Abstract
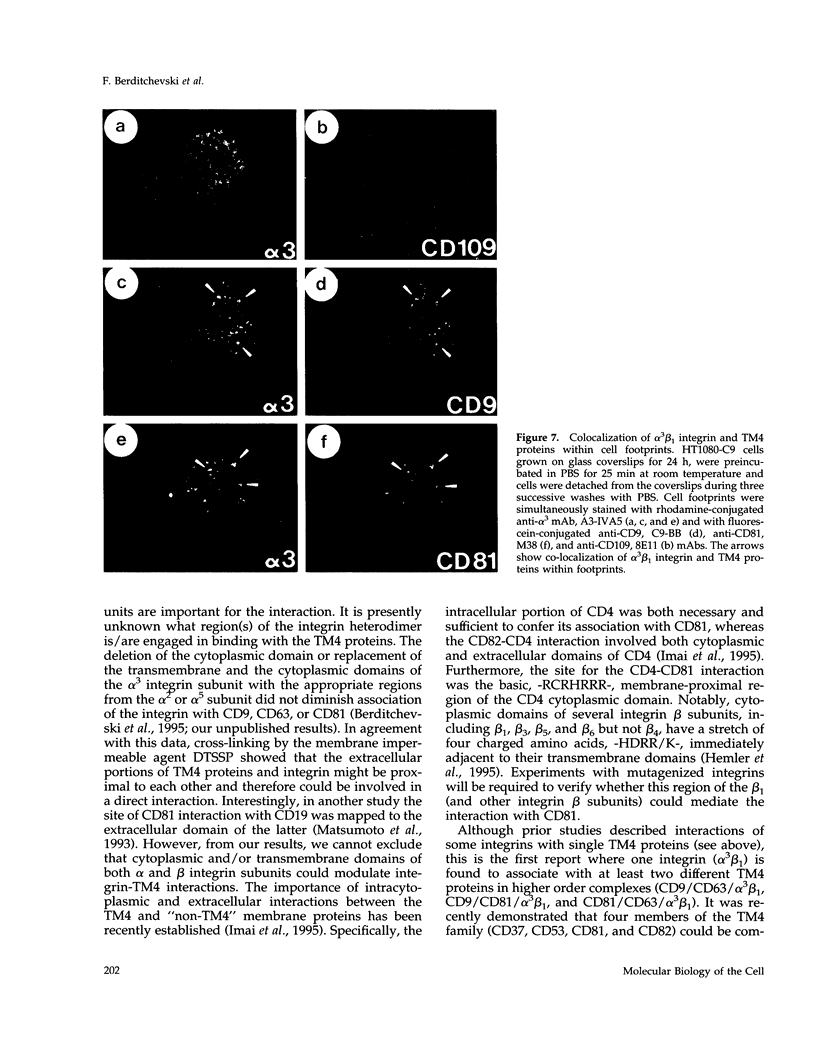
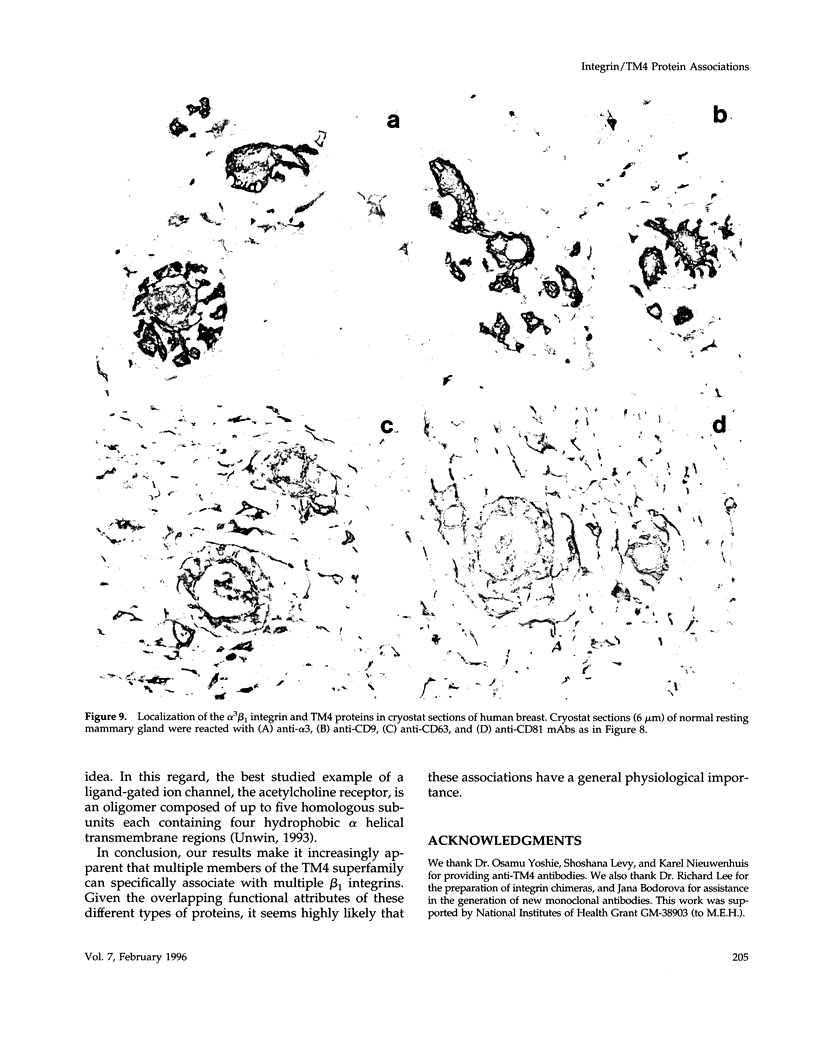
Here we identified several new integrin/TM4 protein complexes on the cell surface. By immunoprecipitation using nonstringent conditions, and by reciprocal immunoprecipitation, we found that alpha 3 beta 1 and alpha 6 beta 1 integrins but not alpha 2 beta 1, alpha 5 beta 1, or alpha 6 beta 4 integrins associated with CD9 and CD81 in alpha 3 beta 1/CD81, alpha 3 beta 1/CD9, alpha 6 beta 1/CD81, and alpha 6 beta 1/CD9 complexes. Also, cross-linking experiments established that alpha 3 beta 1/CD81, alpha 3 beta 1/CD9, and alpha 3 beta 1/CD63 associations occur on the surface of intact cells and suggested that a critical interaction site is located within extracellular domains. Cross-linking in conjunction with reimmunoprecipitation indicated that larger multi-component alpha 3 beta 1/TM4/TM4 complexes (alpha 3 beta 1/CD9/CD63, alpha 3 beta 1/CD81/CD63, and alpha 3 beta 1/CD9/CD81) also could be detected on the cell surface. Immunofluorescent staining showed redistribution of alpha 3 beta 1/TM4 complexes toward the periphery of cells plated on various extracellular matrix substrates and also showed that these complexes were localized in cell footprints. Staining of human tissues yielded additional results consistent with co-localization of alpha 3 beta 1 and CD9, CD63, and CD81 proteins. In conclusion we suggest that the prevalence of integrin/TM4 complexes in diverse cellular environments is indicative of their general physiological importance.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelisová P., Hilgert I., Horejsí V. Association of four antigens of the tetraspans family (CD37, CD53, TAPA-1, and R2/C33) with MHC class II glycoproteins. Immunogenetics. 1994;39(4):249–256. doi: 10.1007/BF00188787. [DOI] [PubMed] [Google Scholar]
- Angelisová P., Vlcek C., Stefanová I., Lipoldová M., Horejsí V. The human leucocyte surface antigen CD53 is a protein structurally similar to the CD37 and MRC OX-44 antigens. Immunogenetics. 1990;32(4):281–285. doi: 10.1007/BF00187099. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bartek J., Bartkova J., Kyprianou N., Lalani E. N., Staskova Z., Shearer M., Chang S., Taylor-Papadimitriou J. Efficient immortalization of luminal epithelial cells from human mammary gland by introduction of simian virus 40 large tumor antigen with a recombinant retrovirus. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3520–3524. doi: 10.1073/pnas.88.9.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F., Bazzoni G., Hemler M. E. Specific association of CD63 with the VLA-3 and VLA-6 integrins. J Biol Chem. 1995 Jul 28;270(30):17784–17790. doi: 10.1074/jbc.270.30.17784. [DOI] [PubMed] [Google Scholar]
- Bergelson J. M., St John N. F., Kawaguchi S., Pasqualini R., Berdichevsky F., Hemler M. E., Finberg R. W. The I domain is essential for echovirus 1 interaction with VLA-2. Cell Adhes Commun. 1994 Oct;2(5):455–464. doi: 10.3109/15419069409004455. [DOI] [PubMed] [Google Scholar]
- Bradbury L. E., Goldmacher V. S., Tedder T. F. The CD19 signal transduction complex of B lymphocytes. Deletion of the CD19 cytoplasmic domain alters signal transduction but not complex formation with TAPA-1 and Leu 13. J Immunol. 1993 Sep 15;151(6):2915–2927. [PubMed] [Google Scholar]
- Brown E., Hooper L., Ho T., Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990 Dec;111(6 Pt 1):2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992 Nov;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J. T., Lamb P. W., Rinker-Schaeffer C. W., Vukanovic J., Ichikawa T., Isaacs J. T., Barrett J. C. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995 May 12;268(5212):884–886. doi: 10.1126/science.7754374. [DOI] [PubMed] [Google Scholar]
- Fukudome K., Furuse M., Imai T., Nishimura M., Takagi S., Hinuma Y., Yoshie O. Identification of membrane antigen C33 recognized by monoclonal antibodies inhibitory to human T-cell leukemia virus type 1 (HTLV-1)-induced syncytium formation: altered glycosylation of C33 antigen in HTLV-1-positive T cells. J Virol. 1992 Mar;66(3):1394–1401. doi: 10.1128/jvi.66.3.1394-1401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiargyrou M., Patterson P. H. An anti-CD9 monoclonal antibody promotes adhesion and induces proliferation of Schwann cells in vitro. J Neurosci. 1995 Jan;15(1 Pt 2):574–583. doi: 10.1523/JNEUROSCI.15-01-00574.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Ware C. F., Strominger J. L. Characterization of a novel differentiation antigen complex recognize by a monoclonal antibody (A-1A5): unique activation-specific molecular forms on stimulated T cells. J Immunol. 1983 Jul;131(1):334–340. [PubMed] [Google Scholar]
- Horejsí V., Vlcek C. Novel structurally distinct family of leucocyte surface glycoproteins including CD9, CD37, CD53 and CD63. FEBS Lett. 1991 Aug 19;288(1-2):1–4. doi: 10.1016/0014-5793(91)80988-f. [DOI] [PubMed] [Google Scholar]
- Hotta H., Ross A. H., Huebner K., Isobe M., Wendeborn S., Chao M. V., Ricciardi R. P., Tsujimoto Y., Croce C. M., Koprowski H. Molecular cloning and characterization of an antigen associated with early stages of melanoma tumor progression. Cancer Res. 1988 Jun 1;48(11):2955–2962. [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ikeyama S., Koyama M., Yamaoko M., Sasada R., Miyake M. Suppression of cell motility and metastasis by transfection with human motility-related protein (MRP-1/CD9) DNA. J Exp Med. 1993 May 1;177(5):1231–1237. doi: 10.1084/jem.177.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T., Fukudome K., Takagi S., Nagira M., Furuse M., Fukuhara N., Nishimura M., Hinuma Y., Yoshie O. C33 antigen recognized by monoclonal antibodies inhibitory to human T cell leukemia virus type 1-induced syncytium formation is a member of a new family of transmembrane proteins including CD9, CD37, CD53, and CD63. J Immunol. 1992 Nov 1;149(9):2879–2886. [PubMed] [Google Scholar]
- Imai T., Kakizaki M., Nishimura M., Yoshie O. Molecular analyses of the association of CD4 with two members of the transmembrane 4 superfamily, CD81 and CD82. J Immunol. 1995 Aug 1;155(3):1229–1239. [PubMed] [Google Scholar]
- Imai T., Yoshie O. C33 antigen and M38 antigen recognized by monoclonal antibodies inhibitory to syncytium formation by human T cell leukemia virus type 1 are both members of the transmembrane 4 superfamily and associate with each other and with CD4 or CD8 in T cells. J Immunol. 1993 Dec 1;151(11):6470–6481. [PubMed] [Google Scholar]
- Kornberg L., Earp H. S., Parsons J. T., Schaller M., Juliano R. L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992 Nov 25;267(33):23439–23442. [PubMed] [Google Scholar]
- Lee R. T., Berditchevski F., Cheng G. C., Hemler M. E. Integrin-mediated collagen matrix reorganization by cultured human vascular smooth muscle cells. Circ Res. 1995 Feb;76(2):209–214. doi: 10.1161/01.res.76.2.209. [DOI] [PubMed] [Google Scholar]
- Lindberg F. P., Gresham H. D., Schwarz E., Brown E. J. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in alpha v beta 3-dependent ligand binding. J Cell Biol. 1993 Oct;123(2):485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marken J. S., Schieven G. L., Hellström I., Hellström K. E., Aruffo A. Cloning and expression of the tumor-associated antigen L6. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3503–3507. doi: 10.1073/pnas.89.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masellis-Smith A., Shaw A. R. CD9-regulated adhesion. Anti-CD9 monoclonal antibody induce pre-B cell adhesion to bone marrow fibroblasts through de novo recognition of fibronectin. J Immunol. 1994 Mar 15;152(6):2768–2777. [PubMed] [Google Scholar]
- Matsumoto A. K., Martin D. R., Carter R. H., Klickstein L. B., Ahearn J. M., Fearon D. T. Functional dissection of the CD21/CD19/TAPA-1/Leu-13 complex of B lymphocytes. J Exp Med. 1993 Oct 1;178(4):1407–1417. doi: 10.1084/jem.178.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee H. P., Ingber D. E., Schwartz M. A. Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-induced inositol lipid breakdown. J Cell Biol. 1993 May;121(3):673–678. doi: 10.1083/jcb.121.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Iwamoto R., Mekada E. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphtheria toxin receptor-associated protein (DRAP27)/CD9 form a complex with integrin alpha 3 beta 1 at cell-cell contact sites. J Cell Biol. 1995 Jun;129(6):1691–1705. doi: 10.1083/jcb.129.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima Y., Hirose T., Tachibana K., Tanaka T., Shi L., Doshen J., Freeman G. J., Schlossman S. F., Morimoto C. The 4F9 antigen is a member of the tetra spans transmembrane protein family and functions as an accessory molecule in T cell activation and adhesion. Cell Immunol. 1993 Nov;152(1):249–260. doi: 10.1006/cimm.1993.1285. [DOI] [PubMed] [Google Scholar]
- Olweus J., Lund-Johansen F., Horejsi V. CD53, a protein with four membrane-spanning domains, mediates signal transduction in human monocytes and B cells. J Immunol. 1993 Jul 15;151(2):707–716. [PubMed] [Google Scholar]
- Oren R., Takahashi S., Doss C., Levy R., Levy S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol Cell Biol. 1990 Aug;10(8):4007–4015. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini R., Bodorova J., Ye S., Hemler M. E. A study of the structure, function and distribution of beta 5 integrins using novel anti-beta 5 monoclonal antibodies. J Cell Sci. 1993 May;105(Pt 1):101–111. doi: 10.1242/jcs.105.1.101. [DOI] [PubMed] [Google Scholar]
- Rubinstein E., Le Naour F., Billard M., Prenant M., Boucheix C. CD9 antigen is an accessory subunit of the VLA integrin complexes. Eur J Immunol. 1994 Dec;24(12):3005–3013. doi: 10.1002/eji.1830241213. [DOI] [PubMed] [Google Scholar]
- Schaller M. D., Otey C. A., Hildebrand J. D., Parsons J. T. Focal adhesion kinase and paxillin bind to peptides mimicking beta integrin cytoplasmic domains. J Cell Biol. 1995 Sep;130(5):1181–1187. doi: 10.1083/jcb.130.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz-Albiez R., Dörken B., Hofmann W., Moldenhauer G. The B cell-associated CD37 antigen (gp40-52). Structure and subcellular expression of an extensively glycosylated glycoprotein. J Immunol. 1988 Feb 1;140(3):905–914. [PubMed] [Google Scholar]
- Schwartz M. A., Brown E. J., Fazeli B. A 50-kDa integrin-associated protein is required for integrin-regulated calcium entry in endothelial cells. J Biol Chem. 1993 Sep 25;268(27):19931–19934. [PubMed] [Google Scholar]
- Seehafer J. G., Shaw A. R. Evidence that the signal-initiating membrane protein CD9 is associated with small GTP-binding proteins. Biochem Biophys Res Commun. 1991 Aug 30;179(1):401–406. doi: 10.1016/0006-291x(91)91384-o. [DOI] [PubMed] [Google Scholar]
- Slupsky J. R., Seehafer J. G., Tang S. C., Masellis-Smith A., Shaw A. R. Evidence that monoclonal antibodies against CD9 antigen induce specific association between CD9 and the platelet glycoprotein IIb-IIIa complex. J Biol Chem. 1989 Jul 25;264(21):12289–12293. [PubMed] [Google Scholar]
- Sutherland D. R., Yeo E., Ryan A., Mills G. B., Bailey D., Baker M. A. Identification of a cell-surface antigen associated with activated T lymphoblasts and activated platelets. Blood. 1991 Jan 1;77(1):84–93. [PubMed] [Google Scholar]
- Szala S., Kasai Y., Steplewski Z., Rodeck U., Koprowski H., Linnenbach A. J. Molecular cloning of cDNA for the human tumor-associated antigen CO-029 and identification of related transmembrane antigens. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6833–6837. doi: 10.1073/pnas.87.17.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S., Fujikawa K., Imai T., Fukuhara N., Fukudome K., Minegishi M., Tsuchiya S., Konno T., Hinuma Y., Yoshie O. Identification of a highly specific surface marker of T-cell acute lymphoblastic leukemia and neuroblastoma as a new member of the transmembrane 4 superfamily. Int J Cancer. 1995 May 29;61(5):706–715. doi: 10.1002/ijc.2910610519. [DOI] [PubMed] [Google Scholar]
- Toothill V. J., Van Mourik J. A., Niewenhuis H. K., Metzelaar M. J., Pearson J. D. Characterization of the enhanced adhesion of neutrophil leukocytes to thrombin-stimulated endothelial cells. J Immunol. 1990 Jul 1;145(1):283–291. [PubMed] [Google Scholar]
- Unwin N. Neurotransmitter action: opening of ligand-gated ion channels. Cell. 1993 Jan;72 (Suppl):31–41. doi: 10.1016/s0092-8674(05)80026-1. [DOI] [PubMed] [Google Scholar]
- Vuori K., Ruoslahti E. Association of insulin receptor substrate-1 with integrins. Science. 1994 Dec 2;266(5190):1576–1578. doi: 10.1126/science.7527156. [DOI] [PubMed] [Google Scholar]
- Weitzman J. B., Pasqualini R., Takada Y., Hemler M. E. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading, and homotypic cell aggregation. J Biol Chem. 1993 Apr 25;268(12):8651–8657. [PubMed] [Google Scholar]
- Wright M. D., Tomlinson M. G. The ins and outs of the transmembrane 4 superfamily. Immunol Today. 1994 Dec;15(12):588–594. doi: 10.1016/0167-5699(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Zutter M. M. Immunolocalization of integrin receptors in normal lymphoid tissues. Blood. 1991 May 15;77(10):2231–2236. [PubMed] [Google Scholar]