Abstract
Context: Data on the presence, extent, and reversibility of cardiovascular disease in primary hyperparathyroidism (PHPT) are conflicting.
Objective: This study evaluated carotid structure and function in PHPT patients compared with population-based controls.
Design: This is a case-control study.
Setting: The study was conducted in a university hospital metabolic bone disease unit.
Participants: Forty-nine men and women with PHPT and 991 controls without PHPT were studied.
Outcome Measures: We measured carotid intima-media thickness (IMT), carotid plaque presence and thickness, and carotid stiffness, strain, and distensibility.
Results: IMT, carotid plaque thickness, carotid stiffness, and distensibility were abnormal in PHPT patients, and IMT was higher in patients than controls (0.959 vs. 0.907 mm, P < 0.0001). In PHPT, PTH levels, but not calcium concentration, predicted carotid stiffness (P = 0.04), strain (P = 0.06), and distensibility (P = 0.07). Patients with increased carotid stiffness had significantly higher PTH levels than did those with normal stiffness (141 ± 48 vs. 94.9 ± 44 pg/ml, P = 0.002), and odds of abnormal stiffness increased 1.91 (confidence interval = 1.09–3.35; P = 0.024) for every 10 pg/ml increase in PTH, adjusted for age, creatinine, and albumin-corrected calcium.
Conclusions: Mild PHPT is associated with subclinical carotid vascular manifestations. IMT, a predictor of cardiovascular outcomes, is increased. Measures of carotid stiffness are associated with extent of PTH elevation, suggesting that those with more severe PHPT may have impaired vascular compliance and that PTH, rather than calcium, is the mediator.
In mild primary hyperparathyroidism, carotid intima-media thickness—a subclinical marker of cardiovascular disease—is increased and PTH level is an independent predictor of carotid vascular compliance.
Classical primary hyperparathyroidism (PHPT) was a symptomatic disease with increased cardiovascular morbidity and mortality (1,2). Today, most patients with PHPT have serum calcium levels within 1 mg/dl above the upper limit of normal, and the majority are without obvious cardiovascular or other symptoms (3). Although epidemiological studies from Scandinavia (1,2,4,5,6,7,8) have continued to report increased cardiovascular mortality in PHPT, the single American epidemiological study assessing patients with more mild hypercalcemia did not confirm this (9). Indeed, those with mild PHPT had reduced cardiovascular mortality (relative risk, 0.6), although mortality was increased in those with the highest calcium levels. As the clinical findings in PHPT have become more subtle over time, the investigation of cardiovascular manifestations of the disease has recently turned to less clinically overt abnormalities.
Increased carotid intima-media thickness (IMT) is an early, subclinical predictor of systemic atherosclerosis as well as clinical coronary and cerebrovascular events (10,11,12). IMT was recently found to be markedly increased in 20 patients with PHPT, but this population had higher calcium levels (range 11.3–13.1 mg/dl) than is typical of most PHPT patients today, making it difficult to extrapolate these data to the majority of patients with PHPT (13). Interpretation of other studies showing no effect of PHPT or its cure on carotid IMT are limited by small sample sizes or IMT measurement in portions of the carotid vascular bed or brachial artery where atherosclerotic disease is rare (14,15,16).
Investigations of other carotid pathology, including carotid plaque thickness and decreased vascular compliance, have not been undertaken in PHPT. Carotid plaque thickness, however, was recently found to be positively associated with serum calcium levels within the normal range (17). Arterial distensibility, a measure of an artery’s ability to expand and contract during systole and diastole, may decrease early in the atherosclerotic process before structural wall changes become detectable (18). Decreased carotid distensibility has been associated with stroke (19), and impaired arterial distensibility has been reported in patients with PHPT in other parts of the vasculature (15,20,21,22,23).
This study was designed to determine whether there are subclinical structural and functional abnormalities within the carotid vasculature in patients with mild PHPT and whether carotid vascular indices are associated with serum calcium and/or PTH levels in this disease.
Subjects and Methods
Measures of carotid structure and function in patients with PHPT were compared with normal values from the literature and to a cohort of non-hyperparathyroid control subjects. All PHPT patients and control subjects gave written, informed consent. This study was approved by the Institutional Review Boards of Columbia University Medical Center and The University of Miami.
Subjects
Participants with PHPT were referred from the Metabolic Bone Diseases Unit at Columbia University Medical Center and represent consecutive cases who agreed to participate in the study. Cases were eligible if they were 45–75 yr of age, in order to study those at risk for cardiac disease and because it includes the vast majority of patients with PHPT. Patients had serum calcium higher than 10.2 but lower than 12.0 mg/dl (to assess the presence of cardiovascular findings in those with mild hypercalcemia) and an elevated or inappropriately normal PTH level. Calcium/creatinine clearance ratio was measured to exclude familial hypocalciuric hypercalcemia, and none had thiazide-induced hyperparathyroidism. Exclusion criteria included current use of bisphosphonates and initiation or changes in cholesterol-lowering medications within 2 yr.
Normal controls were from the Northern Manhattan Study (NOMAS), a population-based study designed to investigate vascular risk factors in 3298 individuals free of stroke (24,25). Carotid duplex Doppler sonography was performed on 2000 subjects, chosen at random. All NOMAS participants who had undergone carotid Doppler imaging, had normal serum calcium concentration, and were between the ages of 45–75 yr and of similar race/ethnicity to cases (Caucasian Hispanic and non-Hispanic) were included (n = 991).
Cardiovascular risk factors
Participants were interviewed to obtain demographic data, cardiac risk factors, and medical history. Race/ethnicity was defined by self-identification. Coronary artery disease was defined as a history of myocardial infarction, angina, angioplasty, or coronary artery bypass surgery. Hypercholesterolemia was defined as being told by a physician of elevated lipid levels or being on a lipid-lowering medication. Hypertension was defined as a systolic blood pressure recording of at least 140 mm Hg, a diastolic blood pressure recording of at least 90 mm Hg, or a patient’s self-report of hypertension or use of antihypertensive medications. Diabetes mellitus was defined as a fasting blood glucose level of at least 126 mg/dl or a patient’s self-report of diabetes or use of insulin or other hypoglycemic medications. Cigarette smoking was categorized as nonsmoker or ever-smoked.
Biochemical evaluation
Fasting samples for serum calcium, albumin, total cholesterol, and triglycerides were measured by an automated chemistry analyzer. Low-density lipoprotein was calculated (26). PTH was measured by immunochemiluminometric assay for intact PTH (Scantibodies Laboratories, Inc., Santee, CA), which detects PTH (1-84) and PTH (7-84).
Carotid ultrasonography
High-resolution B-mode carotid ultrasound was performed using a GE LogIQ 700 system (GE Healthcare, Milwaukee, WI) with a multifrequency 9/13-MHz transducer. With the subject in a supine position, the extracranial carotid arteries were imaged in short axial and long longitudinal planes (anterior, lateral, and posterior views) using standardized carotid ultrasound protocols as previously described (27). Blood pressure was obtained for each subject from the right brachial artery after a minimum of 10 min rest in a supine position with a semiautomated oscillometric blood pressure recorder (Dinamap Pro100; Critikon, Inc., Tampa, FL). Blood pressure was measured before and after each examination and averaged.
Carotid IMT was measured according to the NOMAS protocol (27,28). Total carotid IMT was calculated as a composite measure of the near and far walls IMT of the common carotid artery (CCA), bifurcation, and internal carotid artery from both sides of the neck. Values of IMT of 0.9 mm or higher were considered abnormal (threshold for increased cardiovascular risk in the Cardiovascular Health Study) (11).
Carotid plaque in any of the carotid artery segments (the CCA, bifurcation, and internal carotid artery) was defined as an area of focal wall thickening or protrusion in the arterial lumen more than 50% greater than the surrounding wall thickness. Maximal carotid plaque thickness (MCPT) was measured at the highest plaque prominence from the multi-angled images. If plaque was present in any carotid segments, the plaque boundaries were traced and MCPT was calculated using the semiautomatic Image Pro version 5.2 software (Microsoft Corp., Redmond, WA) by a single trained sonographer.
Carotid distensibility was measured in the 10-mm segment of the right CCA below the origin of the carotid bulb from at least five consecutive cardiac cycles on the B/M-mode imaging recordings using Image Pro analysis software. The systolic diameter and diastolic diameter of the right CCA were measured, and the following metrics were calculated: carotid strain = (systolic diameter − diastolic diameter)/diastolic diameter (percent); stiffness (β) = ln (systolic blood pressure/diastolic blood pressure)/strain; and distensibility (1/β) adjusted for IMT = 1/[ln(systolic blood pressure/diastolic blood pressure)/strain × IMT]. Lower strain, lower distensibility, or higher stiffness indicates impaired vascular compliance. Stiffness of 6 or more, strain less than 6%, and distensibility of 0.425 or less are abnormal and represent the 75th percentile distribution in the NOMAS cohort (n = 2000) for the study age range.
Statistical analysis
Between-group differences in demographic and cardiovascular risk factors were evaluated by independent two-sided t test, χ2, or Fisher’s exact test as appropriate. Critical test values were adjusted for unequal variances when necessary. The five carotid outcomes (IMT, stiffness, strain, distensibility, and maximal carotid plaque thickness) were treated as a vector of within-subject repeated measures, and between-group differences were estimated using a linear mixed model with a fixed effect of group, covariates selected from the cardiovascular risk factors that differed significantly between the groups [gender, diabetes, systolic and diastolic blood pressure, triglycerides, and body mass index (BMI)], and an unstructured covariance matrix. Model-estimated means and sd are presented with P values for the between-group difference based on linear mixed-model differences between groups within measure.
Relationships between albumin-corrected calcium, PTH, and carotid variables were assessed with Pearson correlation. Multiple regression models were created for those variables with significant correlations to assess the effect of PTH on outcomes, independent of confounders (albumin-corrected calcium, creatinine, and age). The contribution of individual subjects’ values to overall variance was assessed using Cook’s distance. No values met the criterion as influence points (Cook’s distance >1). An assessment of colinearity among predictors in the final model was used to establish independence of predictors (variance inflation factors all <10). Because PTH was found to be a significant predictor of stiffness, PTH and calcium levels in those with normal vs. abnormal stiffness and IMT were compared using independent two-sided t tests. For all analyses, a two-tailed P < 0.05 was considered to indicate statistical significance. Statistical analysis was performed using SAS version 9.1.3 (Cary, NC).
Results
Clinical and biochemical data
Consistent with their diagnosis of PHPT, cases were predominantly female (Table 1) and had biochemical evidence typical of mild PHPT [serum calcium (mean ± sd), 10.5 ± 0.5 mg/dl, normal 8.7–10.2 mg/dl; PTH, 116 ± 51, normal 10–66 pg/ml). The control group had more cardiovascular risk factors than did the PHPT patients: a higher proportion of men, higher mean BMI, more participants with diabetes, higher mean systolic and diastolic blood pressure, and higher serum triglycerides. All cases and controls were Caucasian, although more controls were of Hispanic ethnicity. We compared men (PHPT n = 8; control n = 421) and non-Hispanic Caucasian women (PHPT n = 38, 78% of all cases; control n = 100) separately as well.
Table 1.
Participant characteristics and cardiovascular risk factors
| Variables | All participants
|
||
|---|---|---|---|
| PHPT, n = 49 | Controls, n = 991 | P value | |
| Age (yr) | 61.6 ± 7.4 | 63.6 ± 6.0 | 0.07 |
| Male (%) | 16 | 42 | <0.0001 |
| BMI (kg/m2) | 25.6 ± 4.1 | 28.3 ± 4.9 | <0.001 |
| Coronary artery disease (%) | 8 | 9.6 | 1.00 |
| Hypercholesterolemia (%) | 38 | 42 | 0.46 |
| Hypertension (%) | 35 | 46 | 0.12 |
| Diabetes (%) | 2 | 15 | <0.01 |
| Tobacco ever use (%) | 55 | 51 | 0.55 |
| Systolic blood pressure (mm Hg) | 127 ± 19 | 142 ± 21 | <0.0001 |
| Diastolic blood pressure (mm Hg) | 75 ± 11 | 85 ± 11 | <0.0001 |
| Calcium (mg/dl) | 10.5 ± 0.5 | 9.1 ± 0.4 | <0.0001 |
| Total cholesterol (mg/dl) | 211 ± 34 | 203 ± 39 | 0.18 |
| Low-density lipoprotein (mg/dl) | 124 ± 30 | 131 ± 35 | 0.17 |
| Triglycerides (mg/dl) | 98 ± 56 | 144 ± 84 | <0.0001 |
Results are mean ± sd unless indicated otherwise.
Carotid structure and function
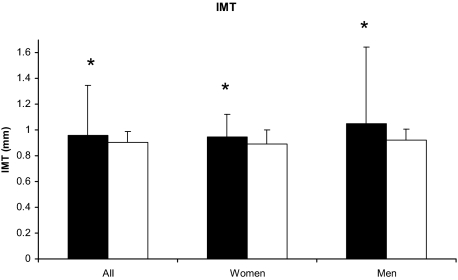
Mean carotid IMT was elevated in PHPT (Table 2), and 69% of cases had high IMT. IMT was also higher in PHPT than controls (all, 0.96 ± 0.39 vs. 0.91 ± 0.09 mm; P < 0.0001; men, 1.05 ± 0.60 vs. 0.93 ± 0.08 mm; P < 0.0001; non-Hispanic Caucasian women, 0.95 ± 0.18 vs. 0.90 ± 0.11 mm; P < 0.0006; Fig. 1). Although carotid plaque was more common in the control group (present in 61 vs. 39% of subjects), plaque thickness was above the threshold associated with deleterious cardiovascular outcomes in both groups. Similarly, although control subjects had higher carotid stiffness than did cases, the mean value for stiffness in PHPT was elevated, and 41% had frankly increased carotid stiffness. There were no between-group differences in carotid strain, distensibility, or maximum plaque thickness in those with carotid plaque (Table 2), however distensibility was abnormal in both groups.
Table 2.
Comparison of carotid measures between PHPT and NOMAS control subjects, adjusted for gender, diabetes, systolic and diastolic blood pressure, triglycerides, and BMI
| PHPT | Controls | Normal range | |
|---|---|---|---|
| IMT (mm)a | 0.96 ± 0.39b | 0.91 ± 0.09 | 0.7–0.9 |
| Strain (%) | 9.1 ± 21.5 | 8.5 ± 5.0 | 6–12 |
| Stiffnessa | 6.2 ± 24.2b | 8.3 ± 5.8 | <6 |
| Distensibilitya | 0.19 ± 0.54 | 0.17 ± 0.13 | >0.425 |
| MCPT (mm)a | 2.09 ± 1.00 | 2.08 ± 0.76 | <1.9 |
Results are mean ± sd. Normal ranges are from Cardiovascular Health Study and NOMAS (11).
Indices that were abnormal in both cases and controls.
PHPT vs. control group: bP < 0.0001; cP < 0.05.
Figure 1.
Comparison of carotid IMT between PHPT (black bar) and control (white bar) subjects. IMT was higher in PHPT than control in all subjects (P < 0.0001), non-Hispanic Caucasian women (P = 0.006), and Hispanic as well as non-Hispanic men (P < 0.0001). Data are presented as mean ± sd adjusted for gender, diabetes, systolic and diastolic blood pressure, triglycerides, and BMI. *, P < 0.01.
Relationship between PTH, calcium, and carotid measures in PHPT
Carotid IMT was not associated with levels of PTH or serum calcium, nor did PTH or serum calcium levels differ in those with abnormal vs. normal IMT (PTH, 109 ± 43 vs. 131 ± 67 pg/ml, P = 0.22; calcium, 10.5 ± 0.6 vs. 10.6 ± 0.4 mg/dl, P = 0.29). There was no association between maximum plaque thickness and PTH or calcium.
Higher levels of PTH were, however, associated with worse carotid stiffness (r = 0.41; P = 0.009), carotid strain (r = −0.45; P = 0.003), and distensibility (r = −0.39; P = 0.02) in patients with PHPT. Serum calcium and albumin-corrected calcium level correlated with carotid strain (r = −0.29; P = 0.04), and the association with distensibility was marginally significant (r = −0.28; P = 0.054).
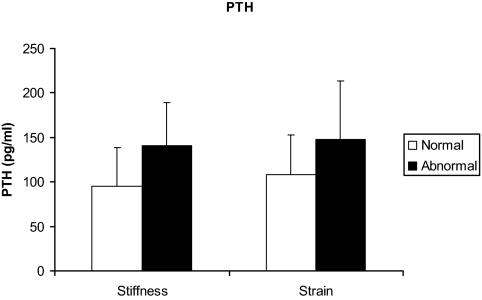
Because increasing age and worsening renal function are associated with rising PTH levels, and PTH and serum calcium are associated with each other in PHPT, we assessed these relationships in a multiple regression model including PTH, albumin-corrected calcium, age, and creatinine. PTH concentration, but not serum calcium level, was a significant predictor of carotid stiffness (Table 3). PTH was the only independent predictor of carotid stiffness, accounting for 11% of the variance when the confounding influence of age, creatinine, and calcium was removed. Patients with increased carotid stiffness had significantly higher PTH levels than did those with normal stiffness (141 ± 48 vs. 94.9 ± 44 pg/ml, P = 0.002; Fig. 2), whereas calcium levels did not differ (10.6 ± 0.5 vs. 10.4 ± 0.5 mg/dl; P = 0.25). The odds of abnormal stiffness increased 1.91-fold (95% confidence interval, 1.09–3.35; P = 0.024) for every 10 pg/ml increase in PTH, adjusted for age, creatinine, and albumin-corrected calcium.
Table 3.
Multiple Regression models of carotid strain, stiffness, and distensibility in PHPT patients
| Variable | β | se | P value | Model R2 |
|---|---|---|---|---|
| Strain | ||||
| PTH | −0.0002 | 0.0782 | 0.06 | 0.34 P value = 0.007 |
| Corrected calcium | −0.0109 | 0.0078 | 0.17 | |
| Age | −0.0005 | 0.0006 | 0.38 | |
| Creatinine | −0.0284 | 0.0158 | 0.09 | |
| Stiffness | ||||
| PTH | 0.0259 | 0.0120 | 0.04 | 0.26 P value = 0.045 |
| Corrected calcium | 0.2861 | 0.9637 | 0.76 | |
| Age | 0.1214 | 0.0735 | 0.11 | |
| Creatinine | 0.8426 | 1.9661 | 0.67 | |
| Distensibility | ||||
| PTH | −0.0005 | 0.0003 | 0.07 | 0.43 P value = 0.0012 |
| Corrected calcium | −0.0160 | 0.0204 | 0.44 | |
| Age | −0.0049 | 0.0016 | 0.004 | |
| Creatinine | −0.0401 | 0.0416 | 0.34 |
Figure 2.
PTH levels (picograms per milliliter) are higher in PHPT patients with abnormal (black bar) vs. normal (white bar) stiffness (P = 0.0022) and strain (P = 0.058). Data are presented as mean ± sd.
Using the same multiple regression (including PTH, albumin-corrected calcium, age, and creatinine), PTH, but not calcium concentration, tended to predict carotid strain (P = 0.06) and distensibility (P = 0.07) as well (Table 3). Those with abnormal strain had a marginally significant higher PTH than those with normal strain (148 ± 66 vs. 108 ± 45 pg/ml; P = 0.058; Fig. 2).
Discussion
These data demonstrate that mild PHPT is associated with subclinical structural changes in the carotid vasculature and functional changes that reflect increased vascular stiffness. Carotid IMT, stiffness, distensibility and plaque thickness were all abnormal in patients with PHPT, although only IMT was higher in PHPT than in a non-PHPT group with many more cardiovascular risk factors. The greater IMT may portend a higher risk of stroke and other cardiovascular events in patients with PHPT, because IMT is a strong cardiovascular risk factor in non-hyperparathyroid populations (10,29). Indices reflecting carotid vascular function (stiffness, strain, and distensibility) were associated with the extent of elevation of PTH, suggesting that those with biochemically more severe PHPT may have impaired vascular compliance. PTH but not serum calcium levels were higher in those with abnormal vs. normal stiffness and strain, and PTH was an independent predictor of stiffness, suggesting that PTH, rather than calcium, is the mediator of these effects.
Patients with classical PHPT clearly demonstrate cardiovascular abnormalities (1,2). Studies conducted in patients with varying severity of PHPT, looking at different portions of the vascular tree, some assessing structural changes and others functional abnormalities, has led to conflicting data in less severe disease. This report, targeting only the carotid vascular bed, assessed for anatomical abnormalities (IMT and plaque) and for early abnormalities in vascular function (carotid stiffness, strain, and distensibility). Altered vascular compliance in PHPT could be due to impaired endothelial function or to calcification of the vascular wall.
In prospective epidemiological studies, IMT is strongly associated with the prevalence and incidence of clinical cardiovascular disease (11,12,30,31,32,33). Our finding of increased IMT extends this finding to PHPT patients with mild disease. As neither calcium nor PTH correlated with IMT, it is unclear how PHPT results in this increase. Impaired carotid compliance may be the pathological mechanism or a precursor to increases in IMT (18). In a recent study, carotid IMT was increased in 20 PHPT patients with marked hypercalcemia (11.4–13.5 mg/dl). IMT was not associated with PTH or calcium either, although the study may have had limited power (13). There was, however, a trend toward more frequent plaque in those with PHPT, raising the possibility that the lower calcium levels may have been responsible for its absence in our cohort. A second study, also in more severely hypercalcemic patients, found increased IMT and plaque frequency only in PHPT patients with traditional cardiovascular risk factors. The authors concluded that traditional cardiovascular risk factors, rather than hypercalcemia and/or PTH elevation, were the determinants of carotid atherosclerosis in PHPT (16). However, the group with more traditional cardiovascular risk factors also had higher mean calcium levels (12.2 ± 1.04 vs. 11.64 ± 0.88 mg/dl). It is therefore impossible to know whether the increased IMT was attributable to the cardiovascular risk factors, higher calcium, PTH, or an interaction between risk factors and the biochemical abnormalities found in PHPT. Another study found no increase in IMT, but it evaluated the brachial artery, an uncommon site for atherosclerotic disease (15).
Although IMT was worse in PHPT, the other structural measure, carotid plaque, was not. The two are generally associated with each other in studies of atherosclerosis but may have different pathogenetic mechanisms (34,35). In the Cardiovascular Health Study, IMT and plaque thickness remained independent risk factors for cardiovascular outcomes and all-cause mortality (11,36). It is also important to note that the mean plaque thickness in both PHPT and control groups was frankly elevated. In 2189 NOMAS subjects, after adjusting for age, gender, race-ethnicity, risk factors (hypertension, diabetes, high-density lipoprotein, low-density lipoprotein, BMI, alcohol intake, current smoking, education, use of aspirin, and lipid-lowering medication), MCPT higher than 1.9 mm (the 75th percentile in NOMAS) was associated with a 2-fold increased risk of ischemic stroke, myocardial infarction, or vascular death (hazard ratio 1.48, 1.05–2.1) (37). The mean plaque thickness in the PHPT group (2.09 mm) was above this threshold and, furthermore, was not different from plaque thickness in a control group at significantly higher cardiovascular risk.
PTH levels predicted carotid compliance in mild PHPT. The nature of the association between PTH and vascular stiffness and/or calcification is a subject of ongoing investigation. PTH increases production and reorganization of collagen by vascular smooth muscle cells in vitro (38). PTH activates the endothelial nitric oxide synthase system (eNOS) and stimulates endothelial expression of receptor of advanced glycation end products (RAGE), which may promote atherogenesis (39). At the same time, PTH decreases osteoprotegerin secretion, which may protect against vascular calcification (39).
In clinical studies, associations between PTH and vascular stiffness or abnormal endothelial function have previously been reported in both primary and secondary hyperparathyroidism (end-stage renal failure) (40,41). Although several studies have reported increased vascular stiffness or impaired endothelium-dependent vasodilatation in patients with PHPT, none have previously evaluated the carotid vasculature, and several studied patients with marked hypercalcemia, limiting the applicability of their conclusions in a less affected cohort (20,21,22,23,42). We previously found that mild PHPT was an independent risk factor for increased aortic stiffness, as assessed by augmentation index and pulse wave velocity (23). In that study, PHPT was a stronger predictor of increased stiffness of the aorta than many traditional cardiovascular risk factors. Similar to the findings of this report, aortic stiffness was positively correlated with the extent of elevation in PTH, but not serum calcium, levels. Although our group and others (42) reported that PHPT was associated with increased vascular stiffness based upon augmentation index, an indirect measure of vascular stiffness, this study is the first to measure vascular compliance in PHPT directly. Somewhat surprisingly, in a multiple regression model that included age, serum creatinine, calcium, and PTH levels, only PTH predicted all three measures of carotid compliance: stiffness, strain, and distensibility. Furthermore, those with abnormal carotid stiffness and strain had higher PTH levels than those who did not.
Our study has a number of limitations. The control group was a free-living population of individuals without stroke, but it was not well matched with our patients with respect to cardiovascular risk factors. We may have been biased against finding carotid abnormalities in PHPT because the traditional cardiovascular risk factors, which we adjusted for, were all greater in the control population. Indeed, mean values for IMT, carotid stiffness, and distensibility were abnormal in both cases and controls compared with normal ranges from the Cardiovascular Health Study and NOMAS, and mean plaque thickness was well above the threshold associated with doubling of risk of ischemic stroke, myocardial infarction, or vascular death in both groups as well. That controls had mean values outside the normal range for some outcomes is consistent with the preponderance of cardiovascular risk factors in this group. More unexpectedly, the PHPT group, who were at much lower cardiovascular risk, also had values outside the reference range. This is particularly striking with regard to the elevation in IMT, which was abnormal despite the normal range having been derived in an older population (subjects in the Cardiovascular Health Study were all >65 yr; mean age 73) in whom more cardiovascular abnormalities might have been expected. The fact that PHPT cases and controls were not ideally matched with regard to cardiovascular risk factors could have impacted our conclusions. Although these differences were taken into account in the data analysis, statistical adjustment may not fully account for these disparities, and it is possible that the data might have suggested more carotid disease in PHPT if cases and controls were more equally matched. We would have expected values for plaque thickness, carotid strain, and distensibility to have been better than those in the control group, given their lower BMI, systolic and diastolic blood pressure, triglyceride levels, and incidence of diabetes. Certainly, the direction of the differences should have biased against our finding of worse IMT in the PHPT group. Finally, the study is also limited because it represents a convenience sample of patients with PHPT, including those who met as well as those who did not meet surgical criteria for parathyroidectomy. Because the size of the PHPT group precludes a subgroup analysis, we cannot address the issue of different carotid findings in those who meet vs. those who do not meet criteria for surgery.
Despite these limitations, the study has several notable strengths. We fully characterized the effect of mild PHPT on the carotid bed in a group of patients with mild PHPT. We used techniques that detect subclinical markers that have been associated with cardiovascular morbidity and mortality. To enhance our confidence in our data, and unlike some previous studies in this area, we measured carotid IMT at 12 carotid sites. The measures we chose assessed end-points reflecting both cardiovascular structure and function, because it was unclear whether the mild increases in serum calcium and PTH might have different effects on these indices. Finally, we had information on demographic and cardiovascular risk factors available for both cases and controls.
In conclusion, this study demonstrates that mild PHPT is associated with cardiovascular manifestations in the carotid vascular bed. Carotid IMT is increased and stiffness of the carotid vasculature is independently associated with the degree of PTH elevation. Although carotid IMT is a strong preclinical marker of atherosclerosis in non-hyperparathyroid populations (43), we do not know that the increased IMT in PHPT necessarily has the same clinical implications. There is, however, a considerable body of data supporting the usefulness of IMT to reflect the response to interventions designed to reduce cardiovascular risk. In particular, carotid IMT has been used as a surrogate outcome in studies of angiotensin-converting enzyme inhibitors, hydroxymethylglutaryl-coenzyme A reductase inhibitors, β-blockers, calcium channel blockers, vitamins, and thiazolidinediones (31,32,33,44,45,46). It will be of significant interest to extend these data to include an investigation of the effect of surgical cure of PHPT on IMT.
Footnotes
This work was supported by National Institutes of Health Grants R01 DK066329, K24 DK074457, and UL1 RR024156.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 15, 2009
Abbreviations: BMI, Body mass index; CCA, common carotid artery; IMT, intima-media thickness; MCPT, maximal carotid plaque thickness; NOMAS, Northern Manhattan Study; PHPT, primary hyperparathyroidism.
References
- Palmér M, Adami HO, Bergström R, Akerström G, Ljunghall S 1987 Mortality after surgery for primary hyperparathyroidism: a follow-up of 441 patients operated on from 1956 to 1979. Surgery 102:1–7 [PubMed] [Google Scholar]
- Hedback G, Oden A, Tisell LE 1991 The influence of surgery on the risk of death in patients with primary hyperparathyroidism. World J Surg 15:399–405; discussion 406–407 [DOI] [PubMed] [Google Scholar]
- Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP 1999 A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med 341:1249–1255 [DOI] [PubMed] [Google Scholar]
- Hedback G, Tisell LE, Bengtsson BA, Hedman I, Oden A 1990 Premature death in patients operated on for primary hyperparathyroidism. World J Surg 14:829–835; discussion 836 [DOI] [PubMed] [Google Scholar]
- Hedbäck G, Oden A 1999 Survival of patients operated on for primary hyperparathyroidism. Surgery 125:240–241 [DOI] [PubMed] [Google Scholar]
- Hedbäck G, Oden A 1998 Increased risk of death from primary hyperparathyroidism: an update. Eur J Clin Invest 28:271–276 [DOI] [PubMed] [Google Scholar]
- Nilsson IL, Yin L, Lundgren E, Rastad J, Ekbom A 2002 Clinical presentation of primary hyperparathyroidism in Europe: nationwide cohort analysis on mortality from nonmalignant causes. J Bone Miner Res 17(Suppl 2):N68–N74 [PubMed] [Google Scholar]
- Øgard CG, Engholm G, Almdal TP, Vestergaard H 2004 Increased mortality in patients hospitalized with primary hyperparathyroidism during the period 1977–1993 in Denmark. World J Surg 28:108–111 [DOI] [PubMed] [Google Scholar]
- Wermers RA, Khosla S, Atkinson EJ, Grant CS, Hodgson SF, O'Fallon WM, Melton 3rd LJ 1998 Survival after the diagnosis of hyperparathyroidism: a population-based study. Am J Med 104:115–122 [DOI] [PubMed] [Google Scholar]
- Chambless LE, Folsom AR, Clegg LX, Sharrett AR, Shahar E, Nieto FJ, Rosamond WD, Evans G 2000 Carotid wall thickness is predictive of incident clinical stroke: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 151:478–487 [DOI] [PubMed] [Google Scholar]
- O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson Jr SK 1999 Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 340:14–22 [DOI] [PubMed] [Google Scholar]
- Burke GL, Evans GW, Riley WA, Sharrett AR, Howard G, Barnes RW, Rosamond W, Crow RS, Rautaharju PM, Heiss G 1995 Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) Study. Stroke 26:386–391 [DOI] [PubMed] [Google Scholar]
- Nuzzo V, Tauchmanovà L, Fonderico F, Trotta R, Fittipaldi MR, Fontana D, Rossi R, Lombardi G, Trimarco B, Lupoli G 2002 Increased intima-media thickness of the carotid artery wall, normal blood pressure profile and normal left ventricular mass in subjects with primary hyperparathyroidism. Eur J Endocrinol 147:453–459 [DOI] [PubMed] [Google Scholar]
- Barletta G, De Feo ML, Del Bene R, Lazzeri C, Vecchiarino S, La Villa G, Brandi ML, Franchi F 2000 Cardiovascular effects of parathyroid hormone: a study in healthy subjects and normotensive patients with mild primary hyperparathyroidism. J Clin Endocrinol Metab 85:1815–1821 [DOI] [PubMed] [Google Scholar]
- Kosch M, Hausberg M, Vormbrock K, Kisters K, Rahn KH, Barenbrock M 2000 Studies on flow-mediated vasodilation and intima-media thickness of the brachial artery in patients with primary hyperparathyroidism. Am J Hypertens 13:759–764 [DOI] [PubMed] [Google Scholar]
- Fallo F, Camporese G, Capitelli E, Andreozzi GM, Mantero F, Lumachi F 2003 Ultrasound evaluation of carotid artery in primary hyperparathyroidism. J Clin Endocrinol Metab 88:2096–2099 [DOI] [PubMed] [Google Scholar]
- Rubin MR, Rundek T, McMahon DJ, Lee HS, Sacco RL, Silverberg SJ 2007 Carotid artery plaque thickness is associated with increased serum calcium levels: the Northern Manhattan study. Atherosclerosis 194:426–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godia EC, Madhok R, Pittman J, Trocio S, Ramas R, Cabral D, Sacco RL, Rundek T 2007 Carotid artery distensibility: a reliability study. J Ultrasound Med 26:1157–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsivgoulis G, Vemmos K, Papamichael C, Spengos K, Daffertshofer M, Cimboneriu A, Zis V, Lekakis J, Zakopoulos N, Mavrikakis M 2006 Common carotid arterial stiffness and the risk of ischaemic stroke. Eur J Neurol 13:475–481 [DOI] [PubMed] [Google Scholar]
- Baykan M, Erem C, Erdođan T, Hacihasanođlu A, Gedikli O, Kiriþ A, Küçükosmanođlu M, Ersöz HO, Celik S 2007 Impairment of flow mediated vasodilatation of brachial artery in patients with primary hyperparathyroidism. Int J Cardiovasc Imaging 23:323–328 [DOI] [PubMed] [Google Scholar]
- Nilsson IL, Aberg J, Rastad J, Lind L 1999 Endothelial vasodilatory dysfunction in primary hyperparathyroidism is reversed after parathyroidectomy. Surgery 126:1049–1055 [DOI] [PubMed] [Google Scholar]
- Kosch M, Hausberg M, Vormbrock K, Kisters K, Gabriels G, Rahn KH, Barenbrock M 2000 Impaired flow-mediated vasodilation of the brachial artery in patients with primary hyperparathyroidism improves after parathyroidectomy. Cardiovasc Res 47:813–818 [DOI] [PubMed] [Google Scholar]
- Rubin MR, Maurer MS, McMahon DJ, Bilezikian JP, Silverberg SJ 2005 Arterial stiffness in mild primary hyperparathyroidism. J Clin Endocrinol Metab 90:3326–3330 [DOI] [PubMed] [Google Scholar]
- Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA 1998 Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol 147:259–268 [DOI] [PubMed] [Google Scholar]
- White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL 2005 Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation 111:1327–1331 [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS 1972 Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18:499–502 [PubMed] [Google Scholar]
- Rundek T, Elkind MS, Pittman J, Boden-Albala B, Martin S, Humphries SE, Juo SH, Sacco RL 2002 Carotid intima-media thickness is associated with allelic variants of stromelysin-1, interleukin-6, and hepatic lipase genes: the Northern Manhattan Prospective Cohort Study. Stroke 33:1420–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juo SH, Lin HF, Rundek T, Sabala EA, Boden-Albala B, Park N, Lan MY, Sacco RL 2004 Genetic and environmental contributions to carotid intima-media thickness and obesity phenotypes in the Northern Manhattan Family Study. Stroke 35:2243–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattanakit K, Folsom AR, Chambless LE, Nieto FJ 2005 Risk factors for cardiovascular event recurrence in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 149:606–612 [DOI] [PubMed] [Google Scholar]
- O'Leary DH, Polak JF, Kronmal RA, Kittner SJ, Bond MG, Wolfson Jr SK, Bommer W, Price TR, Gardin JM, Savage PJ 1992 Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke 23:1752–1760 [DOI] [PubMed] [Google Scholar]
- Furberg CD, Adams Jr HP, Applegate WB, Byington RP, Espeland MA, Hartwell T, Hunninghake DB, Lefkowitz DS, Probstfield J, Riley WA, Young B 1994 Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation 90:1679–1687 [DOI] [PubMed] [Google Scholar]
- Blankenhorn DH, Johnson RL, Nessim SA, Azen SP, Sanmarco ME, Selzer RH 1987 The Cholesterol Lowering Atherosclerosis Study (CLAS): design, methods, and baseline results. Control Clin Trials 8:356–387 [DOI] [PubMed] [Google Scholar]
- Crouse 3rd JR, Byington RP, Bond MG, Espeland MA, Craven TE, Sprinkle JW, McGovern ME, Furberg CD 1995 Pravastatin, lipids, and atherosclerosis in the carotid arteries (PLAC-II). Am J Cardiol 75:455–459 [DOI] [PubMed] [Google Scholar]
- Bonithon-Kopp C, Touboul PJ, Berr C, Leroux C, Mainard F, Courbon D, Ducimetière P 1996 Relation of intima-media thickness to atherosclerotic plaques in carotid arteries. The Vascular Aging (EVA) Study. Arterioscler Thromb Vasc Biol 16:310–316 [DOI] [PubMed] [Google Scholar]
- Homma S, Hirose N, Ishida H, Ishii T, Araki G 2001 Carotid plaque and intima-media thickness assessed by B-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke 32:830–835 [DOI] [PubMed] [Google Scholar]
- Polak JF, Shemanski L, O'Leary DH, Lefkowitz D, Price TR, Savage PJ, Brant WE, Reid C 1998 Hypoechoic plaque at US of the carotid artery: an independent risk factor for incident stroke in adults aged 65 years or older. Cardiovascular Health Study. Radiology 208:649–654 [DOI] [PubMed] [Google Scholar]
- Rundek T, Arif H, Boden-Albala B, Elkind MS, Paik MC, Sacco RL 2008 Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan Study. Neurology 70:1200–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkovic V, Hewitson TD, Kelynack KJ, Martic M, Tait MG, Becker GJ 2003 Parathyroid hormone has a prosclerotic effect on vascular smooth muscle cells. Kidney Blood Press Res 26:27–33 [DOI] [PubMed] [Google Scholar]
- Rashid G, Bernheim J, Green J, Benchetrit S 2007 Parathyroid hormone stimulates the endothelial nitric oxide synthase through protein kinase A and C pathways. Nephrol Dial Transplant 22:2831–2837 [DOI] [PubMed] [Google Scholar]
- Seyrek N, Balal M, Karayaylali I, Payda° S, Aikimbaev K, Cetiner S, Seydaoglu G 2003 Which parameter is more influential on the development of arteriosclerosis in hemodialysis patients? Ren Fail 25:1011–1018 [DOI] [PubMed] [Google Scholar]
- Bortotolotto LA, Costa-Hong V, Jorgetti V, Consolim-Colombo F, Rosa K, Silva BC, Krieger EM, De Lima JJ 2007 Vascular changes in chronic renal disease patients with secondary hyperparathyroidism. J Nephrol 20:66–72 [PubMed] [Google Scholar]
- Smith JC, Page MD, John R, Wheeler MH, Cockcroft JR, Scanlon MF, Davies JS 2000 Augmentation of central arterial pressure in mild primary hyperparathyroidism. J Clin Endocrinol Metab 85:3515–3519 [DOI] [PubMed] [Google Scholar]
- Kuller L, Fisher L, McClelland R, Fried L, Cushman M, Jackson S, Manolio T 1998 Differences in prevalence of and risk factors for subclinical vascular disease among black and white participants in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol 18:283–293 [DOI] [PubMed] [Google Scholar]
- Lonn E, Yusuf S, Dzavik V, Doris C, Yi Q, Smith S, Moore-Cox A, Bosch J, Riley W, Teo K 2001 Effects of ramipril and vitamin E on atherosclerosis: the study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE). Circulation 103:919–925 [DOI] [PubMed] [Google Scholar]
- Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, Ruilope LM 2000 Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT). Lancet 356:366–372 [DOI] [PubMed] [Google Scholar]
- Ubels FL, Terpstra WF, Smit AJ 1999 Carotid intima-media thickness: influence of drug treatment and clinical implications. Neth J Med 55:188–195 [DOI] [PubMed] [Google Scholar]