Abstract
Context: Patients treated with levothyroxine typically ingest it in a fasting state to prevent food impairing its absorption. The serum thyrotropin concentration is the therapeutic index of levothyroxine action.
Objective: The study objective was to determine the effect of the timing of levothyroxine administration in relationship to food on serum thyrotropin levels.
Design: Participants were randomized to one of six sequences, each consisting of three 8-wk regimens in a three-period crossover design. These regimens were in a fasting state, at bedtime, and with breakfast. The concentrations of TSH, free T4, and total T3 during each of the three timing regimens were documented. The primary outcome was the difference between serum TSH concentrations under fasting conditions compared with concentrations during the other 8-wk regimens.
Setting: The study was conducted in an academic medical center.
Participants: Study participants were receiving levothyroxine for treatment of hypothyroidism or thyroid cancer.
Results: Sixty-five patients completed the study. The mean thyrotropin concentration was 1.06 ± 1.23 mIU/liter when levothyroxine was administered in the fasting state. When levothyroxine was taken with breakfast, the serum thyrotropin concentration was significantly higher (2.93 ± 3.29 mIU/liter). When levothyroxine was taken at bedtime, the serum TSH concentration was also significantly higher (2.19 ± 2.66 mIU/liter).
Conclusion: Nonfasting regimens of levothyroxine administration are associated with higher and more variable serum TSH concentrations. If a specific serum TSH goal is desired, thereby avoiding iatrogenic subclinical thyroid disease, then fasting ingestion of levothyroxine ensures that TSH concentrations remain within the narrowest target range.
The timing of levothyroxine administration has a significant effect on the serum thyrotropin concentration.
Studies show optimal intestinal absorption of levothyroxine (LT4) under fasting conditions, with reduction from approximately 80 to 40–64% with concurrent food ingestion (1,2,3,4,5,6). A patient’s biochemical response to LT4 is determined by their serum TSH concentration. LT4 is recognized as a drug with a narrow therapeutic index (7), and its dose can be finely adjusted to keep the serum TSH within the specific range desired for a particular patient’s diagnosis, age, and coexistent medical conditions.
Factors that can hamper the ability to maintain a desired TSH concentration include variable patient adherence (8), conditions that affect LT4 absorption, and medications that affect LT4 absorption and metabolism. Medical conditions affecting absorption include lactose intolerance, celiac sprue, autoimmune gastritis, and impaired gastric acid secretion (9,10,11,12). Medications affecting absorption include ferrous sulfate, calcium carbonate, bile acid sequestrants, aluminum hydroxide antacids, sucralfate, sodium polystyrene sulfonate, cholestyramine, colestipol, and raloxifene (13,14,15,16,17,18). The absorption of LT4 can also be affected by the various inert ingredients with which different brand names are formulated (19,20,21). Additional factors affecting the absorption of LT4 are food (1,2,3,4,22,23), including high-fiber diets (24), soy (25), and beverages (26).
Specific timing of these medications is advised in hypothyroid patients to maintain consistent and optimal LT4 absorption. In addition, patients are generally advised to take their LT4 on an empty stomach. This stringent recommendation could potentially affect compliance. Failure to follow this advice, on the other hand, could impair absorption of LT4. To take LT4 on an empty stomach, patients usually ingest it first thing in the morning. However, patients may wait varying lengths of time until food consumption. Patients may alternatively take their LT4 in the evening or at bedtime, finding such timing convenient. Thus, in practice, patients may be taking their LT4 well separated from breakfast (fasting), close to breakfast (in a fed state), or at bedtime (a few hours after the last meal of the day). Currently it is not known whether selection of these various timing options has a clinically significant impact on serum TSH concentrations.
A case report suggested that close proximation of LT4 within 20 min of a meal, instead of separated by 60 min, resulted in increased TSH values (2). Another study showed that participants’ serum TSH concentrations were unchanged when their time of LT4 administration was altered from 1–2 h after breakfast to midnight (27). An additional recent study showed that the participants had lower serum TSH concentrations when taking their LT4 at bedtime compared with taking it 30 min before breakfast (28). The impact of LT4 timing on serum TSH is thus not clear from consolidating these study results. If serum TSH concentrations were not shifted outside of the desired range by altering the timing of LT4 administration with respect to food ingestion, this would have significant clinical consequences. It would be more convenient for patients to adjust the timing of LT4 with respect only to other medications, without the extra inconvenience of having to also adjust its timing with respect to food consumption. This study manipulates the timing of LT4 administration in relation to food to determine the effect on LT4 absorption, as reflected in serum TSH levels.
Patients and Methods
Study concept
This was a three-period crossover study designed to determine the effect of the timing of LT4 administration on TSH levels in participants after each of three 8-wk regimens. Two regimens were after an overnight fast and at least 1 h before breakfast (BB), and at bedtime and at least 2 h after the last meal of the day (HS). In contrast, the third regimen was in a fed state and within 20 min of breakfast (WB). The difference between the serum TSH concentration under fasting conditions and the concentration measured after each of the other two 8-wk regimens was the primary study outcome. Our hypothesis was that nonfasting regimens would be associated with higher TSH concentrations, even in patients with a subnormal baseline TSH.
Patients
Male or female patients of aged 18–75 yr were recruited for the study. Patients with primary hypothyroidism of any cause who were taking a minimum dose of 75 μg LT4 were eligible. Hypothyroid patients were eligible for the study if their serum TSH was within the laboratory reference range (0.5–4.5 mIU/liter). Patients with thyroid cancer, no evidence of recurrence, and a baseline TSH level of between greater than or equal to 0.01 to less than or equal to 0.5 mIU/liter were also recruited. It was considered unlikely that any changes in TSH during the short duration of this study would be associated with an increased risk of thyroid cancer recurrence.
Women who were pregnant, lactating, or planning pregnancy were ineligible for the study. Patients with changes in their estrogen/progesterone replacement therapy, oral contraceptives, testosterone replacement, or tamoxifen within the last 3 months were excluded. Patients taking bile acid sequestrants, aluminum hydroxide antacids, sodium polystyrene sulfonate, cholestyramine, colestipol, raloxifene, high-fiber diets, and diets high in soy were excluded. Patients with chronic, serious diseases such as diabetes and cardiac, pulmonary, gastrointestinal, and renal diseases were not eligible. Individuals taking medications that might potentially affect serum TSH concentrations, such as steroids, T3 preparations, dopamine analogs, or somatostatin analogs, were not eligible. Patients taking medications affecting thyroid hormone metabolism such as phenytoin, carbamazepine, sertraline, and rifampin were also excluded. In addition, patients who did not eat breakfast were excluded.
Only patients taking the two most commonly used LT4 brands were recruited. Patients taking other brand names of LT4 or generic LT4 were excluded to avoid potential effects of the different absorption or lack of bioequivalence of multiple products on study results (21). Patients were required to have been consistently on the same dose of LT4 for 6 months before study enrollment and also to have hypothyroidism treated with LT4 for at least 2 yr.
Study sequence
The study planned to recruit 42 patients with hypothyroidism and 20 patients with thyroid cancer. Patients had baseline thyroid function tests drawn at their usual clinical laboratory to determine study eligibility. After confirmation of eligibility, the patient signed the written informed consent form. Patients had a medical history and physical examination at the beginning of the study. Each patient’s medications were documented, including calcium carbonate, multivitamins, and other supplements. Patients continued to take the same dose and brand of LT4 supplied by their pharmacy during the 24-wk study.
Patients completed three 8-wk regimens, with regimens defined by the timing of LT4 administration. In one 8-wk block, patients were asked to take their LT4 after an overnight fast at least 1 h BB. In another 8-wk block, patients were asked to take their LT4 WB. In the last 8-wk block, patients were asked to take their LT4 as they retired for bed and at least 2 h after their last meal of the day (HS). Each patient was randomized to one of six possible sequences (see Table 1). These six sequences were all the possible combinations of the three different timing regimens. Each patient served as his or her own control. Blood for thyroid function tests was drawn at study initiation and at the end of each 8-wk period. Blood was split into two aliquots. One aliquot was sent immediately to the clinical laboratory that performed the patient’s initial thyroid function tests. The other aliquot was stored and processed in the General Clinical Research Center (GCRC) laboratory at study completion.
Table 1.
LT4 timing regimens, sequences, and time periods used in the study
| Time period | LT4 timing sequences A–F
|
|||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| Weeks 1–8 | BB | HS | WB | WB | BB | HS |
| Weeks 9–16 | HS | WB | BB | HS | WB | BB |
| Weeks 17–24 | WB | BB | HS | BB | HS | WB |
LT4 timing regimens: BB, WB, and HS.
Patients taking calcium supplements, ferrous sulfate, or multivitamins were asked to take these with meals other than breakfast and at least 4 h apart from their LT4. They were asked to keep a diary of their LT4 ingestion times, breakfast times, dinner times, and bedtimes as well as foods consumed at breakfast, dinner, and after-dinner snacks. Study diaries were provided to patients. At each study visit, participants were given an appointment for their next visit, reminded to complete their diaries, and to comply with the timing and dietary directions. Telephone or E-mail follow-ups were conducted every 4 wk.
Thyroid profiles
Phlebotomy was performed at 0800 h under fasting conditions for the baseline blood tests and during all three LT4 timing regimens. LT4 administration was delayed until after phlebotomy for all circumstances in which LT4 was taken in the morning. Thyroid function was assessed both by a clinical laboratory [Quest Diagnostics (Madison, NJ), LabCorp (Burlington, NC), or Georgetown University Laboratories (Washington, DC)] and the GCRC core laboratory. Each clinical thyroid profile consisted of a serum TSH, free T4 (FT4), and total T3. Clinical laboratories used a third-generation immunochemiluminometric TSH assay with a sensitivity of 0.01 mIU/liter (reference ranges ∼0.4–4.5 mIU/liter). FT4 and T3 levels were measured by the clinical laboratories using chemiluminescent immunoassays. Over the study period, reference ranges were approximately 0.8–1.80 ng/dl (10.29–23.17 pmol/liter) for FT4 and 80–200 ng/dl (1.23–3.08 nmol/liter) for T3. Clinical laboratory data were used to make decisions regarding whether patients could safely continue the study. The GCRC laboratory performed TSH determinations using the Dade Dimension RxL clinical chemistry analyzer (Dade, Newark, DE). This was a colorimetric immunoassay with a sensitivity of 0.01 mIU/liter, a precision of less than 6.2% at all concentrations tested and calibration for the range of 0.01–50 mIU/liter. The manufacturer’s reference range was 0.34–4.82 mIU/liter. The thyroid analytes reported in this study were FT4 and T3 concentrations determined by the clinical laboratory and TSH concentrations determined by the GCRC laboratory.
Statistical analysis
This was a three-period crossover design. There were six combinations of the three timing regimens into which patients were randomized in blocks of six (see Table 1). Power calculations were based on the WB and HS regimens not being inferior to the BB regimen. The TSH concentration that was defined as failure was 1 mIU/liter or greater above the cohort’s mean TSH concentration during the fasting LT4 regimen. Statistical analysis showed that a sample size of 42 patients would be sufficient to detect a difference in TSH of 1.0 mIU/liter between timing regimens with 90% power and α = 0.05. Data for this calculation were generated from cross-sectional chart review examining the variation between TSH concentrations in LT4-treated patients.
Time period, sequence, and LT4 timing variables were used to evaluate the LT4 timing effect for this three-time period, six-sequence study. The variables are shown in Table 1. A multivariate ANOVA model was used to explore the effect of LT4 timing using the proc general linear model and least squares means. Analysis was performed on an intention to treat basis. Bonferroni corrections were used for repeated measures. Other independent variables considered post hoc were diagnosis, etiology of hypothyroidism, gender, age, menstrual status, weight, height, LT4 dose, and LT4 brand. The effect of these independent variables on the response to the LT4 timing regimens was tested using the original data without considering the time period and sequence effects.
Results
Participant recruitment and retention
The charts of all patients followed within the endocrine division with diagnoses of hypothyroidism or thyroid cancer were reviewed for study eligibility. Approximately 450 patients were approached regarding study participation. Eighty-four individuals indicated willingness to participate. The primary reasons given for unwillingness to participate were the extensive time demands of the study, the inconvenience of altering an already-established regimen for LT4 ingestion, and the inconvenience of travel to the GCRC. Four of the 84 patients were excluded after baseline laboratory testing because of TSH values outside the desired range. Of the 80 remaining participants, 15 withdrew during the course of the study (see supplemental Fig. S1). One patient elected to change her LT4 dose despite a normal TSH value, one patient switched to generic LT4, two patients were discontinued because of concern regarding their abnormal TSH values, five patients withdrew because of scheduling difficulties, two patients withdrew because of personal reasons, two withdrew because of the need to travel, and two withdrew because of financial constraints. A further six patients also withdrew but later restarted and completed the study. These patients repeated their entire three-regimen sequence.
Participant characteristics
Sixty-five patients completed the study (see supplemental Fig. S1) and maintained their initial LT4 dose and brand throughout. Forty-two patients had hypothyroidism, whereas 23 patients had thyroid cancer. The patients with thyroid cancer were recruited in addition to the 42 patients required by prestudy power calculations because it was unclear whether the effects of LT4 timing would be of sufficient magnitude to cause such patients’ TSH values to rise out of a subnormal range. Study entry years were 2005 for 18 patients, 2006 for 31 patients, 2007 for two patients, and 2008 for 14 patients. The study ran from August 2005 through December 2008. Of the patients who completed the study, 88% were taking their LT4 in a fasting state, 9% were taking their LT4 at bedtime, and 3% were taking their LT4 within 1 h of breakfast at the time of the baseline TSH determination.
The baseline characteristics of participants who completed the study are shown in Table 2. The gender, age, and LT4 dose of those who declined study participation and who withdrew from the study were similar to the participants who completed the study. Patients without thyroid cancer had the following etiologies of hypothyroidism: 73% Hashimoto’s thyroiditis, 10% radioiodine treatment for hyperthyroidism, and 17% postsurgical. The only significant difference between the patients with hypothyroidism and thyroid cancer was their mean LT4 dose (Table 2) and their mean serum TSH at baseline (Table 3). Documented deviations from protocol instructions regarding meal timing, LT4 compliance, or LT4 timing occurred at a rate of 1.2% (130 of 10,920 LT4 administrations). Completed study diaries were provided by 70% of participants. The number of patients randomized to each of the LT4 timing sequences is indicated in Table 2.
Table 2.
Participant characteristics for those completing the study
| Characteristic | All patients (n = 65) | Patients with hypothyroidism [n = 42 (65%)] | Patients with thyroid cancer [n = 23 (35%)] |
|---|---|---|---|
| Female gender, n/N (%) | 50/65 (77) | 33/42 (79) | 17/23 (74) |
| Mean age (yr) (sd) | 48 (13) | 46 (13) | 51 (11) |
| Premenopausal status, n/N (%) | 27/50 (54) | 18/33 (55) | 9/17 (53) |
| Mean weight (kg) (sd) | 74.1 (14) | 73.0 (13) | 76.0 (15) |
| Mean height (cm) (sd) | 168.9 (8.6) | 169.4 (8.5) | 167.9 (8.7) |
| Mean BMI (kg/m2) (sd) | 25.9 (4.2) | 25.4 (4.2) | 26.8 (3.9) |
| Mean LT4dose (μg) (sd) | 128 (44) | 108 (26) | 165 (47) |
| Mean dose LT4 (μg/kg · d) (sd) | 1.8 (0.6) | 1.6 (0.4) | 2.2 (0.6) |
| LT4 brand 1, n/N (%) | 56/65 (86) | 37/42 (88) | 19/23 (83) |
| LT4 brand 2, n/N (%) | 9/65 (14) | 5/42 (12) | 4/23 (17) |
| Sequence A, n/N (%) | 11/65 (17) | 7/42 (17) | 4/23 (17) |
| Sequence B, n/N (%) | 8/65 (12) | 6/42 (14) | 2/23 (9) |
| Sequence C, n/N (%) | 12/65 (18.5) | 4/42 (10) | 8/23 (35) |
| Sequence D, n/N (%) | 11/65 (17) | 8/42 (19) | 3/23 (13) |
| Sequence E, n/N (%) | 11/65 (17) | 8/42 (19) | 3/23 (13) |
| Sequence F, n/N (%) | 12/65 (18.5) | 9/42 (21) | 3/23 (13) |
Characteristics are shown for all patients combined and for patients with diagnoses of hypothyroidism and thyroid cancer separately (n = number of patients in subgroup, N = total number of patients). BMI, Body mass index.
Table 3.
Effect of timing of LT4 ingestion (fasting, with breakfast, or at bedtime) on the arithmetic mean of thyroid analytes (TSH, FT4, T3) for hypothyroid patients and patients with thyroid cancer
| Analyte | BL | BB | WB | HS |
|---|---|---|---|---|
| Hypothyroid patients | ||||
| Mean TSH, mIU/liter (sd) | 1.77 (1.20) | 1.54 (1.27) | 3.74 (3.55) | 2.79 (2.15) |
| Mean FT4, ng/dl (sd) | 1.20 (0.23) | 1.23 (0.22) | 1.16 (0.22) | 1.2 (0.25) |
| Mean T3, ng/dl (sd) | 128 (24) | 125 (33) | 121 (25) | 123 (24) |
| Thyroid cancer patients | ||||
| Mean TSH, mIU/liter (sd) | 0.29 (0.50) | 0.27 (0.58) | 1.41 (2.02) | 1.14 (3.12) |
| Mean FT4, ng/dl (sd) | 1.45 (0.30) | 1.57 (0.28) | 1.39 (0.24) | 1.50 (0.28) |
| Mean T3, ng/dl (sd) | 136 (29) | 134 (32) | 127 (28) | 129 (29) |
SI conversions: to convert FT4 to picomoles per liter, multiply by 12.871; and to convert T3 to nanomoles per liter, multiply by 0.0154. BL, Baseline.
TSH and FT4 concentrations
The concentration of thyroid analytes according to diagnosis and timing of LT4 administration is shown in Table 3. The independent variable of diagnosis (hypothyroidism vs. thyroid cancer) had no significant effect on any of the dependent variables (differences between thyroid analytes during different LT4 timing regimens). Therefore, patients with both diagnoses were combined for final analysis (see Table 4). There was significant overlap between the serum TSH concentrations at baseline and those during the before breakfast regimen, with respective 95% confidence intervals being 0.95–1.55 mIU/liter and 0.60–1.52 mIU/liter. The TSH concentrations documented during the WB regimen were significantly higher than those in the BB regimen (2.93 vs. 1.06 mIU/liter, P < 0.001). Additionally, the TSH concentrations achieved with the HS regimen were significantly higher from those observed during the BB regimen (2.19 vs. 1.06 mIU/liter, P < 0.001). The WB TSH concentrations were also significantly higher than the TSH values seen with the HS regimen (2.93 vs. 2.19 mIU/liter, P = 0.026).
Table 4.
Effect of timing of LT4 ingestion on the least squares means for thyroid analytes for all patients combined showing the P values for significant differences
| Analyte | BB LT4 timing
|
WB LT4 timing
|
HS LT4 timing
|
|||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| Least squares TSH mean, mIU/liter | 1.06 | 0.60–1.52 | 2.93 | 2.47–3.38 | 2.19 | 1.73–2.65 |
| Difference from BB | n/a | See first column | See first column | |||
| Difference from WB | P < 0.001 | n/a | See previous column | |||
| Difference from HS | P < 0.001 | P = 0.026 | n/a | |||
| Least squares FT4, mean ng/dl | 1.35 | 1.31–1.39 | 1.24 | 1.20–1.28 | 1.34 | 1.30–1.38 |
| Difference from BB | n/a | See first column | See first column | |||
| Difference from WB | P < 0.001 | n/a | See previous column | |||
| Difference from HS | P = 0.72 | P < 0.001 | n/a | |||
| Least squares T3, mean ng/dl | 128.7 | 124.1–133.4 | 123.4 | 118.7–128.0 | 125.5 | 120.8–130.1 |
| Difference from BB | n/a | See first column | See first column | |||
| Difference from WB | P = 0.11 | n/a | See previous column | |||
| Difference from HS | P = 0.33 | P = 0.52 | n/a | |||
SI conversions: to convert FT4 to picomoles per liter, multiply by 12.871; and to convert T3 to nanomoles per liter, multiply by 0.0154.CI, Confidence interval; n/a, not applicable.
With respect to FT4 concentrations, these were significantly lower during the WB regimen than either the BB regimen [1.24 vs. 1.35 ng/dl (15.96 vs. 17.38 pmol/liter), P < 0.001] or the HS regimen [1.24 vs. 1.34 ng/dl (15.96 vs. 17.25 pmol/liter), P < 0.001]. There were no significant differences between the T3 concentrations achieved during any of the LT4 regimens. Patients’ vital signs and weights were not significantly affected by the LT4 timing regimen (data not shown). None of the covariates analyzed influenced the study results.
TSH concentrations according to patient and magnitude of change in TSH
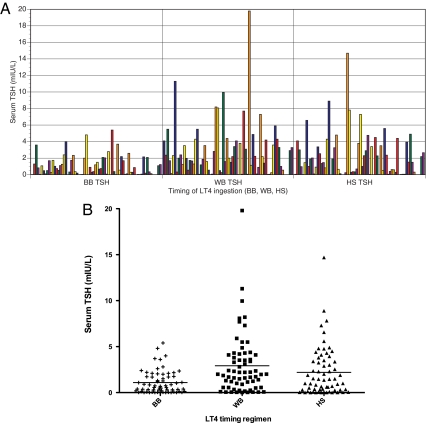
Figure 1A shows the TSH concentrations achieved in each of the LT4 timing regimens for subjects who completed the study. The values for each individual are displayed consecutively across the x-axis, and the patients are displayed in the same order for each regimen. The increased TSH values and greater interindividual TSH variability during the WB regimen is clearly seen. The TSH values appear to be of intermediate magnitude and variability during the HS regimen. TSH values in the range of 0–19 mIU/liter were observed in some patients in the nonfasting regimens. Figure 1B shows the same data displayed as a scatter plot.
Figure 1.
A, Serum TSH concentrations of participants according to their LT4 timing regimen (fasting, with breakfast, or at bedtime) for subjects who completed the study. Patients are displayed in the order and the same color in each of the three levothyroxine timings (each patient is not a unique color). B, Scatter plot showing TSH values during each LT4 timing regimen for subjects who completed the study.
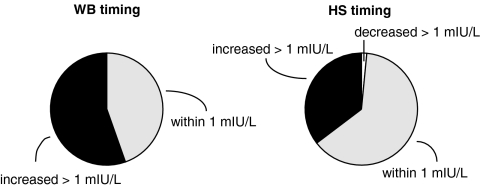
Figure 2 shows the changes in TSH concentrations during the WB and HS LT4 regimen compared with the values attained during the fasting LT4 regimen. The left-sided pie chart shows that TSH values for patients increased by more than 1 mIU/liter in 55% of patients during the WB regimen compared with the values during the fasting regimen. They remained within 1 mIU/liter of the fasting value in 45% of patients. On the other hand, when examining the HS LT4 regimen compared with the fasting LT4 regimen (right sided pie chart), 35% of patients had TSH values that were more than 1 mIU/liter above their fasting values. Sixty-three percent of patients had values that were within 1 mIU/liter of the fasting TSH values.
Figure 2.
Change in serum TSH between a fasting regimen and either a WB regimen (left sided chart) or HS regimen (right sided chart). Pie chart showing percentage of patients whose serum TSH level decreased by more than 1 mIU/liter (white), remained within 1 mIU/liter (gray), and increased by more than 1 mIU/liter (black) when changing from a fasting regimen to a WB regimen and from a fasting regimen to an HS regimen.
Discussion
During our study the TSH concentrations achieved in the fasting state were lower than TSH values during nonfasting conditions. This suggests that the absorption of LT4 is optimum under fasting conditions and translates into a biochemical end point of a lower serum TSH concentration. Obviously achieving a lower TSH during the fasting state than under other conditions is insufficient reason to recommend this particular regimen because if an alternative regimen were more convenient for patients, their LT4 dose could simply be increased to achieve the lower TSH.
However, our study illustrates another feature of the TSH values achieved under nonfasting regimens. As can be seen from the TSH values in Fig. 1A and the sds in Table 3, the TSH values are also more variable during the WB and HS regimens. This suggests that patients taking their LT4 with breakfast or at bedtime had differential absorption, which in turn resulted in fluctuations in serum TSH concentrations. It is noteworthy that higher TSH concentrations were even observed in thyroid cancer patients whose baseline TSH concentrations were subnormal (Table 3). This observation has tremendous implications, given that maintenance of specific TSH goals is beneficial for many thyroid cancer patients (29).
The TSH concentrations in our study could have been affected by patient adherence to their regimen, particularly around the times when the regimens were changed. We believe that we maximized adherence by close monitoring with telephone calls, E-mail contact, study diaries, and follow-up visits. Additionally, patients who contributed their time to a study of this nature were probably self-selected for good adherence. Thus, our study does not address whether more variable absorption during, for example, a WB regimen could be mitigated by better adherence outside a research environment. Thus, it is possible that in a real-life situation, the WB and HS timings may be more convenient for busy patients and be associated with better compliance. In contrast, a fasting regimen may be less convenient and be associated with more variable compliance. In either case, there may be less difference in the TSH variability seen between regimens in a real-life situation. It is interesting that the confidence limits for the TSH concentrations at baseline fell within the confidence limits of the TSH concentrations during the fasting regimen, even though at baseline only 88% of patients were ingesting their LT4 in a fasting state. Perhaps these values were similar because so few patients (3%) were taking their LT4 in close proximity to breakfast at baseline. These data may also reflect the inherent reproducibility or consistency of TSH concentrations.
Interestingly, for both of the nonfasting regimens, there was a significant subset of patients in whom the TSH concentration did not change by more than 1 mIU/liter (see Figs. 1A and 2). In fact, many patients had TSH values that were within 0.1–0.2 mIU/liter of each other (see Fig. 1A). For other patients, nonfasting regimens resulted in considerable increase in the range of TSH values observed. It is possible that such divergent results were due either to individual patient characteristics or consumption of different foods. Examination of patient diaries did not implicate any particular foods or beverages (including coffee) as being associated with greater or lesser TSH changes in the nonfasting regimens. However, it is plausible that meals with different carbohydrate, protein, or fat content are associated with different degrees of impact on LT4 absorption and TSH levels. This theory could be tested in a study similar to the present study but with the addition of prescriptions for either standard breakfast and dinner menus or menus with varying compositions. It is certainly possible that meals with a particular composition may have a lesser impact on serum TSH concentrations. Alternatively, there might be a subset of patients whose TSH concentrations, for other reasons, are less affected by the timing of LT4 ingestion. If either were the case, identification of these conditions or patients would be important because this may allow a less stringent LT4 timing regimen.
Our results initially seem to be different from those of prior studies examining the effect of LT4 timing on serum TSH concentration. Elliott (27) showed that serum TSH concentrations did not differ between morning and bedtime regimens. However, in that study the morning LT4 dose was given an hour after breakfast. With respect to their bedtime regimen, participants ate dinner at 1700–1800 h, had a snack at 2100 h, and were given their LT4 at 2400 h. Thus, both their morning and evening schedules for LT4 ingestion were essentially postprandial. The study by Bolk et al. (28) demonstrated lower serum TSH concentrations when LT4 was taken at bedtime. However, the participants taking their LT4 in the morning took it only 30 min before breakfast. This regimen may have been intermediate between a fasting and fed state. Thus, the results of these three studies may actually be congruent.
Our study had several shortcomings. One of these was that we were unable to collect pharmacokinetic data during each of the three LT4 timing regimens. Such continuous sampling was actually part of the study protocol. Our intention was to admit a subset of 10 hypothyroid participants to the GCRC during the eighth week of each of their three regimens and obtain blood samples for serial TSH, FT4, and T3 determinations at time 0, 1, 2, 3, 4, 6, 9, 12, and 24 h after LT4 administration. We would then have been able to generate parameters such as the maximum serum concentration and area under the concentration-time curve for FT4 and also document serial TSH concentrations. However, this component of the study could not be completed due to lack of funding. Another shortcoming of our study was that we were unable to recruit sufficient patients to study additional LT4 brand names. Analysis of the two brand names studied as covariates did not influence the study conclusions. However, it is possible that other brand names, with different excipients, could have produced different results for the three timing regimens. It is also not clear whether our study results can be generalized to the levothyroxine-treated U.S. population. Given that only 19% of the patients approached regarding the study were interested in participation, it is possible that we studied a group of patients who were particularly rigorous in adhering to their prescribed medication regimen. Finally, we did not perform quality-of-life measures in our patients, and although our study was randomized, it was not blinded, did not use uniform LT4 lots, and had a rather lengthy recruitment period.
The standard of care for patients requiring LT4 is to prescribe its administration on an empty stomach. In conclusion, we believe that our results strongly support this recommendation. This timing closely mimics the endogenous fluctuations in TSH concentrations seen by Andersen et al. (30). In this study of participants with normal thyroid function, the mean TSH was 0.75 mIU/liter with 95% confidence intervals of 0.2–1.6 mIU/liter. The confidence interval we observed during fasting ingestion of LT4 was 0.60–1.52 mIU/liter.
LT4 is generally recognized to be a medication with a relatively narrow toxic to therapeutic ratio (7). During our nonfasting regimens, serum TSH extremes of 0–19 mIU/liter were observed. If the goal of LT4 therapy is maintenance of a specific serum TSH within a relatively narrow range, without significant oscillations, then ingestion of LT4 in the fasting state should be advised. This admonition may be particularly important for patients who are pregnant, elderly, or have diagnoses of thyroid cancer, cardiac disease, or osteoporosis because specific TSH targets are of great importance in these populations. Avoidance of subclinical thyroid disease may be particularly critical in these populations (31). If such a timing regimen is difficult for a patient and an alternative LT4 timing is selected, then the LT4 dose may need to be increased and more variability in the resultant serum TSH values should be anticipated. Bedtime administration of LT4 appears to be a better choice than consumption with breakfast. However, if a change is made to adopt a bedtime regimen, the patient’s serum TSH concentration should be followed more closely for a time to ensure that particular individual is not in that subset of patients in whom the TSH value diverges by more than 1 mIU/liter from the desired range.
Supplementary Material
Acknowledgments
The excellent statistical assistance of Rochelle Tractenberg and Ruihua Xu is appreciated. The authors gratefully acknowledge the dedication of the GCRC nursing staff. This study could not have been completed without the generosity and commitment of the study participants. We also thank Michael Estes, medical student, for his diligent review of patient charts.
Footnotes
This project was conducted through the General Clinical Research Center at Georgetown University and supported by Grant M01-RR-020359 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. J.J. is supported by NCRR Grant K23 RR16524. S.S. is partially supported by NIH GCRC Grant MO1-RR-020359 and by Applied Biosystems/Sciex.
Present address for T.-G.B.-H.: The Endocrinology Group, Arlington, VA.
Present address for B.N.: Veterans Affairs Hospital, Washington, D.C.
Present address for J.L.: Kaiser Permanente, Honolulu, Hawaii.
Presented as an abstract at the 91st Annual Meeting of The Endocrine Society, Washington, DC, 2009.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 7, 2009
Abbreviations: BB, Before breakfast; FT4, free T4; GCRC, General Clinical Research Center; HS, at bedtime and at least 2 h after the last meal of the day; LT4, levothyroxine; WB, within 20 min of breakfast.
References
- Wenzel KW, Kirschsieper HE 1977 Aspects of the absorption of oral l-thyroxine in normal man. Metabolism 26:1–8 [DOI] [PubMed] [Google Scholar]
- Lamson MJ, Pamplin CL, Rolleri RL, Klein I 2004 Quantitation of a substantial reduction in levothyroxine (T4) absorption by food. Thyroid 14:876 [Google Scholar]
- Hays MT 2007 Parenteral thyroxine administration. Thyroid 17:127–129 [DOI] [PubMed] [Google Scholar]
- Wenzel KW 1986 [Optimization of levothyroxine treatment. Dosage dependence on the existing parenchymal mass, age, body weight and fasting intake]. Dtsch Med Wochenschr 111:1356–1362 [DOI] [PubMed] [Google Scholar]
- Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH 1987 Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med 316:764–770 [DOI] [PubMed] [Google Scholar]
- Hays MT 1991 Localization of human thyroxine absorption. Thyroid 1:241–248 [DOI] [PubMed] [Google Scholar]
- Carr D, McLeod DT, Parry G, Thornes HM 1988 Fine adjustment of thyroxine replacement dosage: comparison of the thyrotrophin releasing hormone test using a sensitive thyrotrophin assay with measurement of free thyroid hormones and clinical assessment. Clin Endocrinol (Oxf) 28:325–333 [DOI] [PubMed] [Google Scholar]
- Ain KB, Refetoff S, Fein HG, Weintraub BD 1991 Pseudomalabsorption of levothyroxine. JAMA 266:2118–2120 [PubMed] [Google Scholar]
- Checchi S, Montanaro A, Pasqui L, Ciuoli C, De Palo V, Chiappetta MC, Pacini F 2008 l-thyroxine requirement in patients with autoimmune hypothyroidism and parietal cell antibodies. J Clin Endocrinol Metab 93:465–469 [DOI] [PubMed] [Google Scholar]
- Csako G, McGriff NJ, Rotman-Pikielny P, Sarlis NJ, Pucino F 2001 Exaggerated levothyroxine malabsorption due to calcium carbonate supplementation in gastrointestinal disorders. Ann Pharmacother 35:1578–1583 [DOI] [PubMed] [Google Scholar]
- Munoz-Torres M, Varsavsky M, Alonso G 2006 Lactose intolerance revealed by severe resistance to treatment with levothyroxine. Thyroid 16:1171–1173 [DOI] [PubMed] [Google Scholar]
- Centanni M, Gargano L, Canettieri G, Viceconti N, Franchi A, Delle Fave G, Annibale B 2006 Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med 354:1787–1795 [DOI] [PubMed] [Google Scholar]
- Sherman SI, Tielens ET, Ladenson PW 1994 Sucralfate causes malabsorption of l-thyroxine. Am J Med 96:531–535 [DOI] [PubMed] [Google Scholar]
- John-Kalarickal J, Pearlman G, Carlson HE 2007 New medications which decrease levothyroxine absorption. Thyroid 17:763–765 [DOI] [PubMed] [Google Scholar]
- Sachmechi I, Reich DM, Aninyei M, Wibowo F, Gupta G, Kim PJ 2007 Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract 13:345–349 [DOI] [PubMed] [Google Scholar]
- Siraj ES, Gupta MK, Reddy SS 2003 Raloxifene causing malabsorption of levothyroxine. Arch Intern Med 163:1367–1370 [DOI] [PubMed] [Google Scholar]
- Singh N, Singh PN, Hershman JM 2000 Effect of calcium carbonate on the absorption of levothyroxine. JAMA 283:2822–2825 [DOI] [PubMed] [Google Scholar]
- Campbell NR, Hasinoff BB, Stalts H, Rao B, Wong NC 1992 Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med 117:1010–1013 [DOI] [PubMed] [Google Scholar]
- Berg JA, Mayor GH 1992 A study in normal human volunteers to compare the rate and extent of levothyroxine absorption from Synthroid and Levoxine. J Clin Pharmacol 32:1135–1140 [PubMed] [Google Scholar]
- Blakesley V, Awni W, Locke C, Ludden T, Granneman GR, Braverman LE 2004 Are bioequivalence studies of levothyroxine sodium formulations in euthyroid volunteers reliable? Thyroid 14:191–200 [DOI] [PubMed] [Google Scholar]
- Hennessey JV 2006 Levothyroxine dosage and the limitations of current bioequivalence standards. Nat Clin Pract Endocrinol Metab 2:474–475 [DOI] [PubMed] [Google Scholar]
- Benvenga S, Bartolone L, Squadrito S, Lo Giudice F, Trimarchi F 1995 Delayed intestinal absorption of levothyroxine. Thyroid 5:249–253 [DOI] [PubMed] [Google Scholar]
- Lilja JJ, Laitinen K, Neuvonen PJ 2005 Effects of grapefruit juice on the absorption of levothyroxine. Br J Clin Pharmacol 60:337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liel Y, Harman-Boehm I, Shany S 1996 Evidence for a clinically important adverse effect of fiber-enriched diet on the bioavailability of levothyroxine in adult hypothyroid patients. J Clin Endocrinol Metab 81:857–859 [DOI] [PubMed] [Google Scholar]
- Bell DS, Ovalle F 2001 Use of soy protein supplement and resultant need for increased dose of levothyroxine. Endocr Pract 7:193–194 [DOI] [PubMed] [Google Scholar]
- Benvenga S, Bartolone L, Pappalardo MA, Russo A, Lapa D, Giorgianni G, Saraceno G, Trimarchi F 2008 Altered intestinal absorption of l-thyroxine caused by coffee. Thyroid 18:293–301 [DOI] [PubMed] [Google Scholar]
- Elliott DP 2001 Effect of levothyroxine administration time on serum TSH in elderly patients. Ann Pharmacother 35:529–532 [DOI] [PubMed] [Google Scholar]
- Bolk N, Visser TJ, Kalsbeek A, van Domburg RT, Berghout A 2007 Effects of evening vs morning thyroxine ingestion on serum thyroid hormone profiles in hypothyroid patients. Clin Endocrinol (Oxf) 66:43–48 [DOI] [PubMed] [Google Scholar]
- Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis M, Maxon HR, Sherman SI 2006 Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 16:1229–1242 [DOI] [PubMed] [Google Scholar]
- Andersen S, Pedersen KM, Bruun NH, Laurberg P 2002 Narrow individual variations in serum T4 and T3 in normal subjects: a clue to the understanding of subclinical thyroid disease. J Clin Endocrinol Metab 87:1068–1072 [DOI] [PubMed] [Google Scholar]
- Biondi B, Cooper DS 2008 The clinical significance of subclinical thyroid dysfunction. Endocr Rev 29:76–131 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.