Abstract
Stearoyl-coA desaturase 1 (SCD1) is the rate-limiting enzyme involved in the synthesis of monounsaturated fatty acids, and in mice SCD1 activity is associated with plasma triglyceride levels.
Objective
We used the fatty acid desaturation index (the plasma ratio of 18:1/18:0), as a marker of SCD1 activity to investigate the relationship of SCD1 to familial combined hyperlipidemia (FCHL).
Methods and Results
The fatty acid desaturation index was measured in 400 individuals from 18 extended FCHL pedigrees. FCHL-affected individuals exhibited increased SCD1 activity when compared to unrelated controls (P<0.0001). The fatty acid desaturation index was found to be highly heritable (h2 = 0.48, p= 2.2 × 10−11) in this study sample. QTL analysis in 346 sibling pairs from 18 FCHL families revealed suggestive linkage of the desaturation index to chromosomes 3p26.1-3p13 (z=2.7, P=0.003), containing the peroxisome proliferator-activated receptor gamma (PPARγ) gene, and 20p11.21-20q13.32 (z=1.7, P=0.04), containing the hepatocyte nuclear factor 4, alpha (HNF4α) gene. A specific haplotype of HNF4α was found to be associated with the desaturation index in these FCHL families (P=0.002).
Conclusion
Our results demonstrate that the fatty acid desaturation index is a highly heritable trait that is associated with the dyslipidemia observed in FCHL.
Keywords: familial combined hyperlipidemia, genetics, Stearoyl-coA desaturase 1, peroxisome proliferator-activated receptor gamma, hepatocyte nuclear factor 4 alpha
Stearoyl-CoA desaturase (SCD) is the major enzyme which catalyzes the conversion of saturated fatty acids to monounsaturated fatty acids. The primary products of SCD-1, palmitate and oleate, are the most abundant monounsaturated fatty acids of phospholipids, triglycerides, wax esters, and cholesterol esters 1. Mice homozygous for either a naturally occurring mutation or a targeted disruption of the SCD1 gene exhibit a marked reduction in VLDL triglycerides 2. SCD1−/− mice on a leptin-deficient ob/ob background exhibit reduced adiposity and increased fatty acid oxidation when compared to control animals, demonstrating a key role for SCD1 in the regulation of lipid homeostasis 2.
Familial combined hyperlipidemia (FCHL) is characterized by elevated plasma triglyceride and/or cholesterol levels 3. FCHL often occurs in combination with insulin resistance, central adiposity, altered fatty acid metabolism, and other dyslipidemic phenotypes that predispose to early coronary artery disease (CAD) 4,5. The observed role of SCD1 in the regulation of VLDL metabolism and its involvement in many of the lipid parameters that are perturbed in FCHL suggest that the activity of this enzyme may be altered in FCHL subjects. In fact, the desaturation index was strongly correlated with both triglyceride and HDL levels in a human cohort of which approximately 60% of the subjects had FCHL 6. In mice, studies of SCD activity in the HYPLIP mouse model of hyperlipidemia showed that hepatic SCD activity was increased 4-fold when compared to control animals 6. Taken together these data suggest that SCD1 may contribute to triglyceride levels in FCHL individuals. We analyzed the desaturation index of 400 family members from 18 extended FCHL pedigrees, and performed a genome scan to identify chromosomal regions that may harbor genes that contribute to variation in SCD1 activity.
Methods
Eighteen extended FCHL families of Dutch Caucasian descent were ascertained through probands recruited from the Lipid Clinic of the Utrecht Academic University Hospital, as previously described 7. The probands met the following criteria: (1) a primary combined hyperlipidemia with a fasting plasma cholesterol >6.5 mmol/L, or >90th percentile for age, defined according to tables from the Lipid Research Clinics, and fasting plasma triglycerides >2.3 mmol/L; (2) at least one first degree relative with a different hyperlipidemic phenotype; and (3) a positive family history of premature CAD defined as a myocardial infarction or cardiovascular disease before 60 years of age. Exclusion criteria for the probands included diabetes, obesity (BMI >30), tendon xanthomas, or type III hyperlipidemia (apoE2/E2). None of the subjects included in the study used exogenous insulin or hypoglycemic agents. All subjects gave informed consent, and the study protocol was approved by the Human Investigation Review Committees of Utrecht University Hospital, the Netherlands, and the University of California, Los Angeles.
Genetic Statistical Analyses
To assess the correlation of 18:1/18:0 with 16:1/16:0, Pearson’s correlation was assessed using the R statistical package (http://www.r-project.org/). All traits were transformed to normality using the inverse normal transformation option of SOLAR 4.07 8. The effect of sex, age, sex by age interaction, triglycerides, total cholesterol, LDL-C, HDL-C, apoB, FFA, glucose, and BMI were tested as covariates of the desaturation index. Potential predictors of the desaturation index were considered significant if the regression coefficient reached P<0.05. The contribution of genetic factors to variation in the desaturation index was estimated by calculating the heritability (h2) using the SOLAR software 8. Significance of h2 values was assessed by a likelihood ratio test, in which the likelihood of the model with an additive genetic variance component and covariates was compared with the likelihood model in which the additive genetic variance component was constrained to be 0. However, h2 may be overestimated because only known covariates are entered into the model and unknown environmental effects are not included in this analysis. ANOVA was used to assess the differences in each FCHL characteristic between affected and unaffected relatives after adjustment for age, sex, and family membership where appropriate using the PROC GLM option of SAS v9.1 9. Trait differences were considered significant if the value for this analysis was P<0.05. The QTL analysis of the desaturation index trait was conducted using the nonparametric linkage statistic of the Genehunter 2.1 software10. There were 346 sibling pairs included in this analysis.
Genetic association of the desaturation index was tested using the family-based association test (FBAT) program and the haplotype-based association test (HBAT) program 11. We used the "–o" option of HBAT to assess quantitative association of haplotypes with the desaturation index trait. In order to correct for multiple testing, the "–p" option of HBAT was also used, which performs the Monte-Carlo permutation procedure. The PedCheck program was used to assess pedigree inconsistencies12. Pairwise LD was examined using the JLIN program 13.
Results
We measured the desaturation index, the ratio of plasma 18:1 / 18:0 and 16:1 /16:0, in 39 FCHL probands and 70 normolipidemic control individuals and in 400 family members from 18 extended pedigrees for whom plasma samples were available. The results and relevant clinical characteristics for these study samples are listed in Table 1. The mean 16:1/16:0 and 18:1/18:0 desaturation indices in FCHL probands was significantly higher than that of normolipidemic spouse control individuals (0.134 ± 0.060 vs. 0.101 ± 0.030 for 16:1/16:0, P<0.003, and 3.95 ± 1.08 vs. 2.93 ± 0.882 for 18:1/18:0, P≤0.0001, in probands (n=39) and spouses (n=70), respectively). A similar increase in desaturation indices was also observed in hyperlipidemic relatives when compared to spouse controls (Table 1).
Table 1.
Clinical Characteristics of the 18 FCHL families
| Trait | Hyperlipidemic Individuals† (n=189) | Normolipidemic Individuals (n=226) | Spouses (n=181) | P-value HL vs.NL | P-value HL vs. S |
|---|---|---|---|---|---|
| Male/Female, n | 107/82 | 105/121 | 76/105 | 0.05 | 0.01 |
| Age, y | 46 ± 16 | 32 ± 13 | 46 ± 15 | <0.0001 | NS |
| Body mass index (BMI), kg/m2 | 26.3 ± 3.53 | 23.3 ± 3.25 | 25.2 ± 3.83 | <0.0001 | <0.0001 |
| Triglycerides, mmol/L | 3.74 ± 7.49 | 1.25 ± 0.416 | 1.64 ± 1.09 | <0.0001 | <0.0001 |
| Total cholesterol, mmol/L | 7.51 ± 2.53 | 4.92 ± 0.812 | 5.66 ± 1.09 | <0.0001 | <0.0001 |
| LDL-C, mmol/L | 4.74 ± 1.32 | 3.11 ± 0.728 | 3.70 ± 1.01 | <0.0001 | <0.0001 |
| HDL-C, mmol/L | 1.13 ± 0.328 | 1.24 ± 0.311 | 1.25 ± 0.346 | 0.003 | 0.013 |
| Desaturation Index† | |||||
| 18:1/18:0 | 3.40 ± 0.835 | 3.11 ± 0.728 | 3.02 ± 0.845 | 0.02 | <0.0001 |
| 16:1/16:0 | 0.116 ± 0.043 | 0.098 ± 0.003 | 0.102 ± 0.029 | 0.001 | 0.0001 |
Non-transformed trait values are expressed as mean ± SD.
Includes 18 probands and 171 hyperlipidemic relatives
The desaturation index was measured in 148 hyperlipidemic individuals, 173 normolipidemics, and 79 spouses.
As expected, there was strong correlation between the 18:1/18:0 and 16:1/16:0 values for the 400 FCHL family members (Pearson correlation = 0.53, p=2.2×10−16), therefore for all subsequent analyses we used the 18:1/18:0 molar ratio to define the desaturation index. To assess whether the desaturation index is quantitatively correlated with total cholesterol, triglycerides, HDL-C, as well as other metabolic parameters, we conducted regression analyses in order to identify covariates that are significantly correlated with the desaturation index in these pedigrees. Both triglyceride (β = 0.34, P=2.0 × 10−9) and to a lesser extent, HDLC (β = −0.19, P=0.0005) levels were significant predictors of the desaturation index. Although the desaturation index has been associated with measures of body adiposity in other studies 14–18, BMI was not a significant predictor of the desaturation index in these FCHL families (β = −0.002, P=0.97). This finding could be due to the strong correlation of the desaturation index with plasma triglycerides in this population such that it obscures the relationship of SCD1 activity with BMI. We tested this hypothesis by testing the triglyceride- and HDLC-adjusted desaturation index residuals for correlation with BMI, however BMI was still not a significant predictor of the desaturation index (β =−0.001, P=0.79) in these families.
In order to estimate the heritability of the desaturation index, we used variance components analysis in all 400 individuals that were measured for the desaturation index. Unadjusted heritability was estimated to be 0.47 (P= 1.0 × 10−9). After adjustment for sex (β=0.18,P=0.05), age (β=−0.004, P=0.2), triglyceride (β=0.34, P= 2.0×10−9) and HDL-C (β = −0.19, P= 0.0005) levels, the estimated heritability (h2) of the desaturation index increased slightly to h2=0.48 (P=2.2 × 10−11), indicating that the desaturation index is a highly heritable trait in this FCHL cohort even after adjustment for covariates.
Given the evidence that genetic factors contribute to the desaturation index, we performed quantitative nonparametric sibpair analysis to identify regions of the genome that may harbor genes that contribute to variation in the desaturation index. We performed the analysis using both age- and sex- adjusted and unadjusted desaturation index values. No evidence of genome-wide significant linkage of the desaturation index was obtained in this nonparametric genome scan. We did, however, obtain suggestive evidence for linkage to chromosome 3p26.1-3p13 (z=2.7, P=0.003), 7p22.2-p15.3 (z=2.1, P=0.02), and 20p11.21-20q13.32 (z=1.7, P=0.04). All regions exhibiting a z-score >1.64 (P≤0.05) are shown in Table 2 and Figure 1. Two of these regions, 3p26.1-3p13 and 20p11.21-20q13.32 are located near genes which have been previously associated with either free fatty acid or triglyceride levels in FCHL cohorts 19,20. No evidence for linkage was obtained for the region containing the SCD1 gene on chromosome 10 (Figure 1). Additional adjustment of the desaturation index for triglyceride and BMI levels did not significantly alter the results (Figure 1). Linkage of each region to 16:1/16:0 was consistent with the linkage results of 18:1/18:0, although the overall linkage scores were lower for 16:1/16:0.
Table 2.
Chromosomal Locations of Desaturation Index QTL in 18 FCHL Pedigrees
| Band | Location of Peak* | Peak Z-score | P-value |
|---|---|---|---|
| 3p26.1-3p13 | 76 | 2.7 (2.1) | 0.003 (0.02) |
| 7p22.2-7p15.3 | pter | 1.9 (2.0) | 0.03 (0.02) |
| 7p14.3-q32.3 | 49 | 1.6 (1.9) | 0.02 (0.03) |
| 17p13.3-q21.32 | 14 | 2.1 (1.2) | 0.03 (0.1) |
| 20p11.21-20q13.32 | 69 | 1.7 (1.8) | 0.04 (0.04) |
Z-scores and P-values for age- and sex-adjusted (bold) and for age-, sex-, tg-, and hdlc-( ) adjusted desaturation index trait.
From the p-terminus in centiMorgans
Figure 1.
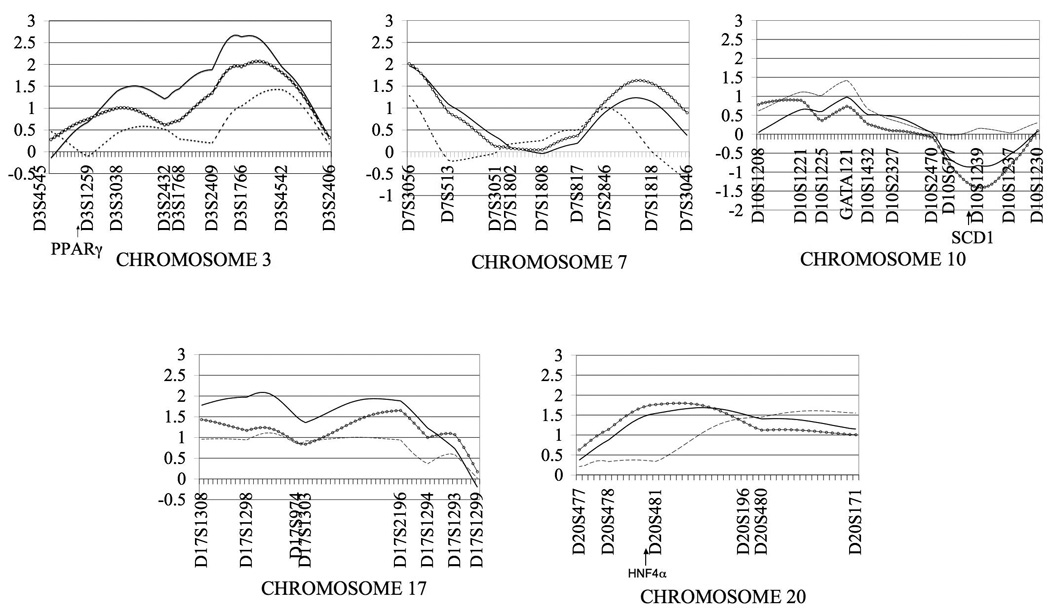
Non-parametric z-scores > 1.64 (p<0.05) obtained for age- and sex- adjusted (solid black line), age- , sex-, tg-, and hdlc- adjusted (open circles) 18:1/18:0 desaturation index values and for 16:1/16:0 (dashed lines) in 18 extended FCHL families. Z-scores for the SCD1 gene locus (Chromosome 10) is also shown.
The strongest evidence for linkage was located on chromosome 3 near the PPARγ gene (z=2.7, P=0.003). The empirical p-value for this linkage region was P=0.01, indicating that there is a 1% chance that the linkage of the desaturation index to this region is a false positive. We identified tagging SNPs in the 148.5 kb genomic region containing the PPARγ gene (+/− 1kb) using HAPMAP data from 30 CEPH trios (Figure 1). Seven tagging SNPs and two coding SNPs (rs1801282, also known as the Pro12Ala polymorphism and rs3856806, also known as C161T), which had previously been studied in our case-control set, were genotyped in our FCHL families 19. These nine SNPs span a total of 141 kb. Pairwise linkage disequilibrium (LD) was similar between FCHL probands and normolipidemic spouses for al SNPs tested (Figure SI). The D’ statistic suggests that there is strong LD among all 9 of the PPARg SNPs in both probands and spouses, whereas the r2 statistics suggest that there is little LD in the region. Since the D’ statistics are not dependent on allele frequencies, the measures of LD indicated by the D’ statistic may be inflated given the small size of our case-control cohort.
We observed only modest association of the desaturation index with four SNPs (rs2972164, rs10510418, rs2938395, rs709157) out of the nine that were genotyped (Table SI, P<0.05), although these results do not remain significant after correction for multiple testing. We tested for haplotype association using the four moderately associated SNPs (P≤0.05 before correction for multiple testing). We observed no significant association of the four-SNP haplotype; however, haplotypes comprised of three SNPs (rs2972164, rs10510418, and rs2938395) defined both a risk and a protective haplotype for the desaturation index trait (Table 3). The most significant association was observed for haplotypes composed of two of these SNPs rs2972164 and rs2938395. Under a dominant model, we observed marginal evidence of association of the T-T haplotype of rs2972164 and rs2938395 with the desaturation index (Table 3, P=0.02), and under an additive model, the C-C haplotype of rs2972164 and rs2938395 was also marginally associated with the desaturation index (P=0.01). The effect of each haplotype can be interpreted by evaluating the sign of the FBAT statistic. For the T-T haplotype, the sign of the FBAT statistic was positive, suggesting that the effect of the haplotype is to raise the desaturation index value. We have therefore designated this haplotype as the risk haplotype. Similarly, the negative FBAT statistic obtained for the C-C haplotype indicates that the effect of the haplotype is to decrease the desaturation index value, and it is therefore designated as a protective haplotype. Although the association of PPARγ with the desaturation index is only weakly significant, linkage analysis of families which contribute to the association signal revealed an increase in the linkage signal to 3.15, suggesting that subsetting families on the basis of PPARg haplotypes strengthens the linkage signal at the chromosome 3 locus. These data suggest that PPARγ haplotypes may contribute to the variation in desaturation indices in FCHL families; however confirmation of these findings in an independent population is warranted.
Table 3.
Haplotype Association of PPARγ and HNF4α with desaturation index in FCHL families.
| PPARγ | ||||
|---|---|---|---|---|
| Markers | Implicated Haplotype | Freq. | P-value* | P-value† |
| rs2972164 - rs10510418 - rs2938395- rs709157 | C-A-C-A | 0.476 | 0.09 | 0.07 |
| ‡rs2972164 - rs10510418 - rs2938395 | T-C-T (risk) | 0.254 | 0.04 | 0.04 |
| §rs2972164 - rs10510418 - rs2938395 | C-A-C (protective) | 0.480 | 0.03 | 0.03 |
| ‡rs2972164- rs2938395 | T-T (risk) | 0.258 | 0.01 | 0.01 |
| §rs2972164- rs2938395 | C-C (protective) | 0.477 | 0.03 | 0.03 |
| HNF4α | ||||
| §rs6031558- rs745975-rs3212198 | G-G-A (risk) | 0.338 | 0.01 | 0.008 |
| §rs6031558- rs745975 | G-G (risk) | 0.463 | 0.002 | 0.002 |
P-value listed is for the -o option of HBAT
P-value listed is for the -p option of HBAT (see Methods)
Dominant Model
Additive Model
We also observed suggestive evidence of linkage of the desaturation index to chromosome 20p11.21-20q13.32 (p= 0.01), a region previously linked to triglycerides and HDL-C in Finnish FCHL and low-HDL families respectively 21,22. The chromosome 20 linkage peak is located directly over the HNF4α gene which has been recently associated with elevated serum lipids in Finnish and Mexican FCHL families 20. We genotyped five SNPs (rs2144908, rs6031558, rs2425640, rs745975, rs3212198) that were associated individually or as haplotypes with triglycerides in the Finnish FCHL cohort and with glucose levels in the Mexican FCHL cohort, in our extended FCHL pedigrees (Table SII). Pairwise LD patterns for the five SNPs tested did not differ from those obtained by Weisglas-Volkov et al. (Figure S1) 20. We observed only marginal association of rs3212198 with the desaturation index in our FCHL families (P=0.03), which does not remain significant after correction for multiple testing. In the study by Weissglas-Volkov et al., a risk haplotype comprised of rs6031558, rs745975, and rs3212198 was significantly associated with serum triglycerides in Finnish and Mexican FCHL families. We tested for association of this haplotype with the desaturation index in our FCHL cohort. We observed quantitative association of the G-G-A haplotype of rs6031558 - rs745975 - rs3212198 with the desaturation index (P=0.01, Table 3) in our FCHL families, although most of the association signal seems to come from rs6031558 - rs745975 (P=0.002). The G-G-A haplotype is the same risk haplotype that was found to be associated with triglycerides in Finnish and Mexican FCHL families20, supporting the hypothesis that specific HNF4α haplotypes contribute to the development of the FCHL phenotype. Subset analysis of the HNF4a associated families revealed that these families provide no evidence for linkage to the peak marker D20S481, however, the linkage signal in the non-associated families remains and even increases to 1.9. These data suggest that although HNF4a haplotypes are associated with the desaturation index, this association does not account for the linkage of the desaturation index to this chromosome 20 region.
Discussion
FCHL individuals present with elevated plasma triglyceride and cholesterol levels, often in conjunction with insulin resistance and altered fatty acid metabolism 23. Identification of biochemical markers of FCHL may help unravel the genetic complexities which underlie the disease 5. Given its central role in the regulation of lipid homeostasis, we examined the role of SCD1 to FCHL and evaluated its correlation with the FCHL phenotype. The results indicate that the desaturation index is heritable and is associated with the dyslipidemic profile of FCHL individuals. Additionally, we provide evidence that this association may be due, at least in part, to common variants HNF4α.
We observed correlations of SCD1 activity with plasma triglyceride and HDL-C levels. BMI was not a significant predictor of the desaturation index in this cohort, as has been reported in studies of metabolic syndrome and obesity 16,17 14,15. This finding could be due to the differences in ascertainment criteria for metabolic syndrome, obesity, and FCHL. Although BMI is increased in FCHL (the mean BMI of hyperlipidemics is 26.3 ± 3.53, Table 1), the hyperlipidemic individuals are not obese (defined as BMI>30). The strong correlation of the desaturation index with plasma triglycerides and HDL-C confirms the findings of Attie et al. who reported that the desaturation ratio accounted for more than 40% of the variation in plasma triglycerides, although in our FCHL families the desaturation index accounts for about 14% (P=1.7 × 10−14) of the variation in triglyceride levels 6.
In the present study, we demonstrate that variation in the desaturation index is highly heritable in FCHL families. We are the first to directly investigate whether or not SCD1 activity is a heritable trait in humans, although some evidence of the genetic determination of SCD1 in humans has been reported 14. In an expression array study of muscle from lean and obese humans, elevated SCD1 expression levels were elevated 3 fold in the muscle of obese subjects when compared to that of lean subjects. The increased SCD1 expression was retained in cultured primary skeletal myocytes obtained from obese and lean individuals, indicating that isolated myocytes cultured under identical environment and stimuli retain SCD1 expression differences in vitro 14. These data are consistent with the notion that SCD1 activity is a genetically driven trait.
The strongest evidence for linkage was obtained for a region on chromosome 3 near the PPARγ gene. PPARγ is a key regulator of lipogenic gene expression, and studies in humans and in tissue culture suggest that the SCD1 gene is up-regulated in response to PPARγ activation24–26. In two independent studies, PPARγ SNPs were marginally associated with free fatty acid and glycerol levels as well as decreased fasting insulin levels in FCHL probands 19,27. We identified two common PPARγ haplotypes, one risk and one protective haplotype, which were associated with the desaturation index, suggesting that common variants of PPARγ may contribute to variation in SCD1 activity, although the observed association was relatively weak. Replication of this finding is warranted.
We also observed linkage of the desaturation index to a locus on chromosome 20 which had previously been linked to triglycerides in FCHL families 28. HNF4α, a highly conserved nuclear receptor, is known to be activated and repressed by the binding of saturated and unsaturated fatty acids respectively, consistent with the hypothesis that HNF4a is involved in the regulation of gene expression by fatty acids29. The role of HNF4a in lipid and glucose homeostasis is highlighted by the identification of loss of function mutations in maturity-onset diabetes of the young (MODY1)30, and by the association of common alleles and haplotypes of HNF4a with T2DM 31 and FCHL20. We observed significant association of an HNF4α haplotype, comprised of SNPs rs6031558-rs745975-rs3212198 with the desaturation index, which was the same triglyceride-associated haplotype originally identified by Weissglas-Volkov et al. These findings suggest that the same genetic variants which contribute to elevated serum triglyceride levels in FCHL may also contribute to increased SCD1 activity.
A recent study by Warensjö et al., reported evidence of association of SCD1 SNPs with the desaturation index. We found no evidence for linkage of the desaturation index trait to the Chromosome 10 region in which the SCD1 gene resides. Since association is generally more powerful than linkage 32, it is possible that the effect of the SCD1 locus on the desaturation index may not be detectable by linkage.
FCHL is genetically complex and is most likely due to the action of genetic and environmental factors, as well as their interactions 23. Identification of new biochemical markers of FCHL could increase our understanding of the underlying mechanisms of the disease. The desaturation index is an attractive candidate marker for FCHL because of the demonstrated effects of SCD1 on VLDL production and fatty acid oxidation 33,34. SCD1 undergoes rapid turnover in response to a variety of nutritional and hormonal signals, and at the transcriptional level it is regulated by a number of factors including the sterol regulatory binding protein-1 (SREBP1) and peroxisome proliferator-activated receptor, alpha (PPARα) 35. Lack of SCD1 expression in mice reduces tissue lipid content and protects against both diet-induced and genetically-induced obesity 2. Recent studies demonstrate that the protection from adiposity is at least partially due to increased fatty acid oxidation in the liver and muscle 34,36. These studies suggests that increased SCD1 activity in humans could result in increased rates of triglyceride synthesis, and decreased rates of fatty acid oxidation. Such metabolic perturbations could lead to increased deposition of lipids in peripheral tissues leading to insulin resistance in the muscle and to steatosis in the liver. Altered fatty acid metabolism, insulin resistance, and hepatic steatosis often occur in combination with FCHL, and SCD1 may contribute to the development of these phenotypes 37–39. Therefore, identification of genes which modulate SCD1 activity in humans could provide new clues to the altered metabolic pathways involved in FCHL and its overlapping syndromes. Our results demonstrate that the desaturation index is significantly associated with FCHL and furthermore suggest that the dyslipidemia observed in FCHL families may be partially due to genetic variations which affect SCD1 activity.
Figure 2.
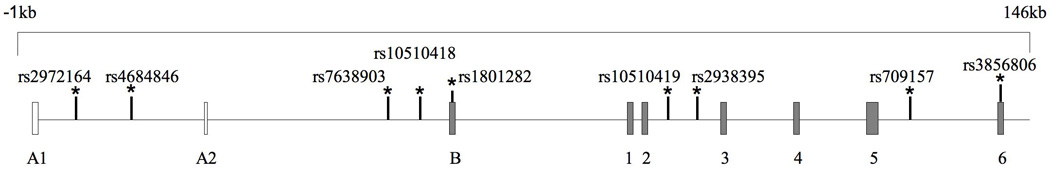
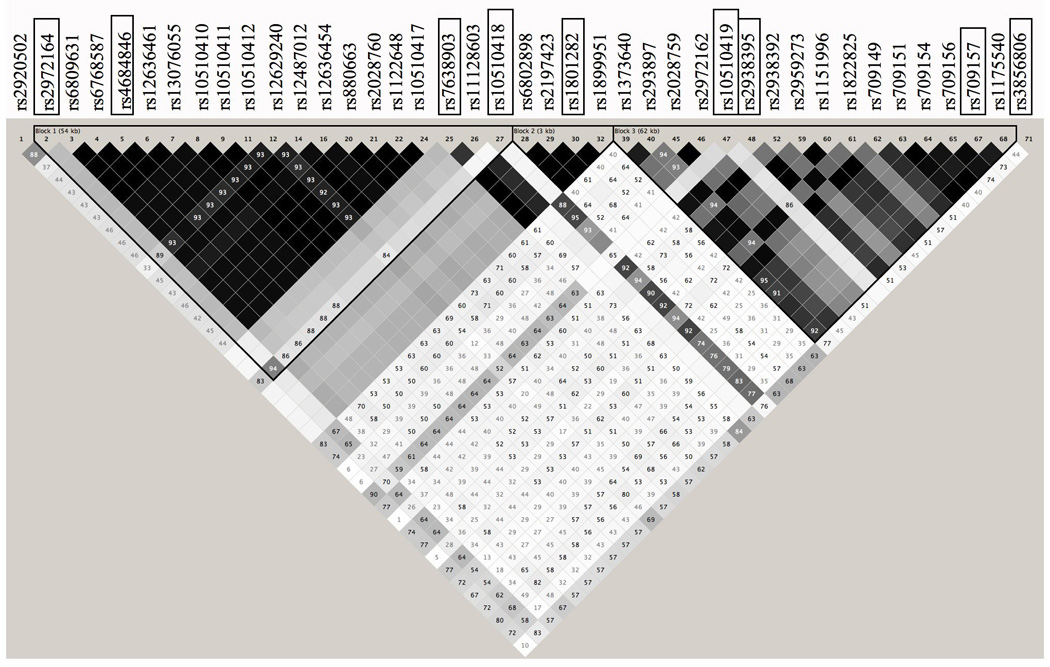
Genomic Structure and Linkage Disequilibrium Map of PPARγ. A) Positions of genotyped SNPs selected for analysis. B) The PPARγ gene +/− 1kb in 30 CEPH trios using the HAPMAP data (release no. 16c.1/phaseI, June 2005). Linkage disequilibrium is displayed using the Haploview Program v. 3.2 (available at www.broad.mit.edu/mpg/haploview/). Genotyped SNPs are boxed. D' values are shown.
Acknowledgements
We would like to thank the patients and relatives who participated in this study and E. Nikkola for laboratory technical assistance.
Sources of Funding
This study was supported by NIH grant PO1 HL28481 (to R.M.C., A.J.L, and P.P). R.M-H. was supported by the National Human Genome Research Institue (NHGRI) of NIH grant T32 HG02536. D.W-V. is supported by NHGRI grant T32H602536. P.P. is also supported by an NIH grant HL082762 and an AHA grant 0430180N. T.W.A.d.B. was supported by the Netherlands Organization for Scientific Research (NOW) grant 900-95-297. J.M.N. and M.M. were supported by NIH Grant NIDDK-R0162388.
Disclosure
T.W.A.d.B. is employed by GlaxoSmithKline. GlaxoSmithKline did not provide financial support for this study.
References
- 1.Enoch HG, Catala A, Strittmatter P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J Biol Chem. 1976;251:5095–5103. [PubMed] [Google Scholar]
- 2.Ntambi JM, Miyazaki M. Recent insights into stearoyl-CoA desaturase-1. Curr Opin Lipidol. 2003;14:255–261. doi: 10.1097/00041433-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG. Hyperlipidemia in coronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest. 1973;52:1544–1568. doi: 10.1172/JCI107332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoulders CC, Jones EL, Naoumova RP. Genetics of familial combined hyperlipidemia and risk of coronary heart disease. Hum Mol Genet. 2004;13(Spec No 1):R149–R160. doi: 10.1093/hmg/ddh069. [DOI] [PubMed] [Google Scholar]
- 5.Badzioch MD, Igo RP, Jr, Gagnon F, Brunzell JD, Krauss RM, Motulsky AG, Wijsman EM, Jarvik GP. Low-density lipoprotein particle size loci in familial combined hyperlipidemia: evidence for multiple loci from a genome scan. Arterioscler Thromb Vasc Biol. 2004;24:1942–1950. doi: 10.1161/01.ATV.0000143499.09575.93. [DOI] [PubMed] [Google Scholar]
- 6.Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR, Ntambi JM. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res. 2002;43:1899–1907. doi: 10.1194/jlr.m200189-jlr200. [DOI] [PubMed] [Google Scholar]
- 7.Dallinga-Thie GM, Bu XD, van Linde-Sibenius Trip M, Rotter JI, Lusis AJ, de Bruin TW. Apolipoprotein A-I/C-III/A-IV gene cluster in familial combined hyperlipidemia: effects on LDL-cholesterol and apolipoproteins B and C-III. J Lipid Res. 1996;37:136–147. [PubMed] [Google Scholar]
- 8.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SAS Institute I. SAS/STAT User's Guide. Cary, NC, USA: SAS Institute, Inc.; 1999–2000. [Google Scholar]
- 10.Kruglyak L, Lander ES. Complete multipoint sib-pair analysis of qualitative and quantitative traits. Am J Hum Genet. 1995;57:439–454. [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath S, Xu X, Lake SL, Silverman EK, Weiss ST, Laird NM. Family-based tests for associating haplotypes with general phenotype data: application to asthma genetics. Genet Epidemiol. 2004;26:61–69. doi: 10.1002/gepi.10295. [DOI] [PubMed] [Google Scholar]
- 12.O'Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter KW, McCaskie PA, Palmer LJ. JLIN: a java based linkage disequilibrium plotter. BMC Bioinformatics. 2006;7:60. doi: 10.1186/1471-2105-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005;2:251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada T, Furuhashi N, Kuromori Y, Miyashita M, Iwata F, Harada K. Plasma palmitoleic acid content and obesity in children. Am J Clin Nutr. 2005;82:747–750. doi: 10.1093/ajcn/82.4.747. [DOI] [PubMed] [Google Scholar]
- 16.Warensjo E, Riserus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia. 2005;48:1999–2005. doi: 10.1007/s00125-005-1897-x. [DOI] [PubMed] [Google Scholar]
- 17.Warensjo E, Ohrvall M, Vessby B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr Metab Cardiovasc Dis. 2006;16:128–136. doi: 10.1016/j.numecd.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Warensjo E, Ingelsson E, Lundmark P, Lannfelt L, Syvanen AC, Vessby B, Riserus U. Polymorphisms in the SCD1 gene: associations with body fat distribution and insulin sensitivity. Obesity (Silver Spring) 2007;15:1732–1740. doi: 10.1038/oby.2007.206. [DOI] [PubMed] [Google Scholar]
- 19.Eurlings PM, van der Kallen CJ, Vermeulen VM, de Bruin TW. Variants in the PPARgamma gene affect fatty acid and glycerol metabolism in familial combined hyperlipidemia. Mol Genet Metab. 2003;80:296–301. doi: 10.1016/S1096-7192(03)00138-0. [DOI] [PubMed] [Google Scholar]
- 20.Weissglas-Volkov D, Huertas-Vazquez A, Suviolahti E, Lee J, Plaisier C, Canizales-Quinteros S, Tusie-Luna T, Aguilar-Salinas C, Taskinen MR, Pajukanta P. Common hepatic nuclear factor-4alpha variants are associated with high serum lipid levels and the metabolic syndrome. Diabetes. 2006;55:1970–1977. doi: 10.2337/db06-0035. [DOI] [PubMed] [Google Scholar]
- 21.Pajukanta P, Nuotio I, Terwilliger JD, Porkka KV, Ylitalo K, Pihlajamaki J, Suomalainen AJ, Syvanen AC, Lehtimaki T, Viikari JS, Laakso M, Taskinen MR, Ehnholm C, Peltonen L. Linkage of familial combined hyperlipidaemia to chromosome 1q21-q23. Nat Genet. 1998;18:369–373. doi: 10.1038/ng0498-369. [DOI] [PubMed] [Google Scholar]
- 22.Soro A, Pajukanta P, Lilja HE, Ylitalo K, Hiekkalinna T, Perola M, Cantor RM, Viikari JS, Taskinen MR, Peltonen L. Genome scans provide evidence for low-HDL-C loci on chromosomes 8q23, 16q24.1-24.2, and 20q13.11 in Finnish families. Am J Hum Genet. 2002;70:1333–1340. doi: 10.1086/339988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aouizerat BE, Allayee H, Bodnar J, Krass KL, Peltonen L, de Bruin TW, Rotter JI, Lusis AJ. Novel genes for familial combined hyperlipidemia. Curr Opin Lipidol. 1999;10:113–122. doi: 10.1097/00041433-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Riserus U, Tan GD, Fielding BA, Neville MJ, Currie J, Savage DB, Chatterjee VK, Frayn KN, O'Rahilly S, Karpe F. Rosiglitazone increases indexes of stearoyl-CoA desaturase activity in humans: link to insulin sensitization and the role of dominant-negative mutation in peroxisome proliferator-activated receptor-gamma. Diabetes. 2005;54:1379–1384. doi: 10.2337/diabetes.54.5.1379. [DOI] [PubMed] [Google Scholar]
- 25.Kim YC, Gomez FE, Fox BG, Ntambi JM. Differential regulation of the stearoyl-CoA desaturase genes by thiazolidinediones in 3T3-L1 adipocytes. J Lipid Res. 2000;41:1310–1316. [PubMed] [Google Scholar]
- 26.Kurebayashi S, Hirose T, Miyashita Y, Kasayama S, Kishimoto T. Thiazolidinediones downregulate stearoyl-CoA desaturase 1 gene expression in 3T3-L1 adipocytes. Diabetes. 1997;46:2115–2118. doi: 10.2337/diab.46.12.2115. [DOI] [PubMed] [Google Scholar]
- 27.Pihlajamaki J, Miettinen R, Valve R, Karjalainen L, Mykkanen L, Kuusisto J, Deeb S, Auwerx J, Laakso M. The Pro12A1a substitution in the peroxisome proliferator activated receptor gamma 2 is associated with an insulin-sensitive phenotype in families with familial combined hyperlipidemia and in nondiabetic elderly subjects with dyslipidemia. Atherosclerosis. 2000;151:567–574. doi: 10.1016/s0021-9150(99)00433-5. [DOI] [PubMed] [Google Scholar]
- 28.Lilja HE, Suviolahti E, Soro-Paavonen A, Hiekkalinna T, Day A, Lange K, Sobel E, Taskinen MR, Peltonen L, Perola M, Pajukanta P. Locus for quantitative HDL-cholesterol on chromosome 10q in Finnish families with dyslipidemia. J Lipid Res. 2004;45:1876–1884. doi: 10.1194/jlr.M400141-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Hertz R, Magenheim J, Berman I, Bar-Tana J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4alpha. Nature. 1998;392:512–516. doi: 10.1038/33185. [DOI] [PubMed] [Google Scholar]
- 30.Pearson ER, Pruhova S, Tack CJ, Johansen A, Castleden HA, Lumb PJ, Wierzbicki AS, Clark PM, Lebl J, Pedersen O, Ellard S, Hansen T, Hattersley AT. Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4alpha mutations in a large European collection. Diabetologia. 2005;48:878–885. doi: 10.1007/s00125-005-1738-y. [DOI] [PubMed] [Google Scholar]
- 31.Love-Gregory L, Permutt MA. HNF4A genetic variants: role in diabetes. Curr Opin Clin Nutr Metab Care. 2007;10:397–402. doi: 10.1097/MCO.0b013e3281e3888d. [DOI] [PubMed] [Google Scholar]
- 32.Botstein D, Risch N. Discovering genotypes underlying human phenotypes: past successes for mendelian disease, future approaches for complex disease. Nat Genet. 2003;33 Suppl:228–237. doi: 10.1038/ng1090. [DOI] [PubMed] [Google Scholar]
- 33.Cohen P, Friedman JM. Leptin and the control of metabolism: role for stearoyl-CoA desaturase-1 (SCD-1) J Nutr. 2004;134:2455S–2463S. doi: 10.1093/jn/134.9.2455S. [DOI] [PubMed] [Google Scholar]
- 34.Dobrzyn P, Dobrzyn A, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver. Proc Natl Acad Sci U S A. 2004;101:6409–6414. doi: 10.1073/pnas.0401627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ntambi JM, Miyazaki M, Dobrzyn A. Regulation of stearoyl-CoA desaturase expression. Lipids. 2004;39:1061–1065. doi: 10.1007/s11745-004-1331-2. [DOI] [PubMed] [Google Scholar]
- 36.Dobrzyn A, Dobrzyn P, Lee SH, Miyazaki M, Cohen P, Asilmaz E, Hardie DG, Friedman JM, Ntambi JM. Stearoyl-CoA desaturase-1 deficiency reduces ceramide synthesis by downregulating serine palmitoyltransferase and increasing beta-oxidation in skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E599–E607. doi: 10.1152/ajpendo.00439.2004. [DOI] [PubMed] [Google Scholar]
- 37.van der Kallen CJ, Voors-Pette C, Bouwman FG, Keizer HA, Lu JY, van de Hulst RR, Bianchi R, Janssen MJ, Keulen ET, Boeckx WD, Rotter JI, de Bruin TW. Evidence of insulin resistant lipid metabolism in adipose tissue in familial combined hyperlipidemia, but not type 2 diabetes mellitus. Atherosclerosis. 2002;164:337–346. doi: 10.1016/s0021-9150(02)00109-0. [DOI] [PubMed] [Google Scholar]
- 38.de Bruin TW, Georgieva AM, Brouwers MC, Heitink MV, van der Kallen CJ, van Greevenbroek MM. Radiological evidence of nonalcoholic fatty liver disease in familial combined hyperlipidemia. Am J Med. 2004;116:847–849. doi: 10.1016/j.amjmed.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 39.Castro Cabezas M, de Bruin TW, de Valk HW, Shoulders CC, Jansen H, Willem Erkelens D. Impaired fatty acid metabolism in familial combined hyperlipidemia. A mechanism associating hepatic apolipoprotein B overproduction and insulin resistance. J Clin Invest. 1993;92:160–168. doi: 10.1172/JCI116544. [DOI] [PMC free article] [PubMed] [Google Scholar]


