Abstract
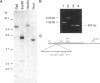
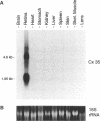
We have used low stringency hybridization to clone a novel connexin from a skate retinal cDNA library. A rat connexin 32 clone was used to isolate a single partial clone that was subsequently used to isolate seven more overlapping clones of the same cDNA. Two clones containing the entire open reading frame have a consensus sequence of 1456 bp and predict a protein of 302 amino acids length and molecular mass of 35,044 daltons, referred to as connexin 35 or Cx35. Southern blot analysis suggests that the cloned sequence lies in a single gene with one intron. Polymerase chain reaction amplification from genomic DNA and partial sequencing of this intron showed that it was approximately 950 bp in length, and located within the coding region 71 bp after the translation start site. Hydropathy analysis of the predicted protein and alignments with previously cloned connexins indicate that Cx35 has a long cytoplasmic loop and a relatively short carboxyl terminal tail. Multiple sequence alignments show that Cx35 has similarities to both alpha and beta groups of connexins and suggests that its origins may be near the divergence point for the two groups. Consensus sequences consistent with sites for phosphorylation by protein kinase C and by cAMP - or cGMP -dependent protein kinase were identified. Two transcripts were detected in Northern blot analysis: a 1.95-kb primary transcript and a 4.6-kb minor transcript. In RNA samples from 10 tissues, transcripts were detected only in the retina.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldridge W. H., Ball A. K. Background illumination reduces horizontal cell receptive-field size in both normal and 6-hydroxydopamine-lesioned goldfish retinas. Vis Neurosci. 1991 Nov;7(5):441–450. doi: 10.1017/s0952523800009731. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Rubin J. B., Bargiello T. A., Verselis V. K. Structure-function studies of voltage sensitivity of connexins, the family of gap junction forming proteins. Jpn J Physiol. 1993;43 (Suppl 1):S301–S310. [PubMed] [Google Scholar]
- Bennett M. V., Zheng X., Sogin M. L. The connexins and their family tree. Soc Gen Physiol Ser. 1994;49:223–233. [PubMed] [Google Scholar]
- Beyer E. C. Gap junctions. Int Rev Cytol. 1993;137C:1–37. [PubMed] [Google Scholar]
- Christ G. J., Brink P. R., Ramanan S. V. Dynamic gap junctional communication: a delimiting model for tissue responses. Biophys J. 1994 Sep;67(3):1335–1344. doi: 10.1016/S0006-3495(94)80605-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church R. L., Wang J. H., Steele E. The human lens intrinsic membrane protein MP70 (Cx50) gene: clonal analysis and chromosome mapping. Curr Eye Res. 1995 Mar;14(3):215–221. doi: 10.3109/02713689509033517. [DOI] [PubMed] [Google Scholar]
- Cook J. E., Becker D. L. Gap junctions in the vertebrate retina. Microsc Res Tech. 1995 Aug 1;31(5):408–419. doi: 10.1002/jemt.1070310510. [DOI] [PubMed] [Google Scholar]
- Cuenca N., Fernández E., García M., De Juan J. Dendrites of rod dominant ON-bipolar cells are coupled by gap junctions in carp retina. Neurosci Lett. 1993 Nov 12;162(1-2):34–38. doi: 10.1016/0304-3940(93)90553-w. [DOI] [PubMed] [Google Scholar]
- DeVries S. H., Schwartz E. A. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989 Jul;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R., Traub O., Hwang T. K., Beyer E., Bennett M. V., Spray D. C., Willecke K. Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10148–10152. doi: 10.1073/pnas.86.24.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. J., McReynolds J. S. The relationship between light, dopamine release and horizontal cell coupling in the mudpuppy retina. J Physiol. 1991;440:291–309. doi: 10.1113/jphysiol.1991.sp018709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman G. I., Spray D. C., Leinwand L. A. Molecular characterization and functional expression of the human cardiac gap junction channel. J Cell Biol. 1990 Aug;111(2):589–598. doi: 10.1083/jcb.111.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie S. C., Gilula N. B. Gap junctional communication and development. Trends Neurosci. 1989 Jan;12(1):12–16. doi: 10.1016/0166-2236(89)90150-1. [DOI] [PubMed] [Google Scholar]
- Hampson E. C., Vaney D. I., Weiler R. Dopaminergic modulation of gap junction permeability between amacrine cells in mammalian retina. J Neurosci. 1992 Dec;12(12):4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennemann H., Dahl E., White J. B., Schwarz H. J., Lalley P. A., Chang S., Nicholson B. J., Willecke K. Two gap junction genes, connexin 31.1 and 30.3, are closely linked on mouse chromosome 4 and preferentially expressed in skin. J Biol Chem. 1992 Aug 25;267(24):17225–17233. [PubMed] [Google Scholar]
- Hidaka S., Maehara M., Umino O., Lu Y., Hashimoto Y. Lateral gap junction connections between retinal amacrine cells summating sustained responses. Neuroreport. 1993 Oct 25;5(1):29–32. doi: 10.1097/00001756-199310000-00007. [DOI] [PubMed] [Google Scholar]
- Kadle R., Zhang J. T., Nicholson B. J. Tissue-specific distribution of differentially phosphorylated forms of Cx43. Mol Cell Biol. 1991 Jan;11(1):363–369. doi: 10.1128/mcb.11.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D. Spatial properties of horizontal cell responses in the turtle retina. J Physiol. 1976 Dec;263(2):239–255. doi: 10.1113/jphysiol.1976.sp011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M. Retinal horizontal cell gap junctional conductance is modulated by dopamine through a cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7319–7323. doi: 10.1073/pnas.84.20.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon D. G., Knapp A. G., Dowling J. E. Horizontal cell gap junctions: single-channel conductance and modulation by dopamine. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7639–7643. doi: 10.1073/pnas.86.19.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiners S., Xu A., Schindler M. Gap junction protein homologue from Arabidopsis thaliana: evidence for connexins in plants. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4119–4122. doi: 10.1073/pnas.88.10.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milks L. C., Kumar N. M., Houghten R., Unwin N., Gilula N. B. Topology of the 32-kd liver gap junction protein determined by site-directed antibody localizations. EMBO J. 1988 Oct;7(10):2967–2975. doi: 10.1002/j.1460-2075.1988.tb03159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T., Dahl G., Werner R. Structure of a gap junction gene: rat connexin-32. Biosci Rep. 1988 Oct;8(5):455–464. doi: 10.1007/BF01121644. [DOI] [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Obaid A. L., Socolar S. J., Rose B. Cell-to-cell channels with two independently regulated gates in series: analysis of junctional conductance modulation by membrane potential, calcium, and pH. J Membr Biol. 1983;73(1):69–89. doi: 10.1007/BF01870342. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Paul D. L. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986 Jul;103(1):123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado A., Yuste R., Katz L. C. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993 Jan;10(1):103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- Penn A. A., Wong R. O., Shatz C. J. Neuronal coupling in the developing mammalian retina. J Neurosci. 1994 Jun;14(6):3805–3815. doi: 10.1523/JNEUROSCI.14-06-03805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino M., Neyton J., Gerschenfeld H. M. Decrease of gap junction permeability induced by dopamine and cyclic adenosine 3':5'-monophosphate in horizontal cells of turtle retina. J Neurosci. 1984 Oct;4(10):2477–2488. doi: 10.1523/JNEUROSCI.04-10-02477.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Malchow R. P., Ripps H. Gap-junctional properties of electrically coupled skate horizontal cells in culture. Vis Neurosci. 1993 Mar-Apr;10(2):287–295. doi: 10.1017/s0952523800003680. [DOI] [PubMed] [Google Scholar]
- Qian H., Ripps H. Receptive field properties of rod-driven horizontal cells in the skate retina. J Gen Physiol. 1992 Sep;100(3):457–478. doi: 10.1085/jgp.100.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E., Gilula N. B. Gap junctions between photoreceptor cells in the vertebrate retina. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1677–1681. doi: 10.1073/pnas.70.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature. 1975 Mar 20;254(5497):250–252. doi: 10.1038/254250a0. [DOI] [PubMed] [Google Scholar]
- Saito T., Kujiraoka T. Characteristics of bipolar-bipolar coupling in the carp retina. J Gen Physiol. 1988 Feb;91(2):275–287. doi: 10.1085/jgp.91.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981 Feb 13;211(4483):712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Suchyna T. M., Xu L. X., Gao F., Fourtner C. R., Nicholson B. J. Identification of a proline residue as a transduction element involved in voltage gating of gap junctions. Nature. 1993 Oct 28;365(6449):847–849. doi: 10.1038/365847a0. [DOI] [PubMed] [Google Scholar]
- Verselis V. K., Ginter C. S., Bargiello T. A. Opposite voltage gating polarities of two closely related connexins. Nature. 1994 Mar 24;368(6469):348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- Weiler R., Akopian A. Effects of background illuminations on the receptive field size of horizontal cells in the turtle retina are mediated by dopamine. Neurosci Lett. 1992 Jun 8;140(1):121–124. doi: 10.1016/0304-3940(92)90696-5. [DOI] [PubMed] [Google Scholar]
- White T. W., Bruzzone R., Wolfram S., Paul D. L., Goodenough D. A. Selective interactions among the multiple connexin proteins expressed in the vertebrate lens: the second extracellular domain is a determinant of compatibility between connexins. J Cell Biol. 1994 May;125(4):879–892. doi: 10.1083/jcb.125.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizaki G., Patiño R., Thomas P. Connexin messenger ribonucleic acids in the ovary of Atlantic croaker: molecular cloning and characterization, hormonal control, and correlation with appearance of oocyte maturational competence. Biol Reprod. 1994 Sep;51(3):493–503. doi: 10.1095/biolreprod51.3.493. [DOI] [PubMed] [Google Scholar]