Abstract
Phenotypic switching of vascular smooth muscle cells (VSMCs) is known to play a critical role in the development of atherosclerosis. However, the factors present within lesions that mediate VSMC phenotypic switching are unclear. Oxidized phospholipids (OxPLs), including 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine (POVPC), are active components of minimally modified low density lipoprotein and have been previously shown to induce multiple proatherogenic events in endothelial cells and macrophages, but their effects on VSMCs have been largely unexplored until recently. We previously showed that OxPLs induced phenotypic switching of VSMCs, including suppression of SMC differentiation marker genes. The goal of the present studies was to test the hypothesis that OxPLs alter extracellular matrix production and VSMC migration. Results showed that POVPC activated expression of several extracellular matrix proteins in VSMC. POVPC increased expression of type VIII collagen α1 chain (Col8a1) mRNA in cultured VSMCs and in vivo in rat carotid arteries by 9-fold and 4-fold, respectively. POVPC-induced activation of Col8a1 gene expression was reduced by small interfering RNA–mediated suppression of Krüppel-like factor 4 (Klf4) and Sp1, and was abolished in Klf4-knockout VSMCs. POVPC increased Klf4 binding to the Col8a1 gene promoter both in vivo in rat carotid arteries and in cultured VSMCs based on chromatin immunoprecipitation assays. Moreover, POVPC-induced VSMC migration was markedly reduced in Klf4- or type VIII collagen–knockout VSMCs. Given evidence that OxPLs are present within atherosclerotic lesions, it is interesting to suggest that OxPL-induced changes in VSMC phenotype may contribute to the pathogenesis of atherosclerosis at least in part through changes in extracellular matrix composition.
Keywords: POVPC, PGPC, PEIPC, type VIII collagen, Klf4, vascular smooth muscle cell migration
Atherosclerosis is a progressive cardiovascular disease that remains the leading cause of death in the Western world. One of the critical factors implicated in the development of atherosclerosis is an accumulation of cholesterol, lipoproteins, and lipid derivatives within large arteries. Oxidized low density lipoprotein (OxLDL) has been shown to elicit a host of responses that contribute to the development of atherosclerosis including inflammation, proliferation, apoptosis, unregulated uptake of LDL, and regulation of gene expression.1,2 Indeed, there is a growing body of evidence that specific oxidized phospholipids (OxPLs) including OxPAPC (l-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine), which are the active part of minimally modified OxLDL and found in high concentrations within atherosclerotic lesions, are the primary stimuli of different proatherogenic events including increased monocyte adherence to endothelial cells, as well as the induction of production of various inflammatory cytokines by macrophages.1,3–5 However, although phenotypic switching of vascular smooth muscle cells (VSMCs) is known to play a key role in the development, progression, and end-stage clinical consequences of atherosclerosis (reviewed by Owens et al6), very little is known regarding the effects of specific OxPLs on VSMCs. Recently we showed that the oxidized phospholipid OxPAPC, as well as the active components of OxPAPC, POVPC (1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine), and PGPC (1 -palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine), induce profound phenotypic switching of VSMCs including suppression of multiple SMC differentiation marker genes in vivo and in vitro.7
Phenotypic switching of VSMCs results in an increase in the rate of cell proliferation and migration and a decrease in the expression of SMC-specific marker genes.6 It has long been recognized that expression of extracellular matrix (ECM) proteins by phenotypically modulated VSMCs within atherosclerotic lesions plays a critical role in the pathogenesis of this disease, as well as its clinical consequences. The normal vessel wall ECM is composed predominantly of type I and III collagens, elastin, and proteoglycans with smaller amounts of fibronectin, laminins, and type IV collagen.8,9 However, the composition of ECM dramatically changes during the development of atherosclerosis. High levels of fibronectin and type VIII collagen are synthesized locally by resident VSMCs in diffuse intimal thickening and atherosclerotic plaques.10–13 In addition, there are major changes in the levels of various laminins and proteoglycans.8 Of major importance, the new synthesized ECM proteins not only play a key role in determining the biomechanical properties of lesions but also modulate the phenotype of VSMCs, including regulation of VSMC proliferation and migration and further ECM synthesis through integrin-mediated signaling.
It was recently reported that OxLDL stimulates collagenous protein production in VSMCs.14 However, virtually nothing is known regarding the effects of specific lipid oxidation products on expression of ECM proteins and what role these processes could play in modulating ECM expression by intimal VSMCs within atherosclerotic lesions. The goal of the present studies was to test the hypothesis that OxPLs including POVPC alter the expression of ECM genes within VSMCs. Results showed that POVPC induced upregulation of several ECM genes including type VIII collagen both in vitro and in vivo and that this expression was mediated, at least in part, by Krüppel-like factor (Klf)4. Moreover, the increase in type VIII collagen expression was required for POVPC-induced VSMC migration, demonstrating that altered ECM expression has important functional consequences.
Materials and Methods
The animal protocols were approved by the Animal Care and Use Committee at the University of Virginia. An expanded Materials and Methods section is available in the online data supplement at http://circres.ahajournals.org.
Results
POVPC Activated Expression of Multiple ECM Genes in Cultured SMCs
As an initial screen of ECM genes modulated by OxPLs in SMCs, we performed Affymetrix GeneChip microarray analyses to compare gene expression profiles between POVPC-and vehicle-treated rat aortic SMCs. Results showed that POVPC significantly increased the expression of type VIII collagen (3.54-fold increase, P=0.01) and the chondroitin sulfate proteoglycan versican (3.04-fold increase, P=0.01). Results also showed trends for increases in several collagens, including type XV, XVIII, XXII, and XXVII, and integrin receptors αv and α8 and for decreased expression of procollagen type XI, although these changes did not achieve statistical significance (P<0.15; Figure I, A, in the online data supplement). POVPC also increased expression of fibronectin 3 by 3-fold, and laminin α2 by 6-fold but did not change the expression of type I collagen, as determined by real-time RT-PCR analyses (supplemental Figure I, B). Taken together, these results clearly demonstrate that POVPC induces selective changes in the expression of multiple ECM genes in cultured rat aortic SMCs. Because type VIII collagen is implicated in cell migration and growth, as well as atherosclerosis, it was chosen to be the major focus of subsequent studies.
POVPC Induced Type VIII Collagen mRNA and Protein Synthesis in Cultured SMCs
Cultured rat aortic SMCs were treated with variable concentrations of OxLDL (10 to 100 μg/mL), OxPAPC (1 to 100 μg/mL), POVPC, PGPC (1 to 25 μg/mL), and PEIPC (0.1 to 3 μg/mL) or vehicle for 24 hours, followed by quantitative real-time RT-PCR analyses. OxLDL and OxPAPC both induced an approximately 4-fold increase in type VIII collagen α1 chain (Col8a1) gene expression when applied at a concentration of 100 μg/mL (Figure 1A and 1B). POVPC was both more potent and efficacious than OxLDL and OxPAPC in inducing Col8a1 gene expression with a maximum increase of more than 9-fold at a concentration of 10 to 25 μg/mL (Figure 1C). PGPC also increased Col8a1 expression by 4-fold at a concentration of 25 μg/mL (Figure 1C).
Figure 1.
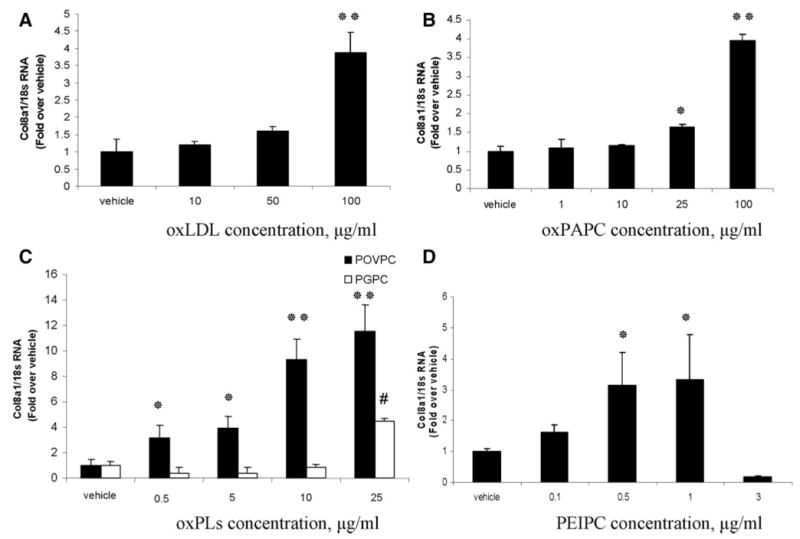
OxPLs induced expression of the endogenous type VIII collagen in cultured rat aortic SMCs, as determined by quantitative real-time RT-PCR. VSMCs were treated with OxLDL (A), OxPAPC (B), POVPC or PGPC (C), and PEIPC (D) or vehicle for 24 hours. #*P<0.05; **P<0.01 vs vehicle.
To extend our recent studies showing POVPC- and PGPC-induced suppression of SMC differentiation marker genes,7 we tested the effect of PEIPC, which has been shown to be the most potent component of OxPAPC for activating endothelial cells.15 Of interest, PEIPC markedly suppressed expression of the SMC marker genes smooth muscle (SM) myosin heavy chain (MHC), SM α-actin, and SM 22α: by 80% to 90% at a concentration of 0.5 to 1 μg/mL (supplemental Figure II). PEIPC also induced a 3-fold increase in type VIII collagen gene expression at concentrations of either 0.5 or 1 μg/mL (Figure 1D). By contrast, VSMCs treated with vehicle or nonoxidized control phospholipids such as LDL, PAPC, and DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine) (supplemental Figure III) showed no change in Col8a1 gene expression, indicating that oxidation is required for the observed effects.
To determine whether POVPC-induced increases in type VIII collagen mRNA were associated with increased protein secretion, Western blot analyses were performed on conditioned media collected from vehicle- or POVPC-treated cultured aortic SMCs. (Figure 2). We tested 2 antibodies raised against bovine type VIII collagen: a guinea pig polyclonal antibody and a mouse monoclonal antibody. Major bands at 50, 65, and 85 kDa and a high-molecular-mass product >250 kDa were visualized by the monoclonal antibody in the SMC conditioned media under nonreduced conditions (Figure 2A). The polyclonal antibody showed bands at 50, 65, 75, 85, and 125 kDa under reduced conditions (Figure 2B). These bands are consistent in size with purified type VIII collagen.16 The multiple bands that we and others16–18 observed for type VIII collagen reflect the well-known molecular heterogeneity of type VIII collagen. Moreover, as noted by Korsching and Rauterberg,16 detection of type VIII collagen protein molecular mass variants differs depending on the source and type of detection antibody. A distinct band at 85 kDa was observed with both antibodies under reduced and nonreduced conditions after 48 to 72 hours of treatment with POVPC as compared to the vehicle-treated control (Figure 2A and 2B). To further validate the specificity of type VIII collagen antibodies, cultured SMCs were treated with a Col8a1 small interfering (si)RNA. These results confirmed that the 85-kDa band corresponded to the α1 chain of type VIII collagen (Figure 2C and 2D).
Figure 2.
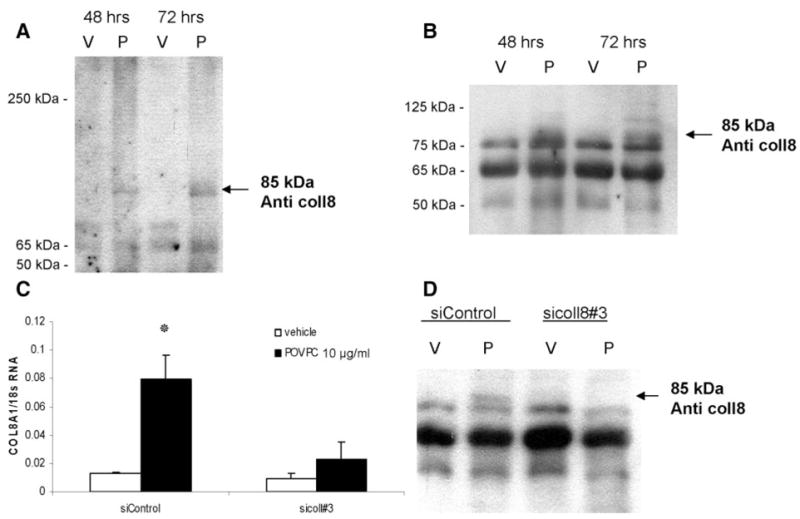
POVPC induced type VIII collagen protein synthesis and secretion in cultured rat aortic SMCs. For Western blot analysis, VSMCs were treated with either POVPC (10 μg/mL) (P) or vehicle (V) for 48 to 72 hours. Conditioned media were collected and concentrated, and 20 μg of total proteins were fractionated by electrophoresis under nondenaturing conditions on a 7.5% polyacrylamide gel (A) or denaturing conditions on a 10% gel (B). Type VIII collagen protein was detected with a guinea pig polyclonal antibody (B) or mouse monoclonal antibody (A) raised against bovine type VIII collagen. To validate the specificity of anti–type VIII collagen antibodies, cultured VSMCs were transfected with Col8a1 siRNA oligonucleotides (sicol8#3) or control siRNA (siControl), followed by either POVPC or vehicle treatment for 24 hours (C). The expression of Col8a1 gene was measured by quantitative real-time RT-PCR. *P<0.05 vs vehicle. After transfection with siRNA and treatment with either POVPC (10 μg/mL) (P) or vehicle (V) for 48 hours, type VIII collagen protein was detected in VSMC conditioned medium by probing Western blots with a guinea pig polyclonal antibody (D) raised against bovine type VIII collagen.
POVPC Induced Type VIII Collagen α1 Chain mRNA Expression in Rat Carotid Arteries In Vivo
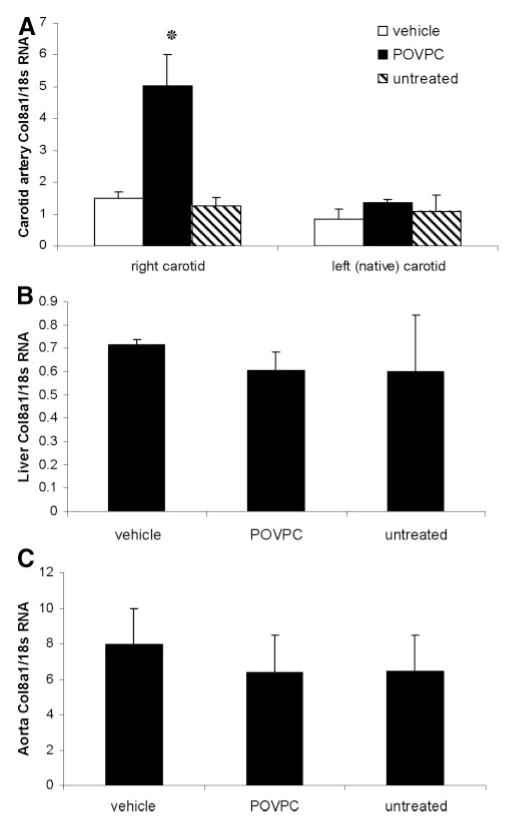
To determine whether POVPC can also activate expression of Col8a1 in vivo, we applied 30 μg of POVPC or DMSO vehicle to the adventitial surface of rat carotid arteries using an F-127 pluronic gel.3,7 Results of real-time RT-PCR demonstrated that the treatment of carotid arteries with POVPC for 24 hours increased expression of Col8a1 mRNA by nearly 3-fold relative to vehicle-treated vessels (Figure 3A). In contrast, no changes in expression of type VIII collagen mRNA were seen in various control tissues, including untreated carotid arteries, or within the liver or aorta of the same rats, indicating that effects were not systemic (Figure 3B and 3C).
Figure 3.
Pluronic gel–based delivery of POVPC increased the expression of the endogenous type VIII collagen gene in vivo. A pluronic gel with either POVPC (30 μg) or vehicle was applied to the adventitial surface of rat right common carotid arteries. Total RNA was isolated from the right treated and left native carotid arteries (A), liver (B), and aorta (C) of the POVPC-treated, vehicle-treated, and untreated animals. Expression of Col8a1 gene was measured by quantitative real-time RT-PCR. *P<0.05 vs vehicle.
Klf4 Was Required for POVPC-Induced Col8a1 Gene Expression In Vivo and In Vitro
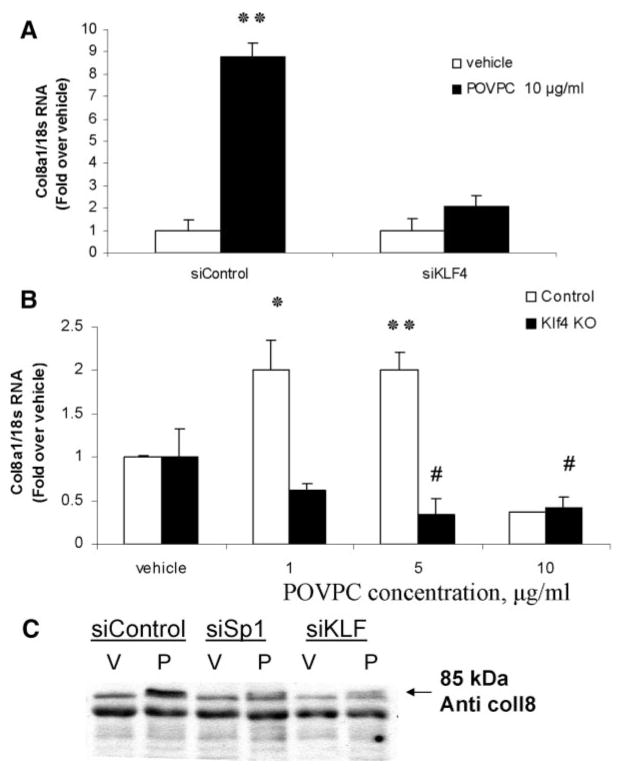
Previous studies have shown that POVPC induces Klf4 synthesis in VSMCs and that Klf4 is required for POVPC-induced repression of SMC marker genes.7 Mutagenesis experiments determined that a Klf4 consensus binding motif has a sequence of 5′-G/A-GA-G-G-C/T-G-C/T-3′ (or 5′-G/A-C-G/A-CC-T/C-T/C-3′).19 Of interest, we found a potential Klf4-binding site (ACACCTT) in the proximal Col8a1 promoter that is completely conserved in the Col8a1 genes in human, mouse, and rat (supplemental Figure IV). The effects of siRNA oligonucleotides specific to Klf4 were used to determine whether Klf4 is required for POVPC-induced increases in Col8a1 gene expression. The specificity of the siRNA has been documented previously.7 Klf4 siRNA, but not a control siRNA, blocked POVPC-induced Col8a1 gene expression in cultured SMC at both the mRNA (Figure 4A) and the protein level (Figure 4C). The siKlf4 also blocked POVPC-induced expression of the LAMA2 gene (supplemental Figure V), which also contains a conserved consensus KLF4 binding site in its 5′ promoter region.
Figure 4.
Klf4 was required for POVPC-induced type VIII collagen gene expression in rat aortic SMCs. A, VSMCs were transfected with Klf4 siRNA oligonucleotides (siKLF4) or control siRNA (siControl) followed by either POVPC (10 μg/mL) or vehicle treatment for 24 hours. B, VSMCs were isolated from the aorta of Klf4-floxed mice and infected with adenovirus expressing Crerecombinase (Klf4 KO) or control adenovirus. KO and control VSMCs were treated with POVPC or vehicle for 24 hours. Expression of Col8a1 gene was measured by quantitative real-time RT-PCR. #*P<0.05 vs vehicle, **P<0.01 vs vehicle. For Western blot analyses, the conditioned media from rat aortic SMCs after transfection with control siRNA (siControl), siSp1, or siKLF4 and treatment with either POVPC (10 μg/mL) (P) or vehicle for 48 hours was fractionated under denaturing conditions on a 10% polyacrylamide gel (C). Type VIII collagen protein was detected with a guinea pig polyclonal antibody raised against bovine type VIII collagen.
To further assess if Klf4 is required for POVPC-induced expression of type VIII collagen, we tested effects of POVPC in Klf4 knockout (KO) mouse aortic SMCs. SMCs were isolated from the aorta of Klf4-floxed mice20 and infected with an adenovirus expressing Cre-recombinase (Klf4 KO) or control adenovirus (control). Control or Klf4 KO SMCs were then treated with POVPC (1 to 10 μg/mL) or vehicle, followed by RNA extraction and real-time RT-PCR analysis of Col8al gene expression (Figure 4B). Results showed significant induction of expression of Col8al in control but not Klf4 KO mouse aortic SMCs.
To determine whether POVPC-induced expression of Col8a1 in vivo was also dependent on Klf4, rat carotid arteries were treated simultaneously with POVPC plus control or Klf4 siRNAs using the pluronic gel system. Of major interest, results showed that the siKlf4 but not the control siRNA partially blocked POVPC-induced expression of Col8a1, as well as POVPC-induced suppression of SM α-actin and SM MHC (supplemental Figure VI).
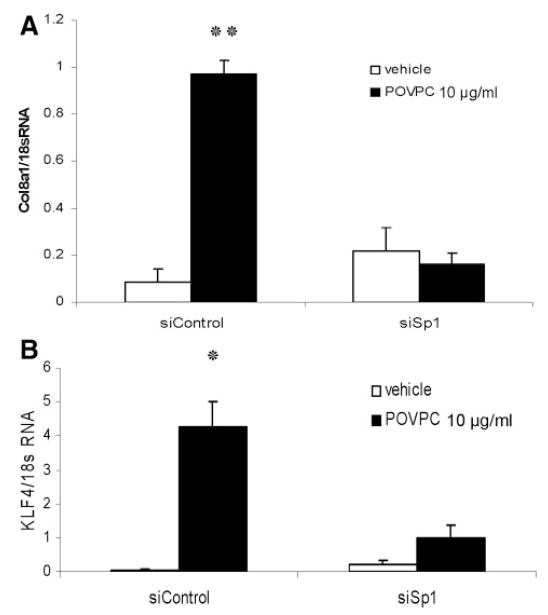
It has been previously reported that Spl transcription factor regulates Klf4 expression in colon cancer cells.21 Figure 5 demonstrates that an Spl siRNA, but not a control siRNA, decreased both the POVPC-induced expression of Klf4 and Col8a1 genes (Figure 5A and 5B), as well as the synthesis of both Klf4 (supplemental Figure VII) and type VIII collagen (Figure 4C) proteins in cultured VSMCs. Thus, we hypothesized that POVPC increased Col8a1 gene expression through Sp1-dependent activation of Klf4.
Figure 5.
POVPC-induced expression of Klf4 and Col8a1 genes was mediated, at least in part, by Sp1. Cultured rat aortic SMCs were transfected with Sp1 siRNA oligonucleotides (siSp1) or control siRNA (siControl), followed by POVPC or vehicle treatment for 24 hours. The expression of Col8a1 (A) and Klf4 (B) genes was measured by quantitative real-time RT-PCR. *P<0.05; **P<0.01 vs vehicle.
The Klf4 promoter contains 3 Sp1 binding sites located in the region between 150 and 50 bp upstream of the transcriptional start site.21 To determine whether Sp1 is required for POVPC-induced Klf4 expression, VSMCs were transfected with different reporter plasmids that contained various lengths of the Klf4 promoter (2200, 515, or 51 bp of the promoter region upstream from the transcriptional site cloned into the pGL3-luciferase vector). POVPC markedly increased both the 2200- and 515-bp Klf4 promoters but not the 51-bp region lacking the 3 Sp1 binding sites (supplemental Figure VIII). These results provide evidence suggesting that POVPC-induced expression of Klf4 in VSMCs is Sp1-dependent.
Klf4 Directly Bound to the Col8a1 Promoter Region In Vitro and In Vivo
We tested whether Klf4 binds to the type VIII collagen promoter region using quantitative chromatin immunoprecipitation (ChIP) assays. Treatment of rat aortic SMCs with POVPC was associated with the recruitment of Klf4 to the Col8a1 gene promoter within 6 hours of POVPC treatment (Figure 6A). To verify our in vitro data, we used the pluronic gel system to determine whether POVPC induced Klf4 binding to the type VIII collagen promoter in vivo. Figure 6B shows that Klf4 was also enriched at the Col8a1 gene promoter in ChIP analyses of chromatin isolated from rat carotid arteries treated with POVPC for 8 hours versus vehicle-treated arteries. In contrast, Klf4 was not changed in ChIP analyses of chromatin isolated from aortas of the same animals (Figure 6B). Taken together, our data provide strong evidence that POVPC-induced increases in type VIII collagen expression are mediated, at least in part, by Klf4 binding to the type VIII collagen promoter.
Figure 6.

Klf4 directly associated with the promoter region of Col8a1 gene within intact chromatin in POVPC-treated rat aortic SMC and rat carotid artery segments in vivo as determined by ChIP. A, Cultured VSMCs were treated with either POVPC (10 μg/mL) or vehicle for 24 hours. Association of Klf4 with the promoter region of Col8a1 gene was determined by ChIP assays. B, Association of Klf4 with the promoter region of Col8a1 gene was also determined by in vivo ChIP assays in carotid arteries of rats after application of pluronic gel containing either 30 μg of POVPC or vehicle for 8 hours. Aortas were used as a control. Chromatin either precipitated with anti-Klf4 antibody (KLF4 AB) or without antibody (no AB) was subjected to the real-time RT-PCR. Results were normalized to the total input and demonstrated as a fold-increase over vehicle. *P<0.05.
POVPC Induced Type VIII Collagen Expression Was Required for Migration of Cultured SMCs
It has been reported that type VIII collagen promotes VSMC migration.22,23 Boyden chamber migration assays were used to determine whether OxPLs increased the migration of cultured SMCs, and if this migration was dependent on both Klf4 and type VIII collagen. As shown in Figure 7A, POVPC and PEIPC, but not PGPC, enhanced the fibronectin-dependent migratory response of VSMCs. Of major interest, POVPC-induced migration of SMCs was inhibited with siRNA to either Klf4 or Col8a1 but not a control siRNA that targets luciferase (Figure 7B).
Figure 7.
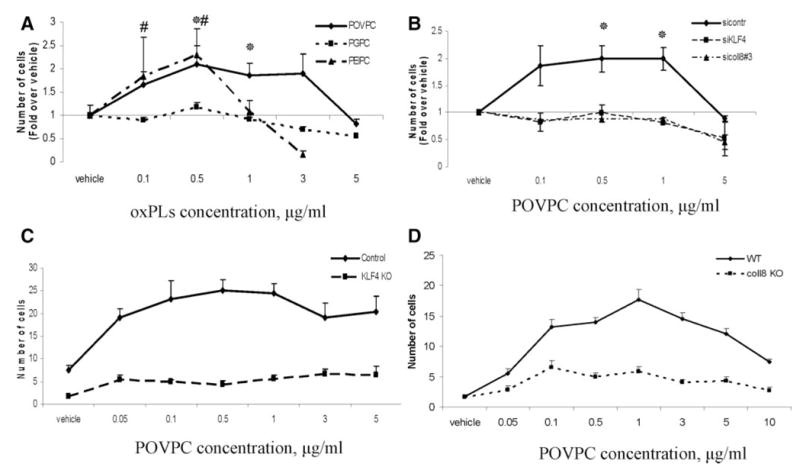
POVPC stimulated VSMC migration through Klf4 and type VIII collagen–dependent mechanisms. A Boyden chamber assay was used to measure VSMC migration through an 8-μm polycarbonate membrane. A, Serum-free media (150-μL volume) with 0.1% BSA, 5 μg/mL fibronectin, and the indicated concentrations of POVPC, PEIPC, or PGPC were added to the bottom chambers. Rat aortic SMC suspension (1×105 cells/mL, 150 μL) was added to the upper wells in serum-free media with 0.1% BSA. The chambers were incubated at 37°C for 18 hours. Data represent means±SEM from 8 to 10 randomly chosen high-power fields. *P<0.05 POVPC vs vehicle, #P<0.05 PEIPC vs vehicle. B, Rat aortic SMCs were transfected with siRNA specific to Col8a1 (sicoll8#3), Klf4 (siKLF4), or control siRNA targeting luciferase (sicontr) and migration assay was performed as described in the legend of Figure 7A. *P<0.05 vs vehicle. Transmigration assay was performed through type I collagen–covered membranes to compare migration of mouse aortic Klf4 KO vs control cells (C) and mouse aortic type VIII collagen KO vs WT cells (D).
To further test the role of type VIII collagen and Klf4 in POVPC-induced SMC migration, we tested migratory effects of POVPC in type VIII collagen and Klf4 KO mouse aortic SMCs. POVPC induced transmigration in Klf4 control and type VIII collagen wild-type (WT) but not Klf4 KO and type VIII collagen KO mouse aortic SMCs (Figure 7C and 7D). Taken together, results of these studies provide evidence that POVPC-induced migration of cultured SMCs is dependent on Klf4 and type VIII collagen.
Endogenous Col8a1 and Klf4 Gene Expression Was Increased in the Aortas of apoE KO Mice
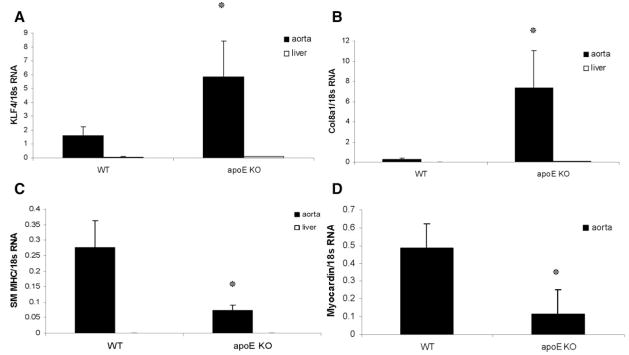
To determine whether Klf4 expression is correlated with Col8a1 gene expression in vivo, we compared the mRNA levels of Col8a1 and Klf4 in the thoracic aortas of WT mice and apoE KO mice after 13 weeks of Western diet feeding. Results of real-time RT-PCR demonstrated that endogenous mRNA levels of Col8a1 and Klf4 were increased in the thoracic aortas of apoE KO mice versus WT mice (Figure 8A and 8B), whereas mRNA levels of SM MHC and myocardin were decreased (Figure 8C and 8D). In contrast, no changes in expression of Col8a1 and Klf4 mRNA were seen in control liver tissue.
Figure 8.
Endogenous mRNA levels of Col8a1 and Klf4 were increased in the aortas of apoE KO after 13 weeks of Western diet vs WT mice. Total RNA was isolated from the thoracic aortas or livers as a control. Expression of Col8a1 (A), Klf4 (B), SM MHC (C), and myocardin (D) mRNA was measured by real-time RT-PCR. Values represent the means for 5 animals per group±SEM. *P<0.05 vs vehicle.
Discussion
In the present study, we tested the hypothesis that OxPLs modulate ECM gene expression in VSMCs. Consistent with this hypothesis, results of real-time RT-PCR analyses of the candidate genes and gene microarray studies showed that POVPC altered expression of a number of ECM or ECM-related genes, including multiple collagens, fibronectin 3, laminin μ2, integrins, and the proteoglycan versican. We also demonstrated that OxPLs, and in particular POVPC, markedly upregulated type VIII collagen expression in cultured VSMCs, as well as in vivo within carotid arteries.
Previous studies have shown that type VIII collagen expression is dramatically increased within VSMCs in response to vascular injury,24,25 as well as in atherosclerotic plaques of the apoE KO mice.13 Type VIII collagen has been shown to stimulate attachment, focal adhesion formation, and chemotaxis of cultured SMC, as well as to modify matrix metalloproteinase synthesis.23 Furthermore, it has been previously shown that VSMCs from type VIII collagen–deficient mice exhibit greater adhesion to type I collagen than WT VSMCs. In contrast, WT VSMCs spread more, migrate further, exhibit increased proliferation, and express higher level of matrix metalloproteinase 2 in comparison with type VIII collagen–deficient VSMCs,22 suggesting that the de novo production of type VIII collagen allows VCMCs to overcome adhesion to type I collagen. A number of factors have been shown to modulate type VIII collagen expression in cultured VSMCs including platelet-derived growth factor-BB,24,25 basic fibroblast growth factor-2,26 angiotensin II,26 transforming growth factor β,25,27 and colony-stimulating factor,27 which increase expression, and interferon γ,25 which decreases expression. However, virtually nothing is known regarding factors that regulate the expression of type VIII collagen or other ECM proteins by VSMCs within atherosclerotic lesions.
Of particular relevance to the present studies, previous studies by Plenz et al28 showed reduced type VIII collagen expression in the media and adventitia, but increased expression in the intima, of carotid arteries in rabbits fed a high-cholesterol diet. However, no direct evidence was presented that these effects were mediated by OxPLs. Our present results clearly demonstrate that OxPAPC, as well as its component phospholipids PGPC, PEIPC, and POVPC, significantly increase type VIII collagen in vitro. Of major significance, we also showed that POVPC increased type VIII collagen expression within carotid arteries in vivo. Taken together, results suggest that the oxidized arachidonoyl phospholipids may be somewhat unique among modified lipids in inducing expression of type VIII collagen and thus implicate these factors as potential mediators of increased type VIII collagen expression in atherosclerotic lesions. A key unresolved question is whether POVPC or other OxPLs directly mediate SMC phenotypic switching in vivo including inducing alterations in expression of type VIII collagen and other ECM components. However, such experiments are not feasible at present for several reasons including: (1) OxPL receptors have not been identified for precluding use of either pharmacological or genetic loss-of-function approaches; (2) there is a lack of highly specific and efficacious inhibitors to block formation of specific OxPL species; and (3) although there are a number of neutralizing antibodies to specific OxPL species,29 it is not clear these can maintain efficacious inhibition in vascular lesions over long periods of time in vivo, and, of course, such approaches would not be selective in inhibiting OxPL responses within VSMCs.
Results of the present study also provide novel insights regarding mechanisms that regulate type VIII collagen expression, in that we show that effects of POVPC are dependent on Sp1-induced activation of Klf4. Klf4 is a member of the Krüppel family of transcription factors.30 Studies in our laboratory have shown that Klf4 expression is normally undetectable in SMC but is rapidly increased in vivo following vascular injury.31,32 Moreover, it was demonstrated that the platelet-derived growth factor-BB– and POVPC-induced downregulation of SMC marker genes is mediated, in part, by Klf4.7,31 In addition, our present results demonstrated that Klf4 and Col8a1 expression are increased in the aortas of apoE KO mice after 13 weeks of Western diet feeding. Finally, results of recent studies in our laboratory showed that conditional knockout of Klf4 in adult mice resulted in a transient delay in downregulation of SMC marker genes, but subsequently enhanced neointimal formation following ligation induced injury of the carotid artery.32 That is, Klf4 appears to be a key rate-limiting factor for initial phenotypic switching of SMC in response to vascular injury but also plays a key role in negative regulation of SMC growth. We found that Klf4 is required for POVPC-induced Col8a1 expression in vivo and in vitro, as well as LAMA2 expression in vitro. Based on the results of the present studies, it is interesting to speculate that Klf4 might also be a key regulator of ECM expression in VSMCs during atherogenesis.
Of interest, although we demonstrated that Sp1 was required for POVPC-induced increases in Klf4 expression, we did not find a significant increase in Sp1 expression or synthesis after POVPC treatment (OA Cherepanova, GK Owens, unpublished data, 2008), suggesting that effects might involve posttranscriptional modification of Sp1 and/or its ability to bind to the Klf4 promoter. Consistent with this possibility, Sp1 has been shown to undergo various posttranscriptional modifications including phosphorylation and O-glycosylation (reviewed by Black et al33) that increase its transcriptional activity, at least in part, by enhancing its binding to DNA.
Previously, we reported that POVPC treatment of cultured rat aortic SMC resulted in enhanced repair in a scratch wound assay.7 Results of the present studies showed that POVPC and PEIPC, but not PGPC, enhanced VSMC migration. Consistent with these observations, previous studies have shown differential effects of POVPC versus PGPC on monocyte and neutrophil binding to endothelium.15,34 Moreover, recent voltage-clamp studies by Leitinger et al34 suggested that POVPC and PGPC recognized different receptors based on mRNA expression studies in Xenopus oocytes. Taken together, results suggest that the stoichiometry of specific OxPLs within lesions may have important functional consequences through differential effects on VSMCs, as well as other vascular cells.
Previous studies by Rocnik et al35 suggested that new collagen synthesis is required for VSMC migration on type I collagen–covered substrata. Results of the present studies showing that POVPC-induced migration of cultured rat aortic SMC was inhibited by siRNA suppression of type VIII collagen and failed to occur in type VIII KO mouse aortic SMC provide evidence that de novo synthesis of type VIII collagen plays an important role in mediating VSMC migration in vitro. Moreover, we also showed that POVPC-induced VSMC migration was dependent on Klf4. These findings are highly intriguing in that they raise the question as to whether Klf4-dependent SMC migration is mediated exclusively through induction of type VIII collagen or whether Klf4 also activates additional signaling pathways important in this response.
Interestingly, we found that POVPC did not induce and actually inhibited VSMC migration at concentrations of >5 μg/mL. From our recent studies,7 we know that this concentration is not toxic for VSMCs. As such, it is possible that activation of additional molecular pathways might be responsible for the inhibition of VSMC migration by the higher concentrations of POVPC.
In conclusion, we provide novel evidence showing that OxPLs activate coordinate expression of a variety of ECM genes in VSMCs including type VIII collagen and that activation of type VIII collagen is dependent on both Klf4 and Sp1. Moreover, we show that increased type VIII collagen expression is required for POVPC-induced migration of VSMCs, indicating that activation of ECM genes has important functional consequences. Future studies are needed to define specific receptors and downstream effector molecules that mediate the effects of specific OxPLs in vivo.
Supplementary Material
Acknowledgments
We thank Rupande Tripathi, Dominique Rose, Diane Raines, and Mary McCanna for expert technical assistance.
Sources of Funding
This work was supported by NIH grants P01HL19242, R01 HL38864, and R01 HL87867 (to G.K.O.); R01 HL064731 (to J.B.); R01 HL084422-01 (to N.L.); Canadian Institutes of Health Research grant MOP81305 (to M.P.B.); and American Heart Association Fellowship Grant 0725373U (to O.A.C.).
Footnotes
Disclosures
None.
References
- 1.Berliner J, Leitinger N, Watson A, Huber J, Fogelman A, Navab M. Oxidized lipids in atherogenesis: Formation, destruction and action. Thromb Haemost. 1997;78:195–199. [PubMed] [Google Scholar]
- 2.Chatterjee S. Role of oxidized human plasma low-density lipoproteins in atherosclerosis - effects on smooth-muscle cell-proliferation. Mol Cell Biochem. 1992;111:143–147. doi: 10.1007/BF00229586. [DOI] [PubMed] [Google Scholar]
- 3.Furnkranz A, Schober A, Bochkov VN, Bashtrykov P, Kronke G, Kadl A, Binder BR, Weber C, Leitinger N. Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler Tromb Vasc Biol. 2005;25:633–638. doi: 10.1161/01.ATV.0000153106.03644.a0. [DOI] [PubMed] [Google Scholar]
- 4.Subbanagounder G, Leitinger N, Schwenke DC, Wong JW, Lee H, Rizza C, Watson A, Faull K, Fogelman A, Berliner J. Determinants of bioactivity of oxidized phospholipids: specific oxidized fatty acyl groups at the sn-2 position. Arterioscler Tromb Vasc Biol. 2000;20:2248–2254. doi: 10.1161/01.atv.20.10.2248. [DOI] [PubMed] [Google Scholar]
- 5.Watson AD, Leitinger N, Navab M, Faull KF, Horkko S, Witztum JL, Palinski W, Schwenke D, Salomon RG, Sha W, Subbanagounder G, Fogelman AM, Berliner JA. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 6.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 7.Pidkovka N, Cherepanova OA, Yoshida T, Alexander M, Deaton RA, Thomas J, Leitinger N, Owens G. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res. 2007;101:792–801. doi: 10.1161/CIRCRESAHA.107.152736. [DOI] [PubMed] [Google Scholar]
- 8.Heeneman S, Cleutjens JP, Faber BC, Creemers EE, van Suylen RJ, Lutgens E, Cleutjens KB, Daemen MJ. The dynamic extracellular matrix: intervention strategies during heart failure and atherosclerosis. J Pathol. 2003;200:516–525. doi: 10.1002/path.1395. [DOI] [PubMed] [Google Scholar]
- 9.Stegemann JP, Hong H, Nerem RM. Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. J Appl Physiol. 2005;98:2321–2327. doi: 10.1152/japplphysiol.01114.2004. [DOI] [PubMed] [Google Scholar]
- 10.Bauters C, Marotte F, Hamon M, Oliviero P, Farhadian F, Robert V, Samuel JL, Rappaport L. Accumulation of fetal fibronectin messenger-RNAs after balloon denudation of rabbit arteries. Circulation. 1995;92:904–911. doi: 10.1161/01.cir.92.4.904. [DOI] [PubMed] [Google Scholar]
- 11.Glukhova MA, Frid MG, Shekhonin BV, Vasilevskaya TD, Grunwald J, Saginati M, Koteliansky VE. Expression of extra domain-A fibronectin sequence in vascular smooth-muscle cells is phenotype dependent. J Cell Biol. 1989;109:357–366. doi: 10.1083/jcb.109.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plenz G, Dorszewski A, Breithardt G, Robenek H. Expression of type VIII collagen after cholesterol diet and injury in the rabbit model of atherosclerosis. Arterioscler Tromb Vasc Biol. 1999;19:1201–1209. doi: 10.1161/01.atv.19.5.1201. [DOI] [PubMed] [Google Scholar]
- 13.Yasuda O, Zhang SH, Miyamoto Y, Maeda N. Differential expression of the alpha 1 type VIII collagen gene by smooth muscle cells from atherosclerotic plaques of apolipoprotein-E-deficient mice. J Vasc Res. 2000;37:158 –169. doi: 10.1159/000025727. [DOI] [PubMed] [Google Scholar]
- 14.Jimi S, Saku K, Uesugi N, Sakata N, Takebayashi S. Oxidized low-density-lipoprotein stimulates collagen production in cultured arterial smooth-muscle cells. Atherosclerosis. 1995;116:15–26. doi: 10.1016/0021-9150(95)05515-x. [DOI] [PubMed] [Google Scholar]
- 15.Cole AL, Subbanagounder G, Mukhopadhyay S, Berliner JA, Vora DK. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler Tromb Vasc Biol. 2003;23:1384 –1390. doi: 10.1161/01.ATV.0000081215.45714.71. [DOI] [PubMed] [Google Scholar]
- 16.Korsching E, Rauterberg J. Generation of type VIII collagen-specific antibodies. J Immunol Methods. 1995;188:51– 62. doi: 10.1016/0022-1759(95)00201-4. [DOI] [PubMed] [Google Scholar]
- 17.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 18.Sage H, Balian G, Vogel AM, Bornstein P. Type VIII collagen -synthesis by normal and malignant cells in culture. Lab Invest. 1984;50:219–231. [PubMed] [Google Scholar]
- 19.Shields JM, Yang VM. Identification of the DNA sequence that interacts with the gut-enriched Kruppel-like factor. Nucleic Acids Res. 1998;26:796 –802. doi: 10.1093/nar/26.3.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen ZY, Rex S, Tseng CC. Kruppel-like factor 4 is transactivated by butyrate in colon cancer cells. J Nutr. 2004;134:792–798. doi: 10.1093/jn/134.4.792. [DOI] [PubMed] [Google Scholar]
- 22.Adiguzel E, Hou GP, Mulholland D, Hopfer U, Fukai N, Olsen B, Bendeck MP. Migration and growth are attenuated in vascular smooth muscle cells with type VIII collagen-null allels. Arterioscler Tromb Vasc Biol. 2006;26:56–61. doi: 10.1161/01.ATV.0000194155.96456.b7. [DOI] [PubMed] [Google Scholar]
- 23.Hou GP, Mulholland D, Gronska MA, Bendeck MP. Type VIII collagen stimulates smooth muscle cell migration and matrix metalloproteinase synthesis after arterial injury. Am J Pathol. 2000;156:467– 476. doi: 10.1016/S0002-9440(10)64751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendeck MP, Regenass S, Tom WD, Giachelli CM, Schwartz SM, Hart C, Reidy MA. Differential expression of alpha(1) type VIII collagen in injured platelet-derived growth factor-BB-stimulated rat carotid arteries. Circ Res. 1996;79:524–531. doi: 10.1161/01.res.79.3.524. [DOI] [PubMed] [Google Scholar]
- 25.Sibinga NES, Foster LC, Hsieh CM, Perrella MA, Lee WS, Endege WO, Sage EH, Lee ME, Haber E. Collagen VIII is expressed by vascular smooth muscle cells in response to vascular injury. Circ Res. 1997;80:532–541. doi: 10.1161/01.res.80.4.532. [DOI] [PubMed] [Google Scholar]
- 26.Plenz GAM, Deng MC, Robenek H, Volker W. Vascular collagens: spotlight on the role of type VIII collagen in atherogenesis. Atherosclerosis. 2003;166:1–11. doi: 10.1016/s0021-9150(01)00766-3. [DOI] [PubMed] [Google Scholar]
- 27.Plenz G, Koenig C, Reichenberg S, Robenek H. Colony stimulating factors modulate the transcription of type VIII collagen in vascular smooth muscle cells. Atherosclerosis. 1999;144:25–32. doi: 10.1016/s0021-9150(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 28.Plenz G, Dorszewski A, Volker W, Ko YS, Severs NJ, Breithardt G, Robenek H. Cholesterol-induced changes of type VIII collagen expression and distribution in carotid arteries of rabbit. Arterioscler Tromb Vasc Biol. 1999;19:2395–2404. doi: 10.1161/01.atv.19.10.2395. [DOI] [PubMed] [Google Scholar]
- 29.Gounopoulos P, Merki E, Hansen LF, Choi SH, Tsimikas S. Antibodies to oxidized low density lipoprotein: epidemiological studies and potential clinical applications in cardiovascular disease. Minerva Cardioangiol. 2007;55:821–837. [PubMed] [Google Scholar]
- 30.Adam PJ, Regan CP, Hautmann MB, Owens GK. Positive- and negative-acting Kruppel-like transcription factors bind a transforming growth factor beta control element required for expression of the smooth muscle cell differentiation marker SM22 alpha in vivo. J Biol Chem. 2000;275:37798–37806. doi: 10.1074/jbc.M006323200. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens KG. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005;280:9719 –9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008;102:1548 –1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 34.Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, Berliner JA, Vora DK. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci U S A. 1999;96:12010–12015. doi: 10.1073/pnas.96.21.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rocnik EF, Chan BMC, Pickering JG. Evidence for a role of collagen synthesis in arterial smooth muscle cell migration. J Clin Invest. 1998;101:1889–1898. doi: 10.1172/JCI1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.