inferences to other and larger cohorts; assess possible distinctions among short-term, prolonged, morning, evening, pulsatile, and constant T-negative feedback; broaden observations to include subjects older than 60 yr, particularly because our population included only five men older than 60 yr; and establish the exact age-dependence of inferred feedback failure via longitudinal studies.
In summary, an experimental paradigm of steroidogenic blockade with glucocorticoid replacement and graded transdermal T supplementation in healthy young and middle-aged men indicates that: 1) total T concentrations within the physiological range suppress mean LH concentrations, pulsatile LH secretion, and basal LH secretion dose-dependently, but not age-dependently; and 2) middle age or factors associated therewith impair T’s conjoint inhibition of LH secretory-burst frequency and its stimulation of LH secretory-burst mass. The discovery that countervailing failure of T feedback on LH burst number and size operates in middle-aged men may explain some earlier reports of both increased and decreased androgen feedback based upon single-sample or mean LH concentrations.
Supplementary Material
Footnotes
P.Y.L. was supported by Career Development Award 511929 from the National Health and Medical Research Council of Australia. P.Y.T. was supported by a Mayo Institutional MIDAS Award. These studies were supported in part by the National Center for Research Resources (Rockville, MD) Grant M01 RR00585 to the General Clinical Research Center of the Mayo Clinic and Foundation; and by National Institutes of Health Grants RO1 AG23133, DK73148, AG29215, R21 AG23777, and AG31763.
Current address for P.Y.L.: Endocrine and Metabolic Group, Woolcock Institute of Medical Research University of Sydney and Concord Hospital Sydney, Sydney 2050, New South Wales, Australia.
Disclosure Summary: The authors have nothing to declare.
First Published Online July 7, 2009
Abbreviations: bio, Bioavailable; BMI, body mass index; CV, coefficient of variation; 5α-DHT, 5α-dihydrotestosterone; T, testosterone.
References
- Liu PY, Swerdloff RS, Veldhuis JD 2004 The rationale, efficacy and safety of androgen therapy in older men: future research and current practice recommendations. J Clin Endocrinol Metab 89:4789–4796 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Urban RJ, Lizarralde G, Johnson ML, Iranmanesh A 1992 Attenuation of luteinizing hormone secretory burst amplitude is a proximate basis for the hypoandrogenism of healthy aging in men. J Clin Endocrinol Metab 75:707–713 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW 2009 Anabolic applications of androgens for functional limitations associated with aging and chronic illness. Front Horm Res 37:163–182 [DOI] [PubMed] [Google Scholar]
- Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD 1995 Amplified nocturnal luteinizing hormone (LH) secretory burst frequency with selective attenuation of pulsatile (but not basal) testosterone secretion in healthy aged men: possible Leydig cell desensitization to endogenous LH signaling—a clinical research center study. J Clin Endocrinol Metab 80:3025–3031 [DOI] [PubMed] [Google Scholar]
- Mulligan T, Iranmanesh A, Kerzner R, Demers LW, Veldhuis JD 1999 Two-week pulsatile gonadotropin releasing hormone infusion unmasks dual (hypothalamic and Leydig-cell) defects in the healthy aging male gonadotropic axis. Eur J Endocrinol 141:257–266 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Veldhuis NJ, Keenan DM, Iranmanesh A 2005 Age diminishes the testicular steroidogenic response to repeated intravenous pulses of recombinant human LH during acute GnRH- receptor blockade in healthy men. Am J Physiol Endocrinol Metab 288:E775–E781 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Veldhuis JD 2001 Hypothesis testing of the aging male gonadal axis via a biomathematical construct. Am J Physiol Regul Integr Comp Physiol 280:R1755–R1771 [DOI] [PubMed] [Google Scholar]
- Winters SJ, Sherins RJ, Troen P 1984 The gonadotropin suppressive activity of androgen is increased in elderly men. Metabolism 33:1052–1059 [DOI] [PubMed] [Google Scholar]
- Winters SJ, Atkinson L 1997 Serum LH concentrations in hypogonadal men during transdermal testosterone replacement through scrotal skin: further evidence that aging enhances testosterone negative feedback. Clin Endocrinol (Oxf) 47:317–322 [DOI] [PubMed] [Google Scholar]
- Deslypere JP, Kaufman JM, Vermeulen T, Vogelaers D, Vandalem JL, Vermeulen A 1987 Influence of age on pulsatile luteinizing hormone release and responsiveness of the gonadotrophs to sex hormone feedback in men. J Clin Endocrinol Metab 64:68–73 [DOI] [PubMed] [Google Scholar]
- Muta K, Kato K, Akamine Y, Ibayashi H 1981 Age-related changes in the feedback regulation of gonadotrophin secretion by sex steroids in men. Acta Endocrinol (Copenh) 96:154–162 [DOI] [PubMed] [Google Scholar]
- Gentili A, Mulligan T, Godschalk M, Clore J, Patrie J, Iranmanesh A, Veldhuis JD 2002 Unequal impact of short-term testosterone repletion on the somatotropic axis of young and older men. J Clin Endocrinol Metab 87:825–834 [DOI] [PubMed] [Google Scholar]
- Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, Veldhuis JD 2006 An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinology 147:2817–2828 [DOI] [PubMed] [Google Scholar]
- Stewart-Bentley M, Odell W, Horton R 1974 The feedback control of luteinizing hormone in normal adult men. J Clin Endocrinol Metab 38:545–553 [DOI] [PubMed] [Google Scholar]
- Marynick SP, Loriaux DL, Sherins RJ, Pita Jr JC, Lipsett MB 1979 Evidence that testosterone can suppress pituitary gonadotropin secretion independently of peripheral aromatization. J Clin Endocrinol Metab 49:396–398 [DOI] [PubMed] [Google Scholar]
- Vigersky RA, Easley RB, Loriaux DL 1976 Effect of fluoxymesterone on the pituitary-gonadal axis: the role of testosterone-estradiol-binding globulin. J Clin Endocrinol Metab 43:1–9 [DOI] [PubMed] [Google Scholar]
- Kuhn JM, Rieu M, Laudat MH, Forest MG, Pugeat M, Bricaire H, Luton JP 1984 Effects of 10 days of administration of percutaneous dihydrotestosterone on the pituitary-testicular axis in normal men. J Clin Endocrinol Metab 58:231–235 [DOI] [PubMed] [Google Scholar]
- Miossec P, Archambeaud-Mouveroux F, Teissier MP 1997 Inhibition of steroidogenesis by ketoconazole. Therapeutic uses. Ann Endocrinol (Paris) 58:494–502 [PubMed] [Google Scholar]
- Zwart AD, Iranmanesh A, Veldhuis JD 1997 Disparate serum free testosterone concentrations and degrees of hypothalamo-pituitary-LH suppression are achieved by continuous versus pulsatile intravenous androgen replacement in men: a clinical experimental model of ketoconazole-induced reversible hypoandrogenemia with controlled testosterone add-back. J Clin Endocrinol Metab 82:2062–2069 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Lizarralde G, Iranmanesh A 1992 Divergent effects of short-term glucocorticoid excess on the gonadotropic and somatotropic axes in normal men. J Clin Endocrinol Metab 74:96–102 [DOI] [PubMed] [Google Scholar]
- Fitzgerald RL, Herold DA 1996 Serum total testosterone: immunoassay compared with negative chemical ionization gas chromatography-mass spectrometry. Clin Chem 42:749–755 [PubMed] [Google Scholar]
- Elmlinger MW, Kühnel W, Wormstall H, Döller PC 2005 Reference intervals for testosterone, androstenedione and SHBG levels in healthy females and males from birth until old age. Clin Lab 51:625–632 [PubMed] [Google Scholar]
- Ledue TB, Collins MF, Ritchie RF 2002 Development of immunoturbidimetric assays for fourteen human serum proteins on the Hitachi 912. Clin Chem Lab Med 40:520–528 [DOI] [PubMed] [Google Scholar]
- Takahashi PY, Votruba P, Abu-Rub M, Mielke K, Veldhuis JD 2007 Age attenuates testosterone secretion driven by amplitude-varying pulses of recombinant human luteinizing hormone during acute gonadotrope inhibition in healthy men. J Clin Endocrinol Metab 92:3626–3632 [DOI] [PubMed] [Google Scholar]
- Södergård R, Bäckström T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Veldhuis JD, Keenan DM 2008 Probabilistic recovery of pulsatile, secretory and kinetic structure: an alternating discrete and continuous schema. Quarterly Appl Math 66:401–421 [Google Scholar]
- Veldhuis JD, Fraioli F, Rogol AD, Dufau ML 1986 Metabolic clearance of biologically active luteinizing hormone in man. J Clin Invest 77:1122–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H 1974 A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723 [Google Scholar]
- Liu PY, Keenan DM, Kok P, Padmanabhan V, O'Byrne KT, Veldhuis JD 2009 Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endocrinol Metab 297:E538–E544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan DM, Chattopadhyay S, Veldhuis JD 2005 Composite model of time-varying appearance and disappearance of neurohormone pulse signals in blood. J Theor Biol 236:242–255 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Keenan DM, Pincus SM 2008 Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev 29:823–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ 1971 Statistical principles in experimental design. New York: McGraw Hill; 232–250 [Google Scholar]
- Clarke IJ, Cummins JT 1985 GnRH pulse frequency determines LH pulse amplitude by altering the amount of releasable LH in the pituitary glands of ewes. J Reprod Fertil 73:425–431 [DOI] [PubMed] [Google Scholar]
- Urban RJ, Evans WS, Rogol AD, Kaiser DL, Johnson ML, Veldhuis JD 1988 Contemporary aspects of discrete peak detection algorithms. I. The paradigm of the luteinizing hormone pulse signal in men. Endocr Rev 9:3–37 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT 1982 The temporal relationship between gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 111:1737–1739 [DOI] [PubMed] [Google Scholar]
- Pirke KM, Baraño JL, Calandra R, Lüthy I, Spyra B 1982 Influence of starvation on the dihydrotestosterone-luteinizing hormone feedback in the male rat. J Steroid Biochem 16:403–406 [DOI] [PubMed] [Google Scholar]
- Baker HW, Burger HG, de Kretser DM, Hudson B, O'Connor S, Wang C, Mirovics A, Court J, Dunlop M, Rennie GC 1976 Changes in the pituitary-testicular system with age. Clin Endocrinol (Oxf) 5:349–372 [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Nakamura K, Fujita Y, Guengerich FP, Horiuchi R, Yamamoto K 2008 Defective activity of recombinant cytochromes P450 3A4.2 and 3A4.16 in oxidation of midazolam, nifedipine, and testosterone. Drug Metab Dispos 36:2287–2291 [DOI] [PubMed] [Google Scholar]
- Canovatchel WJ, Volquez D, Huang S, Wood E, Lesser ML, Gautier T, Imperato-McGinley J 1994 Luteinizing hormone pulsatility in subjects with 5-α-reductase deficiency and decreased dihydrotestosterone production. J Clin Endocrinol Metab 78:916–921 [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Naai MA, Hess DL, Matsumoto AM 1994 The Brown Norway rat as a model of male reproductive aging: evidence for both primary and secondary testicular failure. J Gerontol 49:B42–B50 [DOI] [PubMed] [Google Scholar]
- Haji M, Kato KI, Nawata H, Ibayashi H 1981 Age-related changes in the concentrations of cytosol receptors for sex steroid hormones in the hypothalamus and pituitary gland of the rat. Brain Res 204:373–386 [DOI] [PubMed] [Google Scholar]
- Wu D, Lin G, Gore AC 2009 Age-related changes in hypothalamic androgen receptor and estrogen receptor α in male rats. J Comp Neurol 512:688–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorr JA, Bray MJ, Veldhuis JD 2001 Aromatization mediates testosterone’s short-term feedback restraint of 24-hour endogenously driven and acute exogenous GnRH-stimulated LH and FSH secretion in young men. J Clin Endocrinol Metab 86:2600–2606 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.