Abstract
Context: The incidence of pediatric type 2 diabetes (T2D) is rising, with unclear effects on the cardiovascular system. Cardiopulmonary fitness, a marker of morbidity and mortality, is abnormal in adults with T2D, yet the mechanisms are incompletely understood.
Objective: We hypothesized that cardiopulmonary fitness would be reduced in youth with T2D in association with insulin resistance (IR) and cardiovascular dysfunction.
Design, Setting, and Participants: We conducted a cross-sectional study at an academic hospital that included 14 adolescents (age range, 12–19 yr) with T2D, 13 equally obese adolescents and 12 lean adolescents similar in age, pubertal stage, and activity level.
Main Outcome Measures: Cardiopulmonary fitness was measured by peak oxygen consumption (VO2peak) and oxygen uptake kinetics (VO2kinetics), IR by hyperinsulinemic clamp, cardiac function by echocardiography, vascular function by venous occlusion plethysmography, body composition by dual-energy x-ray absorptiometry, intramyocellular lipid by magnetic resonance spectroscopy, and inflammation by serum markers.
Results: Adolescents with T2D had significantly decreased VO2peak and insulin sensitivity, and increased soleus intramyocellular lipid, C-reactive protein, and IL-6 compared to obese or lean adolescents. Adolescents with T2D also had significantly prolonged VO2kinetics, decreased work rate, vascular reactivity, and adiponectin, and increased left ventricular mass and fatty acids compared to lean adolescents. In multivariate linear regression analysis, IR primarily, and fasting free fatty acids and forearm blood flow secondarily, were significant independent predictors of VO2peak.
Conclusions: Given the strong relationship between decreased cardiopulmonary fitness and increased mortality, these findings in children are especially concerning and represent early signs of impaired cardiac function.
Adolescents with type 2 diabetes have abnormal exercise function, vascular reactivity, and increased left ventricular mass.
The incidence of pediatric type 2 diabetes (T2D) increased 10-fold from 1982 to 1994 (1), and it continues to rise in parallel with increases in pediatric obesity (2). However, little is known about how T2D affects the cardiovascular system in youth. Because cardiopulmonary fitness is directly linked to mortality, we sought to investigate exercise function in T2D youth. We previously observed that T2D adults have significantly reduced cardiopulmonary fitness (3,4), but younger subjects, with generally lower cardiopulmonary risks, and who are influenced by growth and pubertal hormone changes, may not mirror adults. We hypothesized that cardiopulmonary fitness would be reduced in T2D youth in association with insulin resistance (IR) and markers of cardiovascular function. To test this hypothesis, we measured peak oxygen uptake (VO2peak), oxygen uptake kinetics (VO2kinetics) during submaximal constant-load exercise, and IR in obese T2D adolescents and in activity-matched healthy normal-weight and obese adolescents without diabetes. Resting echocardiography and forearm reactive hyperemia were measured to assess possible cardiac and hemodynamic contributors to exercise dysfunction. Additionally, intramyocellular (IMCL) and extramyocellular (EMCL) lipid, serum lipids, and inflammatory markers were measured to assess the potential relations of ectopic fat and inflammation, typically associated with IR, to the observed exercise responses.
Subjects and Methods
Subjects
Adolescents were recruited from the University of Colorado Denver (UCD) pediatric diabetes and obesity clinics and by advertisement. By design, obese and T2D subjects had similar body mass index (BMI). A total of 39 subjects were enrolled: 12 controls, BMI 15–85%ile (six males and six females; one Black, one Hispanic, nine White, one other), 13 obese (six males and seven females; one Black, five Hispanic, one Asian, six White), and 14 T2D (seven males and seven females; one Black, nine Hispanic, three White, one other). Mean age of participants was 15 ± 2 yr (range, 12–19 yr), and mean Tanner stage was 4.7 ± 0.5 for females and 4.1 ± 0.8 for males. Family history of diabetes was similar in obese and T2D subjects, but negative in control subjects. The study was approved by the UCD institutional review board, and appropriate consent was obtained.
Screening included a history, physical examination, Tanner staging and fasting laboratory testing to confirm the absence of comorbid conditions. T2D was defined by American Diabetes Association criteria and the absence of glutamic acid decarboxylase, islet cell or insulin autoantibodies, insulin requirement, or secondary causes of diabetes. Absence of diabetes was confirmed in the nondiabetic groups by a 2-h, 75-g oral glucose tolerance test.
Inclusion criteria included pubertal status (Tanner stage >1) to minimize pubertal effects, and sedentary status (<3 h of exercise per week) to minimize training effects. Exclusions included body weight greater than 300 pounds, blood pressure greater than 140/90 mm Hg at rest or greater than 190/100 mm Hg during exercise, hemoglobin less than 9 mg/dl, serum creatinine greater than 1.5 mg/dl, hemoglobin A-1c (HbA-1c) above 12%, smoking, medication-dependent asthma, respiratory or orthopedic conditions precluding exercise testing, medications known to affect IR other than metformin (oral or inhaled steroids, thiazolidinediones, atypical antipsychotics), insulin, other antidiabetic drugs, antihypertensive drugs, pregnancy, breastfeeding, or plans to alter exercise or diet during the study.
All subsequent tests were performed after a 12-h fast, preceded by 3 d of restricted physical activity and a fixed-macronutrient, weight-maintenance diet provided by the Clinical Translational Research Center (CTRC) metabolic kitchen (55% carbohydrates, 30% fat, 15% protein). Metformin was withdrawn during the 3-d study diet. T2D subjects were instructed to monitor blood glucose levels at least four times daily and were excluded for fasting blood glucose greater than 200 mg/dl or random blood glucose greater than 300 mg/dl during the study diet, or if urine ketones were present on admission. There were no significant changes in blood glucose during the study diet.
Activity and diet questionnaires
A 3-d pediatric physical activity recall questionnaire was administered by interview to estimate habitual physical activity (5), reported as a 3-d average of metabolic equivalents (METs) per day, where one MET equals resting VO2 (3.5 ml · kg−1 · min−1). A food frequency questionnaire designed for lean and obese youth was administered by interview to estimate calorie and macronutrient intake (6).
Insulin sensitivity
Subjects were admitted overnight to the Pediatric CTRC to ensure fasting. Fasting laboratory evaluation included: total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, glucose, insulin, HbA-1c, C-peptide, blood urea nitrogen, creatinine, complete blood count, alanine aminotransferase, aspartate aminotransferase, highly sensitive C-reactive protein (hsCRP), IL-6, myeloperoxidase, and adiponectin; assays were performed by standard methods at the UCD CTRC Laboratory. A 3-h hyperinsulinemic euglycemic clamp (80 mU · m−2 · min−1 insulin) was performed to estimate IR (7). Glycemia was monitored every 5 min using a Yellow Springs Instruments (YSI, Yellow Springs, OH) glucose analyzer, and 20% dextrose infusion was titrated to maintain a blood glucose concentration of 95–105 mg/dl. Blood samples were drawn at baseline, 90, 150, 160, 165, 170, 175, and 180 min to determine glucose, insulin, and free fatty acids (FFA). Glucose disposal rate was determined from the average glucose infusion rate during the last 20 min of the clamp and expressed as milligrams per kilogram body mass and milligrams per kilogram fat-free mass (obtained from dual-energy x-ray absorptiometry scan). There were no significant differences in mean blood glucose or mean insulin levels between the groups at the conclusion of the clamp (Table 1).
Table 1.
Demographic characteristics
| Control | Obese | T2D | |
|---|---|---|---|
| n | 12 | 13 | 14 |
| Sex (% female) | 50 | 46 | 50 |
| Age (yr) | 15.6 ± 1.8 | 14.9 ± 2.1 | 14.6 ± 2.2 |
| Tanner stage | 4.4 ± 0.7 | 4.6 ± 0.6 | 4.5 ± 0.8 |
| BMI (kg/m2) | 21.0 ± 2.4 | 32.1 ± 5.4a | 33.4 ± 6.2a |
| BMI percentile | 45% | 96% | 97% |
| Diabetes duration (yr) | 1.6 ± 1.6 | ||
| HbA-1c (%) | 4.9 ± 0.3 | 5 ± 0.3 | 8 ± 2.2a,h |
| Cholesterol (mg/dl) | 132 ± 22 | 178 ± 40b | 176 ± 35b |
| Triglycerides (mg/dl) | 82 ± 37 | 211 ± 168c | 200 ± 136d |
| HDL cholesterol (mg/dl) | 45 ± 8 | 42 ± 14 | 42 ± 10 |
| Fasting C-peptide (ng/ml) | 1.7 ± 0.6 | 3.8 ± 2.3 | 7 ± 5.5d |
| Fasting FFA (μEq/liter) | 491 ± 310 | 583 ± 145 | 619 ± 87d |
| FFA during euglycemic clamp (μEq/liter) | 48.9 ± 38.9 | 357 ± 484 | 241.4 ± 201e |
| Fasting insulin (μU/ml) | 7.7 ± 3.5 | 27 ± 37 | 31 ± 20 |
| Insulin during euglycemic clamp (μU/ml) | 105 ± 35 | 134 ± 41 | 109 ± 44 |
| Fasting glucose (mg/dl) | 83 ± 5 | 86 ± 9 | 121 ± 26a,g |
| Glucose during euglycemic clamp (mg/dl) | 100 ± 4 | 100 ± 4 | 99 ± 3 |
| Glucose disposal rate (mg · kg · min) | 15.3 ± 4.7 | 8.1 ± 2.7a | 4.1 ± 2.3a,f |
| Glucose disposal rate (mg · kg lean · body mass · min) | 19.8 ± 4.6 | 14.6 ± 4.9c | 7.8 ± 3.9a,g |
P < 0.001 vs. controls;
P < 0.01 vs. controls;
P < 0.05 vs. controls;
P < 0.02 vs. controls;
P < 0.04 vs. controls;
P < 0.02 vs. obese;
P < 0.01 vs. obese;
P < 0.001 vs. obese.
Autonomic insufficiency
Variation in R-R intervals with cycled breathing were obtained in supine subjects breathing five times per minute for 5 min, coordinated with an electronic signal (8). Blood pressure and heart rate responses to standing and R-R variations with Valsalva breathing were measured. Average tachycardic beats per minute/bradycardic beats per minute value of 1.21 or less during three 15-sec Valsalvas or each following 45 sec was considered abnormal. No subjects had autonomic dysfunction by these methods (data not shown).
Echocardiograms
Resting two-dimensional and tissue Doppler echocardiography was performed using Vivid 7 and Echopac (BTO6 6.1.3, General Electric, Milwaukee, WI) to exclude left ventricular systolic dysfunction (ejection fraction <50%), regional wall motion abnormalities, pericardial disease, or significant valvular pathology. Electrocardiograms excluded ischemia. Left ventricular end-systolic and diastolic chamber dimensions and wall thickness, fractional shortening, and biplane Simpson’s method (chamber volumes) were quantitated by standard techniques. Diastolic filling measurements were obtained by velocity of propagation (Vp) and tissue Doppler at the septal and lateral mitral annuli. Mitral valve deceleration time, Vp, peak early mitral inflow velocity:peak late inflow velocity (E/A), and peak early mitral annular velocity (E/E’ septal and E:E’ lateral) were assessed for diastolic dysfunction (9).
Left ventricular posterior wall thickness in diastole (LVPWd), interventricular septal thickness in diastole (IVSd), left ventricular end-diastolic dimension (LVED), and end-systolic dimension were assessed for left ventricular hypertrophy. Left ventricular mass (LVM) was calculated as LVM(g) = (0.8) (1.04) × [(LVED + LVPWd + IVSd)3 − LVED3] + 0.6, and indexed by dividing by height in meters2.7 (10).
Plethysmography
Forearm reactive hyperemic blood flow was determined in supine participants by venous occlusion strain-gauge plethysmography (D. E. Hokanson, Issaquah, WA) with calibrated mercury SILASTIC (Dow Corning Corp., Midland, MI) strain gauges using standard methods (11). At least five measurements were performed at 10- to 15-sec intervals. The difference between peak (after releasing arterial occluding cuff) and resting forearm blood flow was considered peak hyperemic flow (ml/100ml/min).
Exercise testing
A graded cycle ergometer (Lode, Groningen, The Netherlands) protocol to exhaustion was used to determine VO2peak using standard methods (12). Oxygen consumption (VO2), carbon dioxide production, and minute ventilation were measured continuously and recorded breath-by-breath using a metabolic cart (Ultima CPX; Medical Graphics, St. Paul, MN), and perceived exertion was measured every minute using the Borg scale (13). The respiratory exchange ratio (RER) was calculated as carbon dioxide production/VO2. Work rate was increased in 10, 15, or 20 W/min increments, depending on age and sex, while subjects maintained a speed of 65 rotations per minute. VO2peak (ml/kg/min) was defined as the highest VO2 and heart rate averaged over 10 sec at an RER of 1.1 or greater. Subjects were excluded if RER was less than 1.1.
Kinetic measurements during constant-load exercise
Subjects performed three identical exercise bouts on a cycle ergometer (Lode) at a constant work rate equal to 85% of their lactate threshold (14), as estimated by the V-slope method during maximal testing (15). Each bout consisted of 2 min of resting gas exchange, 6 min of exercise at 65 rotations per minute, and 10 min of rest. Oxygen consumption data were processed and analyzed using nonlinear regression techniques for kinetic parameters as previously described (14). The phase 2 time constant of oxygen uptake (τ2), a coordinated marker of the ability to deliver and use oxygen at the level of the exercising skeletal muscle, is reported in seconds.
Fat deposition
Body composition by dual-energy x-ray absorptiometry was performed according to standard methods (16). IMCL and EMCL were measured via 1H magnetic resonance spectroscopy using 3.0 T whole-body magnetic resonance imaging (GE Healthcare, Waukesha, WI) (17,18). The soleus muscle was centered in a standard quadrature lower extremity coil. Scout images obtained in all three orthogonal planes determined the approximately 5-cm3 spectroscopy voxel in soleus or tibialis anterior muscle, avoiding visible adipose, vascular structures, and fasciae lines. Spectroscopic acquisitions with PROBE-P (PRESS) (GE Healthcare) used pulse sequence parameters (repetition time/echo time = 2000/100 msec; 128 averages; total acquisition time, 7 min) optimized to avoid contaminating bulk fat signals and a clean spectral baseline. The PROBE-P sequence automatically collects non-water suppressed frames for internal water referencing (see below). Spectra degraded by subject motion were repeated.
Spectra were analyzed with LCModel software (version 6.1-4A) (Provencher, SW, Gottingen, Germany), widely used for analysis of brain 1H magnetic resonance spectroscopy, using solution metabolite spectra as basis sets for Bayesian analysis to provide automatic quantification of in vivo proton magnetic resonance spectra without operator bias or intervention using time domain input (19,20,21). More recent versions of LCModel accommodate muscle spectra analysis, using a simulated muscle basis set analyzing for EMCL, IMCL, creatine, choline, and taurine. IMCL and EMCL concentrations were obtained by referencing spectra peak areas at 1.3 ppm and 1.5 ppm to the unsuppressed water peak area at 4.7 ppm (internal water referencing) (22,23) using the unsuppressed water reference frames from the PROBE-P acquisition (18,22,24,25,26). Magnetic resonance relaxation times of water and lipid signals are not corrected for because repetition time/echo time remains constant (18,22,23,24,25,27). Results are reported as concentrations of methylene (-CH2) groups in arbitrary concentration units, not directly comparable to traditional water/fat % absolute concentrations (mm). Arbitrary units are useful for accessing differences between individuals and groups (24,25,28) but did not allow reporting of IMCL or EMCL fat measurements as a percentage of water signal.
Statistical analysis
Data are reported as mean ± sd. An α level of less than 0.05 was considered statistically significant. Using SPSS 13.0 (SPSS, Inc., Chicago, IL), variances between groups were compared using the Lavine statistic and normality assessed by the Shapiro-Wilks test. A priori comparisons were made using ANOVA and post hoc analysis to correct for multiple comparisons (Bonferroni when variances were equal, Dunnett T3 when unequal). Planned univariate correlations between VO2peak and variables hypothesized to be causally associated with VO2peak were analyzed (Pearson correlation coefficients when data were normally distributed, Spearman’s rho when lacking normality). Step-down multivariate linear regression analysis was performed with SAS 9.1 (SAS Institute, Inc., Cary, NC), including biologically plausible variables univariately correlated with VO2peak, with an α of less than 0.01 or smaller, to determine independent predictors of VO2peak.
Results
Metabolic parameters
Groups were similar in age, Tanner stage, gender (Table 1), blood urea nitrogen, creatinine, aspartate aminotransferase, alanine aminotransferase, hemoglobin, hematocrit, platelets, urine microalbumin:creatinine, HDL, calorie and macronutrient intake, and no subjects had anemia (data not shown) (Table 1). T2D subjects had significantly higher fasting glucose and HbA-1c than control or obese subjects (Table 1).
BMI, total cholesterol, and triglyceride concentrations were significantly lower in control subjects than T2D or obese subjects. T2D subjects had higher fasting FFA, C-peptide, and FFA at 180 min of the insulin clamp vs. lean controls (Table 1). BMI, total cholesterol, triglycerides, fasting FFA, and FFA at 180 min of the hyperinsulinemic clamp were similar in T2D and obese subjects (Table 1). Baseline biochemical parameters did not differ in T2D subjects on metformin vs. those not on metformin.
Exercise testing
Peak RER and heart rate were similar between groups, indicating similar maximal effort at VO2peak (Table 2). There were no significant differences between groups in baseline habitual physical activity (Table 2).
Table 2.
Exercise and cardiovascular results
| Control | Obese | T2D | |
|---|---|---|---|
| Mean habitual activity (METs) | 64 ± 11.2 | 59 ± 14.6 | 64 ± 18.8 |
| RER | 1.18 ± 0.07 | 1.13 ± 0.07 | 1.13 ± 0.05 |
| Peak HR (beats/min) | 184 ± 11 | 188 ± 13 | 183 ± 8 |
| VO2 (ml/kg body mass/min) | 40.4 ± 9.9 | 27.2 ± 5.3a | 21.8 ± 4.2a,b |
| VO2 (ml/kg lean body mass/min) | 53.1 ± 8.5 | 49.5 ± 5.6 | 42.8 ± 7.6c |
| Peak work rate (Watts) | 220 ± 48.7 | 167 ± 44.5 | 149 ± 33.6d |
| τ2 (sec) | 28.6±10 | 34.9±10.5 | 40.2±9.7e |
| Resting systolic blood pressure (mm Hg) | 109 ± 6 | 119 ± 7e | 124 ± 10e |
| Resting diastolic blood pressure (mm Hg) | 63 ± 6 | 74 ± 7e | 72 ± 11b |
| Limb blood flow (ml/100 ml/min) | 17.6 ± 6.9 | 10 ± 5e | 10 ± 5.6e |
| IVSd (cm) | 0.71 ± 0.12 | 0.69 ± 0.13 | 0.78 ± 0.15 |
| LVPWd (cm) | 0.76 ± 0.10 | 0.8 ± 0.17 | 0.9 ± 0.18b |
| LVM (g) | 100 ± 20.7 | 107 ± 25.6 | 123 ± 35.8 |
| Indexed LVM (g/m2.7) | 24 ± 4.3 | 27 ± 7.6 | 32 ± 8e |
HR, Heart rate; VO2, oxygen uptake.
P < 0.0001 vs. controls;
P < 0.04 vs. obese;
P < 0.05 vs. obese;
P < 0.02 vs. controls;
P < 0.01 vs. controls.
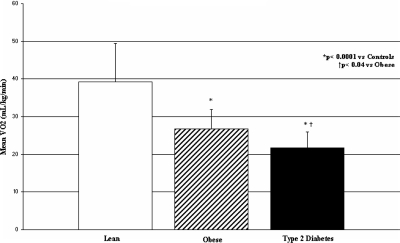
VO2peak was significantly lower in T2D subjects vs. control or obese subjects (Fig. 1). VO2peak per kilogram of fat-free mass was also significantly lower in T2D subjects vs. control or obese subjects. Peak work rate was significantly less in T2D subjects than controls (Table 2). No significant differences were noted in resting VO2, heart rate, recovery heart rate at 2 min, or perceived exertion between groups (data not shown).
Figure 1.
Maximal exercise capacity (VO2peak) in control (white bar), obese (hatched bar), and T2D (black bar) adolescents, expressed in milliliters per kilogram per minute. *, P values compared to control adolescents; †, P values compared to obese adolescents.
T2D subjects had significantly prolonged VO2 kinetics, (e.g. τ2) vs. controls (Table 2).
Insulin sensitivity
Glucose disposal rates during the hyperinsulinemic euglycemic clamp expressed as milligrams per kilogram per minute or as milligrams per kilogram of fat-free mass per minute were significantly lower in obese and T2D subjects than controls. Glucose disposal rates in milligrams per kilogram per minute and milligrams per kilogram of fat-free mass per minute were also significantly lower in T2D than obese subjects (Table 1).
Fat deposition
There were no significant differences between groups in fat-free mass (Table 3). Percentage body fat and fat-free mass were similar in obese and T2D groups and significantly higher in obese and T2D groups compared with controls (Table 3). Adiponectin was significantly lower in T2D subjects than controls (Table 3). EMCL content of the soleus muscle was similar in T2D and obese subjects, with both groups being significantly higher than controls (Table 3). IMCL content of the soleus and tibialis anterior muscles (Table 3) was significantly higher only in the T2D subjects. T2D subjects also had higher waist:hip ratio (Table 3) when compared with obese or control subjects.
Table 3.
Body composition, muscle fat distribution, and inflammation
| Control | Obese | T2D | |
|---|---|---|---|
| Fat-free mass (kg) | 44.8 ± 8.4 | 47.3 ± 10.1 | 47.0 ± 10.8 |
| Fat mass (kg) | 11.01 ± 6.4 | 33.3 ± 11.3a | 37.6 ± 13.1a |
| % Fat-free | 79.4 ± 10.6 | 59.2 ± 5.8a | 56.8 ± 7.3a |
| % Fat | 20.6 ± 10.6 | 40.8 ± 5.8a | 43.2 ± 7.3a |
| Waist circumference (cm) | 72.9 ± 7.5 | 97.0 ± 12.5a | 105.3 ± 15.4a |
| Hip circumference (cm) | 83.2 ± 6.1 | 106.5 ± 11.3a | 107.3 ± 12.7a |
| Waist:hip | 0.88 ± 0.06 | 0.91 ± 0.06 | 0.98 ± 0.05a,b |
| Soleus EMCL (ACU) | 1450 ± 863 | 2863 ± 1312c | 3169 ± 1014d |
| Tibialis EMCL (ACU) | 926 ± 723 | 1763 ± 1664e | 2220 ± 1027f |
| Soleus IMCL (ACU) | 1225 ± 476 | 1788 ± 792 | 2539 ± 910a,h |
| Tibialis IMCL (ACU) | 373 ± 116 | 434 ± 268 | 657 ± 256f |
| Adiponectin (μg/ml) | 8.4 ± 3.1 | 9.9 ± 3.3 | 6.4 ± 1.4e |
| WBC (103/μl) | 6.0 ± 1.0 | 6.9 ± 1.9 | 8.8 ± 2.9f |
| hsCRP (mg/liter) | 0.57 ± 0.3 | 1.1 ± 1.5 | 5.2 ± 4.4b,f |
| Myeloperoxidase (pmol/liter) | 694 ± 396 | 651 ± 270 | 740 ± 216 |
| IL-6 (pg/ml) | 1.2 ± 1.8 | 0.8 ± 1.6 | 3.4 ± 1.8g |
ACU, Arbitrary concentration units, WBC, white blood cells.
P < 0.0001 vs. controls;
P < 0.01 vs. obese;
P < 0.02 vs. controls;
P < 0.002 vs. controls;
P < 0.05 vs. controls;
P < 0.01 vs. controls;
P < 0.03 vs. obese;
P < 0.05 vs. obese.
Cardiovascular function
Systolic blood pressure and diastolic blood pressure were similar in the T2D and obese groups (Table 2), and significantly higher than controls. Peak forearm vascular reactivity was significantly reduced in the T2D and obese groups compared with controls (Table 2).
LVPWd and indexed LVM were significantly greater in T2D subjects vs. controls (Table 2). There was evidence of left ventricular hypertrophy (IVSd or LVPWd >1 cm) in 29% of T2D subjects. Using tissue Doppler, measurement of systolic and diastolic parameters (fractional shortening, E/A, Vp, and E/E’) was not significantly different between the three groups (data not shown).
Inflammation
hsCRP and IL-6 levels were significantly higher in T2D subjects compared with obese and control subjects (Table 3). White blood cell count was also significantly higher in T2D subjects than controls (Table 3).
Correlates of VO2peak
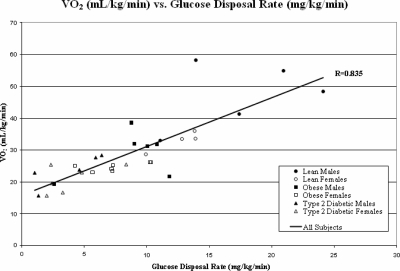
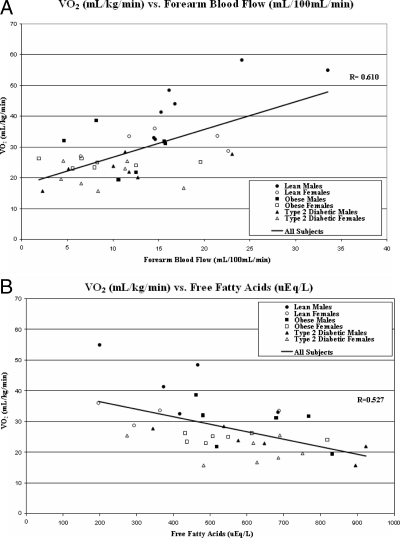
Insulin sensitivity (mg/kg/min) was the strongest predictor of VO2peak (r = 0.84; P < 0.0001) (Fig. 2) in a multivariate regression analysis controlled for age, Tanner stage, BMI, and baseline habitual physical activity. Other a priori univariate correlates of VO2peak included fasting insulin (r = −0.75; P < 0.0001), FFA (r = −0.527; P = 0.001), HbA-1c (r = −0.654; P < 0.0001), IMCL (soleus, r = −0.63, P < 0.0001; tibialis anterior, r = −0.46, P < 0.01), forearm blood flow (r = 0.610; P < 0.0001), and hsCRP (r = −0.578; P < 0.0001). Waist:hip ratio did not correlate with VO2peak. Among T2D subjects, HbA-1c and diabetes duration were not correlated with VO2peak. In a step-down multivariate linear regression analysis, IR (Fig. 2), forearm blood flow (Fig. 3A), and fasting FFA (Fig. 3B) remained as independent predictors of VO2peak.
Figure 2.
Correlation between maximal exercise capacity (VO2peak) expressed in milliliters per kilogram per minute and glucose disposal rate from the hyperinsulinemic euglycemic clamp, expressed in milligrams per kilogram per minute. •, Control males; ○, control females; ▪, obese males; □, obese females; ▴, T2D males; ▵, T2D females.
Figure 3.
A, Correlation between maximal exercise capacity (VO2peak) expressed in milliliters per kilogram per minute and peak hyperemic forearm blood flow from venous plethysmography, expressed in milliliters per 100 ml per minute. B, Correlation between maximal exercise capacity (VO2peak) expressed in milliliters per kilogram per minute and fasting FFA, expressed in microequivalents per liter. •, Control males; ○, control females; ▪, obese males; □, obese females; ▴, T2D males; ▵, T2D females.
Discussion
We report significant impairment in functional exercise capacity in well-characterized T2D youth, compared with similarly obese or lean nondiabetic controls of similar pubertal stage and baseline activity level. Our results indicate that the presence of T2D in youth confers an additional reduction in exercise capacity, beyond that seen with obesity alone, despite a relatively short duration of disease. Supporting this observation is the key finding that VO2peak correlated strongly with IR and its manifestations. Additionally, the exclusion of other univariate correlates from multivariate analysis can potentially be explained by their correlations with IR. Early cardiovascular abnormalities, demonstrated by impaired vascular reactivity and cardiac hypertrophy, are consistent with observations in adults with more advanced T2D and may negatively impact exercise function of T2D youth. Together, these findings in children represent early signs of impaired cardiovascular function and a poor prognosis that accompanies decreased cardiovascular fitness (29,30).
Some abnormalities noted may be more related to obesity, and others specific to T2D. Obese and T2D subjects had similarly abnormal cholesterol, triglycerides, HDL, fat mass, EMCL, blood pressure, and vascular reactivity. Exercise capacity and IR were more abnormal in T2D than obese youth. Only T2D subjects had significantly abnormal FFA, HbA-1c, C-peptide, IMCL, inflammation, waist:hip circumference, adiponectin, VO2kinetics, and left ventricular hypertrophy.
Limited data are available regarding exercise function in T2D youth. Gusso et al. (31) reported no differences between VO2peak or peak watts in eight T2D adolescents relative to 10 obese or 10 control adolescents, yet lower VO2peak in T2D vs. 12 adolescents with type 1 diabetes. Combining all diabetic subjects, VO2peak (ml/fat-free kg/min but not ml/kg/min or peak watts) was lower than all nondiabetic subjects, suggesting an effect of diabetes independent of body weight. Similarly, Faulkner et al. (32) found that fitness and habitual physical activity were lower in T2D adolescents compared with adolescents with type 1 diabetes, but baseline physical activity and BMI were not controlled. In contrast, controlling for these variables as well as pubertal stage and dietary intake, we found reduced VO2peak in T2D youth vs. obese or lean controls, despite below average VO2peak in controls due to their sedentary status (12). These results are consistent with the findings of Regensteiner et al. (3,4) and others, who demonstrated that adults with uncomplicated T2D have reduced exercise function. Moreover, even when corrected for fat-free mass, T2D youth display significant performance deficits that cannot be explained by sedentary or obese status.
Our pulmonary VO2kinetic data are the first to extend the findings of abnormally slowed oxygen uptake kinetics during submaximal exercise in T2D adults (4) to youth and support the observed peak exercise defect. The practical implication of low-level exercise impairment is a decreased ability to accommodate and perform simple physical tasks, which may decrease the likelihood of children engaging in exercise. The same independent predictors reported for VO2peak were also predictors of VO2kinetics (τ2): IR (r = −0.36; P = 0.04), reactive hyperemia (r = −0.412; P = 0.01), and FFA after the hyperinsulinemic clamp (r = 0.582; P = 0.001), indicating that similar components may affect VO2kinetics and VO2peak.
The mechanisms of exercise dysfunction in T2D are not well understood. Our previous findings in adults treated with rosiglitazone showed that change in fasting insulin and homeostasis model of assessment for insulin resistance, but not glucose, correlated with improvements in exercise capacity, implying that IR may predominate over glycemia (33). Several studies examined the relationship between surrogate IR markers and exercise in obese nondiabetic youth. Physical work capacity was decreased in children with hyperinsulinemia (34), and exercise duration and VO2peak were reduced in adolescent boys with metabolic syndrome, vs. obese controls (35). Furthermore, baseline and change in predicted VO2peak correlated with fasting insulin levels in 11 to 14 yr olds (36). These BMI-controlled studies support our finding of IR rather than obesity, per se, as a potential contributor to exercise defects. However, Shaibi et al. (37) found no differences in treadmill VO2peak between obese Hispanic 8 to 13 yr olds with a family history of T2D, with or without the metabolic syndrome, and no correlation between VO2peak and IR (measured by iv glucose tolerance test) (38). Possible explanations for this discrepancy include much younger subjects, who therefore had not yet developed IR comorbidities, and no control for pubertal stage or dietary intake. Through intensive control of diet and pubertal stage and direct measurement of IR by hyperinsulinemic euglycemic clamp, our present data provide compelling support for the role of IR as an independent contributor to exercise dysfunction in youth, and further research will help clarify this question.
IMCL is typically associated with obesity and IR (39,40) but has not been studied in T2D youth. We now report the novel finding of increased IMCL in T2D youth relative to controls and even similarly obese adolescents. These results cannot be explained by training differences because all subjects were similarly inactive. This finding is important because decreased turnover and accumulation of lipid byproducts reportedly impairs muscle insulin signaling (41) and mitochondrial function (42), which could affect exercise performance in these youth.
Cardiovascular abnormalities are notable as known contributors to exercise dysfunction. In this study, we report concerning evidence of left ventricular hypertrophy in T2D youth. In support of these findings, left ventricular hypertrophy was reported in 28% of T2D adults (43) and in extremely morbidly obese adolescents (BMI = 60 ± 9 kg/m2) (44). However, unlike reports in T2D adults (45), we found no resting diastolic abnormalities, potentially because of shorter T2D duration and less obesity than was observed in these previous studies. Alternatively, resting echocardiography may be inadequate to exclude diastolic dysfunction in T2D youth. For example, we previously reported that adults with T2D of short duration had normal resting echocardiograms, yet increased pulmonary capillary wedge pressure during exercise, possible early evidence of diastolic dysfunction (46). Indeed, reduced central hemodynamic responses during submaximal exercise have been reported in T2D adolescents. These data argue that cardiac abnormalities secondary to T2D may significantly contribute to the observed exercise defects in youth.
Vascular dysfunction is associated with obesity and T2D (47,48). In the present study, reactive hyperemia was reduced in obese and T2D adolescents, correlating independently with exercise dysfunction. Our results also show a relationship between IR and inflammation in T2D youth, independent of obesity. Increased visceral fat in T2D youth, implied by increased waist:hip circumference, is a possible explanation for inflammation. A provocative question is whether inflammation is a central mechanism uniting vascular and cardiac dysfunction in T2D, making systemic inflammation a potential therapeutic target for improving exercise capacity.
The lack of differences in resting heart rate, peak heart rate, or recovery heart rate after exercise, or abnormalities in autonomic screening make autonomic dysfunction an unlikely explanation for the reduced exercise capacity we observed, but it cannot be entirely excluded. Inclusion criteria and 3-d pediatric physical activity recalls revealed no differences in baseline physical activity, but undetected differences could still impact exercise function. Future studies will employ accelerometers to monitor physical activity.
Taken together, our data demonstrate that T2D youth have decreased exercise performance, potentially explained by IR and its manifestations, as well as vascular dysfunction. T2D adolescents also have left ventricular hypertrophy, increased IMCL, inflammation, and hypoadiponectinemia. Importantly, these abnormalities extend beyond what might be contributed by inactivity or obesity alone. These findings are especially concerning in very young patients, with no other comorbidities to explain exercise dysfunction, because they forecast early morbidity and mortality. Studies in T2D adults suggest the reversibility of exercise defects (33), although acute and chronic metformin treatment failed to improve peak exercise capacity in normal adults (49). Future studies are needed to further evaluate the cardiac abnormalities in T2D adolescents and the effects of interventions aimed at improving exercise capacity to determine the reversibility of exercise defects in youth.
Acknowledgments
We thank our study participants for their time and efforts and Vermed, Inc., for providing exercise electrodes. We also thank Gerald Reaven, Lindsay Ehlers, Leah Herlache, Lisa Herbert, Erik Sorenson, and Amber Hull.
Footnotes
K.J.N. is supported by a career development award from the National Institutes of Health (NIH/NCRR 1 K23 RR020038-01). T.A.B. was supported by NIH Postdoctoral Fellowship Grant F32 DK078413-01. B.D. is supported by a Merit Review Award from the Department of Veterans Affairs. P.S.Z. is supported by a cooperative research project grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (5 U01 DK61242-02). J.E.B.R. is supported by Veterans Affairs merit review, NIH DK-64741, HL56481, and DK 57516. J.G.R. is supported by an American Diabetes Association Award. This research was also supported by Adult General Clinical Research Center NIH Grant M01-RR00051 and by Pediatric Clinical Translational Research Center NIH Grant 5MO1 RR00069.
Disclosure Summary: The authors have nothing to declare.
First Published Online July 7, 2009
Abbreviations: BMI, Body mass index; EMCL, extramyocellular; FFA, free fatty acids; HbA-1c, hemoglobin A-1c; HDL, high-density lipoprotein; hsCRP, highly sensitive C-reactive protein; IMCL, intramyocellular; IR, insulin resistance; IVSd, interventricular septal thickness in diastole; LVED, left ventricular end-diastolic dimension; LVM, left ventricular mass; LVPWd, left ventricular posterior wall thickness in diastole; MET, metabolic equivalent; RER, respiratory exchange ratio; τ2, phase 2 time constant of oxygen uptake; T2D, type 2 diabetes; VO2kinetics, oxygen uptake kinetics; VO2peak, peak oxygen uptake; Vp, velocity of propagation.
References
- Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P 1996 Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr 128:608–615 [DOI] [PubMed] [Google Scholar]
- Pinhas-Hamiel O, Zeitler P 2005 The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr 146:693–700 [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Shetterly SM, Mayer EJ, Eckel RH, Haskell WL, Baxter J, Hamman RF 1995 Relationship between habitual physical activity and insulin area among individuals with impaired glucose tolerance. The San Luis Valley Diabetes Study. Diabetes Care 18:490–497 [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Bauer TA, Reusch JE, Brandenburg SL, Sippel JM, Vogelsong AM, Smith S, Wolfel EE, Eckel RH, Hiatt WR 1998 Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J Appl Physiol 85:310–317 [DOI] [PubMed] [Google Scholar]
- Weston AT, Petosa R, Pate RR 1997 Validation of an instrument for measurement of physical activity in youth. Med Sci Sports Exerc 29:138–143 [DOI] [PubMed] [Google Scholar]
- Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S 1999 Validity and reproducibility of a food frequency interview in a multi-cultural epidemiology study. Ann Epidemiol 9:314–324 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Tobin JD, Andres R 1979 Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223 [DOI] [PubMed] [Google Scholar]
- Barbato AL 1990 Bedside evaluation of the autonomic nervous system. In: Walker HK, Hall WD, Hurst JW, eds. Clinical methods: the history, physical and laboratory examinations. 3rd ed. Butterworth-Heinemann Ltd. Publishers: Oxford, UK; 404–406 [Google Scholar]
- Rakowski H, Appleton C, Chan KL, Dumesnil JG, Honos G, Jue J, Koilpillai C, Lepage S, Martin RP, Mercier LA, O'Kelly B, Prieur T, Sanfilippo A, Sasson Z, Alvarez N, Pruitt R, Thompson C, Tomlinson C 1996 Canadian consensus recommendations for the measurement and reporting of diastolic dysfunction by echocardiography: from the Investigators of Consensus on Diastolic Dysfunction by Echocardiography. J Am Soc Echocardiogr 9:736–760 [DOI] [PubMed] [Google Scholar]
- de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH 1992 Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 20:1251–1260 [DOI] [PubMed] [Google Scholar]
- Stanton AW, Holroyd B, Northfield JW, Levick JR, Mortimer PS 1998 Forearm blood flow measured by venous occlusion plethysmography in healthy subjects and in women with postmastectomy oedema. Vasc Med 3:3–8 [DOI] [PubMed] [Google Scholar]
- Cooper CB, Storer TW 2001 Exercise testing and interpretation: a practical approach. Cambridge, UK: Cambridge University Press [Google Scholar]
- Borg G 1998 Borg’s perceived exertion and pain scales. 1st ed. Champaign, IL: Human Kinetics Publishers [Google Scholar]
- Bauer TA, Reusch JE, Levi M, Regensteiner JG 2007 Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care 30:2880–2885 [DOI] [PubMed] [Google Scholar]
- Wasserman K, Whipp BJ, Koyl SN, Beaver WL 1973 Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol 35:236–243 [DOI] [PubMed] [Google Scholar]
- Kamel EG, McNeill G, Han TS, Smith FW, Avenell A, Davidson L, Tothill P 1999 Measurement of abdominal fat by magnetic resonance imaging, dual-energy X-ray absorptiometry and anthropometry in non-obese men and women. Int J Obes Relat Metab Disord 23:686–692 [DOI] [PubMed] [Google Scholar]
- Schick F, Eismann B, Jung WI, Bongers H, Bunse M, Lutz O 1993 Comparison of localized proton NMR signals of skeletal muscle and fat tissue in vivo: two lipid compartments in muscle tissue. Magn Reson Med 29:158–167 [DOI] [PubMed] [Google Scholar]
- Boesch C, Slotboom J, Hoppeler H, Kreis R 1997 In vivo determination of intra-myocellular lipids in human muscle by means of localized 1H-MR-spectroscopy. Magn Reson Med 37:484–493 [DOI] [PubMed] [Google Scholar]
- Provencher SW 2001 Automatic quantitation of localized in-vivo 1H spectra with LCModel. NMR Biomed 14:260–264 [DOI] [PubMed] [Google Scholar]
- Provencher SW 1993 Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679 [DOI] [PubMed] [Google Scholar]
- Mierisová S, Ala-Korpela M 2001 MR spectroscopy quantitation: a review of frequency domain methods. NMR Biomed 14:247–259 [DOI] [PubMed] [Google Scholar]
- White LJ, Ferguson MA, McCoy SC, Kim HS 2003 Intramyocellular lipid changes in men and women during aerobic exercise: a 1H-magnetic resonance spectroscopy study. J Clin Endocrinol Metab 88:5638–5643 [DOI] [PubMed] [Google Scholar]
- Shen W, Mao X, Wolper C, Heshka S, Dashnaw S, Hirsch J, Heymsfield SB, Shungu DC 2008 Reproducibility of single- and multi-voxel 1H MRS measurements of intramyocellular lipid in overweight and lean subjects under conditions of controlled dietary calorie and fat intake. NMR Biomed 21:498–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermathen P, Kreis R, Boesch C 2004 Distribution of intramyocellular lipids in human calf muscles as determined by MR spectroscopic imaging. Magn Reson Med 51:253–262 [DOI] [PubMed] [Google Scholar]
- Thamer C, Machann J, Bachmann O, Haap M, Dahl D, Wietek B, Tschritter O, Niess A, Brechtel K, Fritsche A, Claussen C, Jacob S, Schick F, Häring HU, Stumvoll M 2003 Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab 88:1785–1791 [DOI] [PubMed] [Google Scholar]
- De Bock K, Dresselaers T, Kiens B, Richter EA, Van Hecke P, Hespel P 2007 Evaluation of intramyocellular lipid breakdown during exercise by biochemical assay, NMR spectroscopy, and Oil Red O staining. Am J Physiol Endocrinol Metab 293:E428–E434 [DOI] [PubMed] [Google Scholar]
- Sleigh A, Regan F, Harding S, Williams R, Salgin B, Saukkonen T, Dunger D, Carpenter A, IMCL is increased in adolescent type 1 diabetics—comparison with both age matched and adult controls. Proc of Annual Meeting of International Society for Magnetic Resonance in Medicine, Berlin, 2007 (Poster 2609) [Google Scholar]
- St-Onge MP, Newcomer BR, Buchthal S, Aban I, Allison DB, Bosarge A, Gower B 2007 Intramyocellular lipid content is lower with a low-fat diet than with high-fat diets, but that may not be relevant for health. Am J Clin Nutr 86:1316–1322 [DOI] [PubMed] [Google Scholar]
- Seyoum B, Estacio RO, Berhanu P, Schrier RW 2006 Exercise capacity is a predictor of cardiovascular events in patients with type 2 diabetes mellitus. Diab Vasc Dis Res 3:197–201 [DOI] [PubMed] [Google Scholar]
- Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN 2000 Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 132:605–611 [DOI] [PubMed] [Google Scholar]
- Gusso S, Hofman P, Lalande S, Cutfield W, Robinson E, Baldi JC 2008 Impaired stroke volume and aerobic capacity in female adolescents with type 1 and type 2 diabetes mellitus. Diabetologia 51:1317–1320 [DOI] [PubMed] [Google Scholar]
- Faulkner MS, Quinn L, Rimmer JH, Rich BH 2005 Cardiovascular endurance and heart rate variability in adolescents with type 1 or type 2 diabetes. Biol Res Nurs 7:16–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensteiner JG, Bauer TA, Reusch JE 2005 Rosiglitazone improves exercise capacity in individuals with type 2 diabetes. Diabetes Care 28:2877–2883 [DOI] [PubMed] [Google Scholar]
- Molnàr D, Pòrszàsz J 1990 The effect of fasting hyperinsulinaemia on physical fitness in obese children. Eur J Pediatr 149:570–573 [DOI] [PubMed] [Google Scholar]
- Török K, Szelényi Z, Pórszász J, Molnár D 2001 Low physical performance in obese adolescent boys with metabolic syndrome. Int J Obes Relat Metab Disord 25:966–970 [DOI] [PubMed] [Google Scholar]
- McMurray RG, Bauman MJ, Harrell JS, Brown S, Bangdiwala SI 2000 Effects of improvement in aerobic power on resting insulin and glucose concentrations in children. Eur J Appl Physiol 81:132–139 [DOI] [PubMed] [Google Scholar]
- Shaibi GQ, Cruz ML, Ball GD, Weigensberg MJ, Kobaissi HA, Salem GJ, Goran MI 2005 Cardiovascular fitness and the metabolic syndrome in overweight Latino youths. Med Sci Sports Exerc 37:922–928 [PubMed] [Google Scholar]
- Ball GD, Shaibi GQ, Cruz ML, Watkins MP, Weigensberg MJ, Goran MI 2004 Insulin sensitivity, cardiorespiratory fitness, and physical activity in overweight Hispanic youth. Obes Res 12:77–85 [DOI] [PubMed] [Google Scholar]
- Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L 1999 Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H–13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48:1600–1606 [DOI] [PubMed] [Google Scholar]
- Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S 2002 Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 51:1022–1027 [DOI] [PubMed] [Google Scholar]
- Boden G 2006 Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diab Rep 6:177–181 [DOI] [PubMed] [Google Scholar]
- Schrauwen P 2007 High-fat diet, muscular lipotoxicity and insulin resistance. Proc Nutr Soc 66:33–41 [DOI] [PubMed] [Google Scholar]
- Robillon JF, Sadoul JL, Jullien D, Morand P, Freychet P 1994 Abnormalities suggestive of cardiomyopathy in patients with type 2 diabetes of relatively short duration. Diabete Metab 20:473–480 [PubMed] [Google Scholar]
- Ippisch HM, Inge TH, Daniels SR, Wang B, Khoury PR, Witt SA, Glascock BJ, Garcia VF, Kimball TR 2008 Reversibility of cardiac abnormalities in morbidly obese adolescents. J Am Coll Cardiol 51:1342–1348 [DOI] [PubMed] [Google Scholar]
- Baldi JC, Aoina JL, Whalley GA, Carrick-Ranson G, Walsh HA, O'Shaughnessy H, Bagg W, Doughty RN 2006 The effect of type 2 diabetes on diastolic function. Med Sci Sports Exerc 38:1384–1388 [DOI] [PubMed] [Google Scholar]
- Regensteiner JG, Groves BM, Bauer TA, Reusch JE, Smith SC, Wolfel EE 2002 Recently diagnosed type 2 diabetes mellitus adversely affects cardiac function during exercise. Diabetes 51(Suppl 2):A59 [Google Scholar]
- McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR 1992 Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 35:771–776 [DOI] [PubMed] [Google Scholar]
- Caballero AE, Bousquet-Santos K, Robles-Osorio L, Montagnani V, Soodini G, Porramatikul S, Hamdy O, Nobrega AC, Horton ES 2008 Overweight Latino children and adolescents have marked endothelial dysfunction and subclinical vascular inflammation in association with excess body fat and insulin resistance. Diabetes Care 31:576–582 [DOI] [PubMed] [Google Scholar]
- Braun B, Eze P, Stephens BR, Hagobian TA, Sharoff CG, Chipkin SR, Goldstein B 2008 Impact of metformin on peak aerobic capacity. Appl Physiol Nutr Metab 33:61–67 [DOI] [PubMed] [Google Scholar]