Abstract
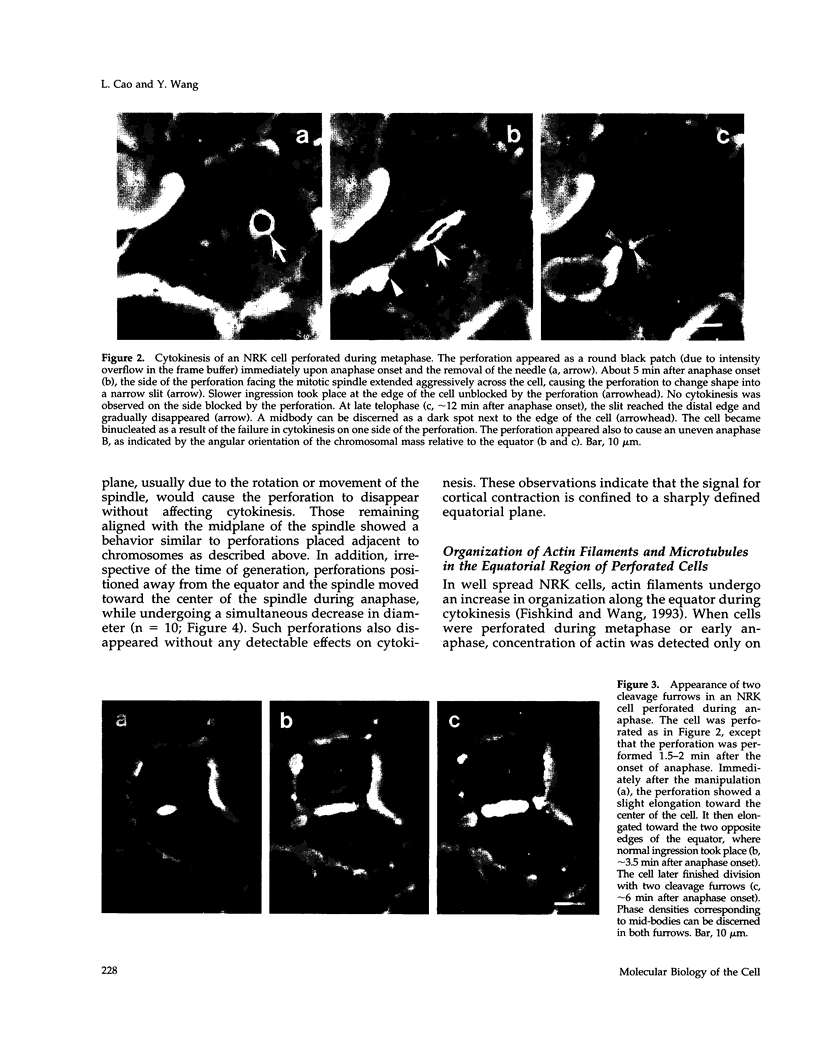
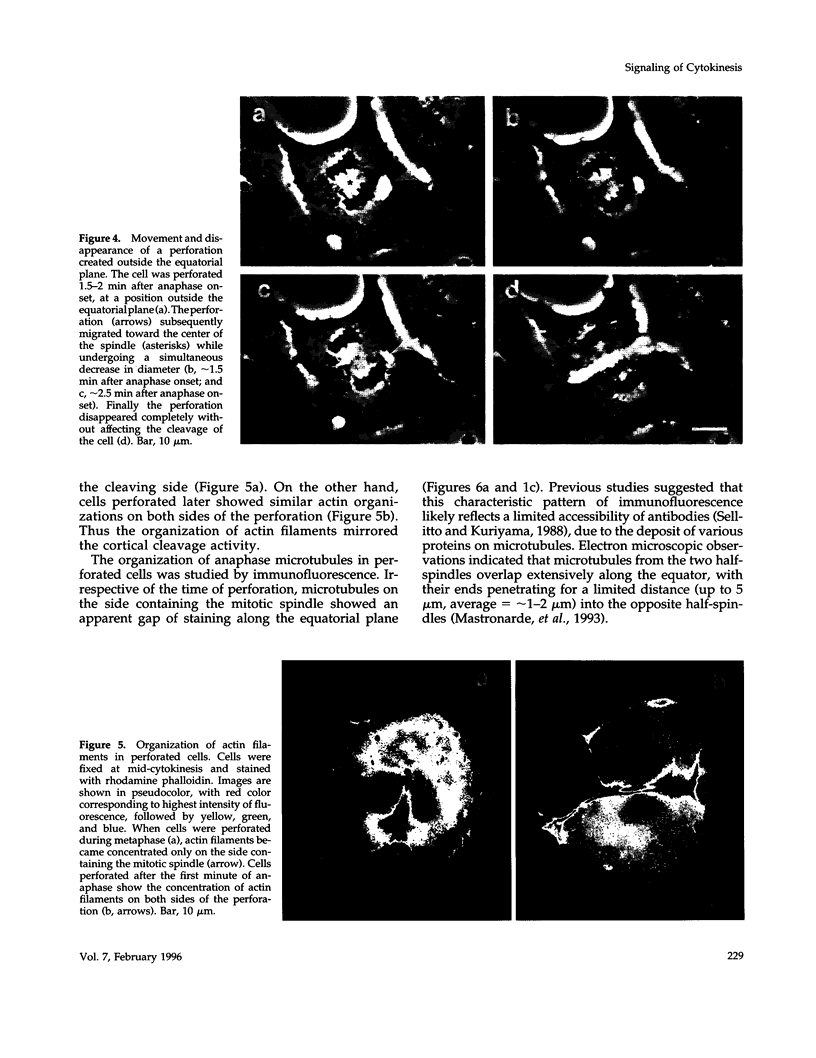
The interaction between the mitotic spindle and the cellular cortex is thought to play a critical role in stimulating cell cleavage. However, little is understood about the nature of such interactions, particularly in tissue culture cells. We have investigated the role of the spindle midzone in signaling cytokinesis by creating a barrier in cultured epithelial cells with a blunted needle, to block signals that may emanate from this region. When the barrier was created during metaphase or early anaphase, cleavage took place only on the sides of the cortex facing the mitotic spindle. Microtubules on the cleaving side showed organization typical of that in normal dividing cells. On the noncleaving side, most microtubules passed from one side of the equator into the other without any apparent organization, and actin filaments failed to organize in the equatorial region. When the barrier was created after the first minute of anaphase, cells showed successful cytokinesis, with normal organization of microtubules and actin filaments on both sides of the barrier. Our study suggests that transient signals from the midzone of early anaphase spindles are required for equatorial contraction in cultured cells and that such signaling may involve the organization of microtubules near the equator.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray D., White J. G. Cortical flow in animal cells. Science. 1988 Feb 19;239(4842):883–888. doi: 10.1126/science.3277283. [DOI] [PubMed] [Google Scholar]
- Devore J. J., Conrad G. W., Rappaport R. A model for astral stimulation of cytokinesis in animal cells. J Cell Biol. 1989 Nov;109(5):2225–2232. doi: 10.1083/jcb.109.5.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishkind D. J., Wang Y. L. New horizons for cytokinesis. Curr Opin Cell Biol. 1995 Feb;7(1):23–31. doi: 10.1016/0955-0674(95)80041-7. [DOI] [PubMed] [Google Scholar]
- Fishkind D. J., Wang Y. L. Orientation and three-dimensional organization of actin filaments in dividing cultured cells. J Cell Biol. 1993 Nov;123(4):837–848. doi: 10.1083/jcb.123.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. K., Gewalt S. L. Simulation testing of mechanisms for inducing the formation of the contractile ring in cytokinesis. J Cell Biol. 1989 Nov;109(5):2215–2223. doi: 10.1083/jcb.109.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiramoto Y. Analysis of cleavage stimulus by means of micromanipulation of sea urchin eggs. Exp Cell Res. 1971 Oct;68(2):291–298. doi: 10.1016/0014-4827(71)90153-4. [DOI] [PubMed] [Google Scholar]
- Hird S. N., White J. G. Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J Cell Biol. 1993 Jun;121(6):1343–1355. doi: 10.1083/jcb.121.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K. Microdissection studies on the dividing neuroblast of the grasshopper, with special reference to the mechanism of unequal cytokinesis. Exp Cell Res. 1977 Apr;106(1):127–137. doi: 10.1016/0014-4827(77)90249-x. [DOI] [PubMed] [Google Scholar]
- Lee G. M., Zhang F., Ishihara A., McNeil C. L., Jacobson K. A. Unconfined lateral diffusion and an estimate of pericellular matrix viscosity revealed by measuring the mobility of gold-tagged lipids. J Cell Biol. 1993 Jan;120(1):25–35. doi: 10.1083/jcb.120.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Andreassen P. R. The telophase disc: its possible role in mammalian cell cleavage. Bioessays. 1993 Mar;15(3):201–207. doi: 10.1002/bies.950150310. [DOI] [PubMed] [Google Scholar]
- Mastronarde D. N., McDonald K. L., Ding R., McIntosh J. R. Interpolar spindle microtubules in PTK cells. J Cell Biol. 1993 Dec;123(6 Pt 1):1475–1489. doi: 10.1083/jcb.123.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckenna N. M., Wang Y. L. Culturing cells on the microscope stage. Methods Cell Biol. 1989;29:195–205. doi: 10.1016/s0091-679x(08)60195-8. [DOI] [PubMed] [Google Scholar]
- Miller A. L., Fluck R. A., McLaughlin J. A., Jaffe L. F. Calcium buffer injections inhibit cytokinesis in Xenopus eggs. J Cell Sci. 1993 Oct;106(Pt 2):523–534. doi: 10.1242/jcs.106.2.523. [DOI] [PubMed] [Google Scholar]
- Mullins J. M., Snyder J. A. Anaphase progression and furrow establishment in nocodazole-arrested PtK1 cells. Chromosoma. 1981;83(4):493–505. doi: 10.1007/BF00328275. [DOI] [PubMed] [Google Scholar]
- RAPPAPORT R. Experiments concerning the cleavage stimulus in sand dollar eggs. J Exp Zool. 1961 Oct;148:81–89. doi: 10.1002/jez.1401480107. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Establishment of the mechanism of cytokinesis in animal cells. Int Rev Cytol. 1986;105:245–281. doi: 10.1016/s0074-7696(08)61065-7. [DOI] [PubMed] [Google Scholar]
- Rappaport R., Rappaport B. N. Cleavage in conical sand dollar eggs. Dev Biol. 1994 Jul;164(1):258–266. doi: 10.1006/dbio.1994.1196. [DOI] [PubMed] [Google Scholar]
- Rappaport R., Rappaport B. N. Establishment of cleavage furrows by the mitotic spindle. J Exp Zool. 1974 Aug;189(2):189–196. doi: 10.1002/jez.1401890206. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Repeated furrow formation from a single mitotic apparatus in cylindrical sand dollar eggs. J Exp Zool. 1985 Apr;234(1):167–171. doi: 10.1002/jez.1402340120. [DOI] [PubMed] [Google Scholar]
- Salmon E. D. Cytokinesis in animal cells. Curr Opin Cell Biol. 1989 Jun;1(3):541–547. doi: 10.1016/0955-0674(89)90018-5. [DOI] [PubMed] [Google Scholar]
- Satterwhite L. L., Pollard T. D. Cytokinesis. Curr Opin Cell Biol. 1992 Feb;4(1):43–52. doi: 10.1016/0955-0674(92)90057-j. [DOI] [PubMed] [Google Scholar]
- Sellitto C., Kuriyama R. Distribution of a matrix component of the midbody during the cell cycle in Chinese hamster ovary cells. J Cell Biol. 1988 Feb;106(2):431–439. doi: 10.1083/jcb.106.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V. Organization of actin in the leading edge of cultured cells: influence of osmium tetroxide and dehydration on the ultrastructure of actin meshworks. J Cell Biol. 1981 Dec;91(3 Pt 1):695–705. doi: 10.1083/jcb.91.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Borisy G. G. On the mechanisms of cytokinesis in animal cells. J Theor Biol. 1983 Mar 21;101(2):289–316. doi: 10.1016/0022-5193(83)90342-9. [DOI] [PubMed] [Google Scholar]