Abstract
The purpose of this study was to examine the impact of age, sex, and education on category and letter verbal fluency task performance. A secondary goal was to examine whether resting EEG theta power in bilateral frontal and temporal lobes impacts age-associated decline in verbal fluency task performance. A large sample (N=471) of healthy, normal participants, age 21–82, was assessed for letter fluency (i.e., FAS), and for category fluency (i.e., Animal Naming), and with a 32-channel EEG system for ‘eyes-open’ resting theta power. The effects of age, sex, and education were examined using analyses of variance. Correlation analyses were used to test the impact of theta power on age and fluency performance by controlling for the effects of theta when examining the relationship between the other two variables. The results indicated that performance on both fluency tests declined linearly with age, but that the rate of decline was greater for category fluency. These age changes were not associated with education level, and there were no sex differences. While theta power was negatively associated with age and positively associated with Animal Naming performance, it did not moderate the relationship between the two. The differential age-associated decline between category and letter fluency suggests separate neurobiological substrates underlying the two domains of performance, which is not related to theta activity.
Keywords: Normal aging, Category fluency, Letter fluency, EEG, Theta
1. Introduction
Assessment of verbal fluency has long been an important component of clinical neuropsychological evaluation (Benton, 1968; Benton & Hamsher, 1976; Lezak, 1995). Verbal fluency is typically tested in letter and category domains. Letter fluency is most commonly assessed by forms of the Controlled OralWord Association Test (COWAT; Benton & Hamsher, 1976), which requires subjects to produce words beginning with a certain letter of the alphabet, usually ‘F,’ ‘A,’ and ‘S’ (Thurstone, 1938), in three respective 60-s trials. In tasks of category fluency, subjects are asked to produce exemplars of a particular category in a 60-s trial. Animal Naming, which requires the subject to generate as many animals as possible in 60 s, is by far the most common test of category fluency (Goodglass & Kaplan, 1972; Rosen, 1980).
Both measures of verbal fluency have been shown to be sensitive to brain damage (Butters, Granholm, Salmon, Grant, & Wolfe, 1987; Janowsky, Shimamura, Kritchevsky, & Squire, 1989; Stuss et al., 1998). Differential performance on measures of letter versus category fluency has also been demonstrated to be of diagnostic utility in aging-related disorders, such as Alzheimer’s disease (AD; Barr & Brandt, 1996; Hodges, Salmon, & Butters, 1992; Ober, Dronkers, Koss, Delis, & Friedland, 1986; Rosen, 1980). In AD, category fluency is particularly affected, whereas letter fluency remains relatively intact (Monsch et al., 1992). These observations are consistent with findings that temporal systems mediate category fluency performance (Pihlajamaki et al., 2000) and frontal systems underlie letter fluency performance (e.g., Abrahams et al., 2003). Despite the clinical implications for assessment of category and letter verbal fluency, there has been relatively little investigation of changes in both letter and category fluency across the adult lifespan in the same sample.
Based on existing studies examining the impact of age on verbal fluency, it appears that category but not letter fluency is typically poorer in elderly as compared to younger adults (Bolla, Gray, Resnick, Galante, & Kawas, 1998; Crossley, D’Arcy, & Rawson, 1997; Kozora & Cullum, 1995; Tombaugh, Kozak, & Rees, 1999; Tomer & Levin, 1993). This pattern of poorer performance on tests of category fluency is reproduced in AD, but with a greater effect (Monsch et al., 1992). Several reports show a generally negative association between age and category fluency measures in adults (Borod, Goodglass, & Kaplan, 1980; Whelihan & Lesher, 1985). Although there is a larger literature on letter fluency in normal adults, the findings concerning age-related change are less consistent. A recent meta-analysis of the FAS task in normal, healthy adults across the lifespan demonstrated a fairly robust age-associated decline in the task (Loonstra, Tarlow, & Sellers, 2001). However, not all authors have reported this pattern (Perlmuter & Tun, 1987).
There is general agreement across studies that verbal fluency is impacted by education level. Most studies report a positive association between number of years of formal education and performance on tasks of letter and category verbal fluency (Benton, Hamsher, & Sivan, 1983; Crossley et al., 1997; Tombaugh et al., 1999; Yeudall, Fromm, Reddon, & Stefanyk, 1986). Relationships between IQ and fluency also feasibly reflect the impact of education. Bolla, Lindgren, Bonaccorsy, and Bleecker (1990) used a regression model, including years of education, an estimate of verbal IQ, age, and sex, to predict performance on tasks of verbal fluency. While they found that the estimate of IQ but not education significantly predicted performance, this finding is most likely due to the high association between IQ and education level (i.e., collinearity between the two factors).
While there is fairly consistent evidence that education impacts fluency output, it is less clear whether fluency performance across the lifespan varies as a function of education. Education may be protective against age-associated decline in cognitive performance, as suggested by the concept of cognitive reserve (Stern, 2002; Stern, 2003). Using regression-based approaches, normative data studies (e.g., Tombaugh et al., 1999) have shown that both age and education account for a significant amount of variance in fluency performance. In one study, Gladsjo and colleagues (1999) found a significant interaction between age and number of years of education for FAS but not Animal Naming performance. In contrast, Bolla and colleagues (1990) did not find a significant influence of education on fluency performance after correcting for the effects of age, sex, and IQ. Similarly, Kempler, Teng, Dick, Taussig, and Davis (1998) did not find a significant interaction between age and education on fluency performance.
The impact of sex differences on fluency performance, and potential changes in these differences across the lifespan, has not been clearly elucidated. Some studies have reported that women perform better on tasks of letter verbal fluency (Bolla et al., 1990; Crossley et al., 1997), while others (Borod et al., 1980; Gladsjo et al., 1999; Yeudall et al., 1986) report no differences between men and women. There is little evidence in the literature for sex differences on tasks of category fluency. In terms of the stability of sex differences across the lifespan, Crossley and colleagues (1997) did not find a significant interaction between sex and age on either letter or category fluency. However, Capitani, Laiacona, and Basso (1998) demonstrated that there was a greater age-associated decline on a task of letter fluency in men.
Electroencephalography (EEG) is a method that is sensitive to cerebral functioning and can provide important insights into neurophysiological correlates of age-related cognitive decline. It has been used to differentiate patients with AD from normal comparison subjects (Duffy, McAnulty, & Albert, 1995), but the pattern of physiological change and its relationship to cognition is less understood across the normal, healthy adult lifespan. Age-associated increases in slow wave activity, characterized by an increase in delta and theta band measurements, have been reported (for review, see Dustman, Shearer, & Emmerson, 1993). Studies of clinical populations have shown theta increases in AD patients as compared to age-matched normal controls (Duffy et al., 1995) and individuals with mild cognitive impairment (Huang et al., 2000), as well as in AD patients who were impaired on a task of verbal fluency compared to those who were not (Le Roc’h, Rancurel, Poitrenaud, Bourgin, & Sebban, 1993).
In healthy individuals, theta wave activity has been implicated in a number of other language-based cognitive tasks (e.g., Weiss & Rappelsberger, 1998). For example, increases in theta rhythm have been reported during processing of words (Bastiaansen, van Berkum, & Hagoort, 2002a) and theta power has been shown to increase during the perception of syntactic violations (Bastiaansen, van Berkum, & Hagoort, 2002b). In children with dyslexia, theta band power was shown to be lower than in matched controls (Klimesch et al., 2001). Theta wave activity has been linked to both verbal and non-verbal memory (Kahana, Seelig, & Madsen, 2001) and may be particularly associated with retrieval (Ward, 2003), which is required for successful performance on tasks of verbal fluency. Further, Uclés, Almárcegui, Lorente, Romero, and Marco (1997) demonstrated a significant improvement in theta power following carotid endarterectomy in patients with carotid artery stenosis, which correlated significantly with an improvement in category verbal fluency. In light of the evidence linking theta band activity to aging and to language-based cognitive tasks, the current study focused on theta power as a potential neurophysiological moderator of age-related change on tasks of verbal fluency. That is, resting theta may be reflective of underlying neural networks involved in age-related decline in fluency performance and a purpose of our study was to explore these potential correlates of theta power. The focal regions of interest were the frontal and temporal lobes given that these areas have also been implicated in tasks of verbal fluency (Phelps, Hyder, Blamire, & Shulman, 1997; Pihlajamaki et al., 2000).
The purpose of the current study was to examine category and letter verbal fluency performance across the adult lifespan in a large sample of normal, healthy adults. Concurrent assessment of both types of fluency meant that differential performance as a function of age could be determined. We hypothesized that category, but not letter, fluency task performance would decline as a function of age. We also examined the effects of education and sex on verbal fluency, and their contribution to changes in performance across the lifespan. It was hypothesized that higher levels of education would be associated with better fluency performance and less age-related decline. Our secondary aim was to determine whether fronto-temporal EEG theta power moderates the relationship between age and verbal fluency performance. We predicted that theta power would contribute significantly to this relationship.
2. Methods
2.1. Subjects
The present study makes use of data from 471 healthy adults tested in collaboration with the Brain Resource International Database (www.brainresource.com; Clark et al., 2004). Subjects included in the analyses were age 21–82 and had complete fluency and EEG data at the time of analyses. Table 1 displays education and sex distribution as a function of age group. Six laboratories (London, New York, Providence, Holland, Adelaide, and Sydney) participated in the data acquisition in a total quality-controlled manner with identical assessment and data acquisition systems. Participants were recruited with fliers, advertisements, and word-of-mouth. Exclusion criteria included a personal history of mental illness, signs of anxiety or depression (Hickie et al., 1998), physical brain injury, neurological disorder or other medical condition (e.g., hypertension, diabetes, cardiac disease, and thyroid disease), and a personal history of drug or alcohol addiction. All subjects voluntarily gave written informed consent to participate in the testing sessions. Subjects were asked to refrain from smoking and caffeine for 2 h prior to testing, and to refrain from alcohol 12 h prior to testing.
Table 1.
Subject characteristics
| Age group | N | Mean (S.D.) years of education |
Percent with >15 years of education |
Percent female |
|---|---|---|---|---|
| 21–30 | 196 | 15.30 (2.54) | 58 | 52 |
| 31–40 | 87 | 14.83 (2.75) | 51 | 39 |
| 41–50 | 72 | 14.09 (3.09) | 42 | 63 |
| 51–60 | 61 | 14.36 (2.59) | 40 | 56 |
| 61–70 | 35 | 12.74 (3.17) | 20 | 51 |
| 71–82 | 20 | 13.35 (3.38) | 30 | 35 |
| Total sample | 471 | 14.64 (2.85) | 48 | 51 |
2.2. Procedures
2.2.1. Measures of FAS and Animal Naming
Participants were seated in a sound attenuated room, in front of a touchscreen computer (NEC MultiSync LCD 1530V). All participants completed the measures of letter fluency and category fluency as part of a computerized test battery. The tests were administered using pre-recorded task instructions (via headphones), and voice recording was used for answers. Instructions for English-speaking locales were administered with region-specific accents; tests were administered in Dutch for the laboratory in Holland. For the letter fluency task, participants were instructed to verbally generate words that began with the letters F, A, and S in three separate, 1-min trials. Proper nouns were scored as incorrect. Similarly, for the Category Fluency Test, participants were instructed to generate exemplars of animals within a 60-s time period. The dependent variable for the letter fluency taskwas the mean correct responses across the three trials. The dependent variable for animal fluency was total number of correct animal exemplars.
2.2.2. Psychophysiology data acquisition
EEG data were acquired in a sound and light attenuated room using a 32-channel Quikcap, according to the 10–20 electrode international system, and with a NuAmps system. Of these channels, we focused on average theta power from frontal (left: Fp1, F3, F7; right: Fp2, F4, F8) and temporal (left: T3, T5; right: T4, T6) electrode sites. Horizontal and vertical eye movements were recorded with electrodes placed 1.5 cm lateral to the outer canthus of each eye and 3mm above the middle of the left eyebrow and 1.5 cm below the middle of the left bottom eyelid, respectively. EEG data were acquired relative to virtual ground and referenced offline to linked mastoids. Data were continuously acquired and corrected offline, following Gratton, Coles, and Donchin (1983).
In this study, we used data from a resting eyes-open paradigm. During EEG recording for 2 min, subjects sat quietly with their eyes-open and fixated on a red dot presented on a computer monitor. To compute average power spectra, the 2 min of EEG were divided into adjacent intervals of 4 s. A Welch window and then a Fast Fourier Transformation (FFT) was applied on each interval. The power spectrum of interest was the theta frequency band between 4 and 7.5 Hz. The resulting power data were square-root transformed to approximate normal distribution and facilitate the use of parametric statistical analytic procedures.
2.2.3. Statistical analytic approach
Subjects were divided into six age groups, organized by decade (e.g., 21–30) and demographic data, including education and sex distribution, were analyzed with analysis of variance (ANOVA) and the χ2 procedures, respectively. Age stratification by decade allows for trend analysis and is a common approach to examining age effects in cross-sectional studies (see Brickman et al., 2003). Repeated measures ANOVAs were used to examine the main effects of age and its interactions. For these analyses, Age Group (6) and Sex (2: male, female) were between-subjects factors and Fluency Task (2: Animal Naming, FAS) was a repeated-measure. Significant effects involving Age Group were followed-up with trend analyses to determine which polynomial function best fit the data. The relationship between Education and performance on the two fluency tasks was examined with Pearson Product Moment correlations. Our initial intention was to covary number of years of education in an Age Group × Fluency Task ANOVA to examine the impact of education on age-associated decline in fluency. However, number of years of education significantly interacted with Age Group (F(4, 464) = 13.624, P < .001). Thus, the assumption of a lack of heterogeneity of regression slope in a covariate analysis would be violated (Tabachnick & Fidell, 2001). To deal with this issue, the median number of years of education was calculated and a median-split-determined variable of Education was entered into the ANOVA.
Pearson correlation coefficients were calculated to examine the relationship among Age, Animal Naming performance, FAS performance, and frontal and temporal theta power. Theta power was considered separately for each hemisphere, as well as collapsed across both hemispheres. To test whether theta power moderates the relationship between age and fluency performance, partial correlations were conducted.
3. Results
3.1. Demographic data
There was a significant linear decline in education across the age groups (F(1, 465) = 30.97, P < .001). Sex distribution varied somewhat unsystematically across age groups (χ2(5) = 12.35, P = .03; see Table 1), although as a group the sample comprised 51% women.
3.2. Age and sex differences in fluency
As the main effect of Sex (F(1, 459) = 2.11, P = .15), the Sex × Fluency Type interaction (F(1, 459) = 0.427, P = .51), and Age Group × Sex × Fluency Task interaction (F(5, 459) = 0.778, P = .57) were not statistically significant, the repeated-measures ANOVA was rerun without Sex as an independent variable.
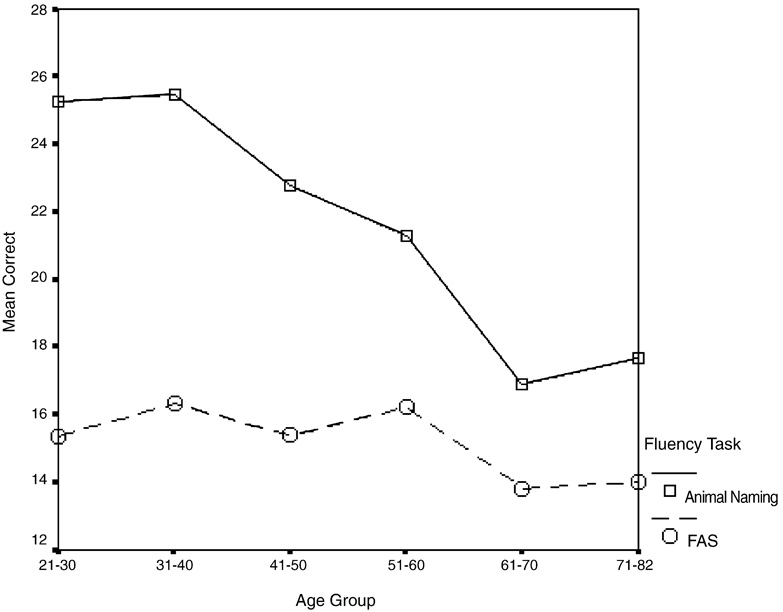
Across both fluency types, significantly fewer words were produced as a function of age (Main effect of Age Group, F(5, 465) = 15.48, P < .001). A significant main effect of Fluency Task (F(1, 465) = 408.219, P < .001) indicated that participants produced more words on the Animal Naming trial (mean ± S.E. = 21.75 ± 0.31) than average words per trial on the FAS task (mean ± S.E. = 15.19 ± 0.24). There was a significant linear interaction between Age Group and Fluency Type (F(5, 465) = 18.034, P < .001). As can be seen in Figure 1, the degree of age-associated fluency decline was greater for Animal Naming than for FAS. Follow-up ANOVAs demonstrated that the age-associated decline was linear for both Animal Naming (F(1, 470) = 82.663, P < .001) and for FAS (F(1, 470) = 6.183, P = .013). Thus, both Animal Naming and FAS declined linearly with age, but with the former at a higher rate.
Fig. 1.
Significant age group by fluency type interaction (F(5, 465) = 18.034, P < .001).
3.3. Effect of education
Number of years of education was significantly correlated with performance on both Animal Naming (r = .19, P < .001) and FAS (r = .20, P < .001). The median number of years of education was 15. Individuals with lower education (≤15 years) produced fewer words across fluency type (mean ± S.E. = 18.02 ± 0.29) than individuals with higher education (mean ± S.E. = 19.17 ± 0.43; Main effect of Education, F(1, 459) = 5.036, P = .025). However, there was not a significant interaction between Age Group and Education (F(5, 459) = 0.666, P = .65), or among Age Group, Education, and Fluency Task (F(5, 459) = 0.773, P = .57). Therefore, the effects of education did not moderate the age-associated decline in fluency or the differential age-associated decline in Category versus letter output.
3.4. Contribution of frontal and temporal EEG theta power
As can be seen in Table 2, there were statistically significant negative associations of medium effect size between age and theta power in both frontal and temporal lobes. Small, but statistically significant, positive associations were found between Animal Naming performance and frontal and temporal theta power, whereas there were essentially no meaningful relationships between FAS performance and theta power.
Table 2.
Correlation coefficients between age, Animal Naming performance, FAS performance, and measures of frontal and temporal theta wave activity
| Left frontal theta |
Right frontal theta |
Total frontal theta |
Left temporal theta |
Right temporal theta |
Total temporal theta |
|
|---|---|---|---|---|---|---|
| Age | −0.274** | −0.244** | −0.269** | −0.257** | −0.296** | −0.299** |
| Animal Naming | 0.144** | 0.114* | 0.135** | 0.152** | 0.167** | 0.173** |
| FAS | 0.007 | 0.000 | 0.003 | 0.022 | 0.031 | 0.029 |
P < .001.
P < .01.
As the association between theta power and FAS performance did not approach statistical significance, only performance on Animal Naming was considered for the mediational analyses. We collapsed activity in left and right hemispheres by taking the average of the two values, and examined the relationship. Controlling for frontal theta power, the significant negative correlation between Age and Animal Naming performance (r =−.417, P < .001) was not significantly modified (r =−.407, P < .001). Similarly, the correlation between Age and Animal Naming performance was not markedly altered when temporal theta power was partialled out (r =−.407, P < .001). Thus, theta power did not appear to significantly moderate the relationship between age and fluency performance.
4. Discussion
The results of the current study demonstrate that fluency performance declines significantly with age. The age-associated decline for the category fluency task was greater than that for letter fluency, though both declined linearly. In general, individuals with higher education performed better across fluency tasks. However, the decline in fluency performance did not vary as a function of education. There were no sex differences in overall performance or in differential performance across the lifespan. Finally, while theta power was negatively associated with age and positively associated with Animal Naming performance, it did not account for the relationship between the two.
Our finding of a greater age-associated decline in category fluency performance replicates other studies that have found a similar effect (e.g., Bolla et al., 1998). Age-associated patterns on these two tasks may point to underlying brain regions that are differentially affected in normal aging. Evidence from functional neuroimaging studies suggests that inferior and mid-frontal regions mediate letter fluency tasks (Abrahams et al., 2003; Gaillard et al., 2003; Phelps et al., 1997), whereas both frontal and temporal structures underlie tasks of category fluency (Pihlajamaki et al., 2000). Similarly, Moskovitch (1995) has proposed a neuropsychological model in which letter fluency is mediated by the frontal lobes and category fluency is mediated by temporal lobe structures. In AD, a disorder that impacts semantic networks, category fluency is more severely affected than letter fluency (Monsch et al., 1992). Thus, like in AD, frontal–temporal semantic networks may be vulnerable to the effects of the normal aging process. Neuroimaging studies examining category and letter fluency tasks in the same subjects across the lifespan would help clarify this issue. Even in the absence of correlative neuroimaging data, however, the results from this study suggest that the two fluency tasks are mediated by different neuroanatomical substrates.
Although the behavioral findings of varying category and letter fluency performance across the lifespan suggest differential neurobiologic substrates, this was not captured by EEG theta band power. Our finding that theta power decreased as a function of age was inconsistent with what has been reported in the literature, which has generally suggested an increase in slow wave activity (e.g., theta) with advancing age (Dustman et al., 1993). Discrepancies with the extant literature could be due to several factors. The participants in the current study were very well screened and the elderly participants did not evidence any symptoms of a neurodegenerative or medical condition that might impact cognitive or brain functioning. Thus, a subject-selection bias could have been operative; that is, older subjects in the study could have been “supranormal.” The findings of age-associated decline in verbal fluency speak against this possibility. Furthermore, the relationships between theta power and fluency were in the expected positive direction, which is consistent with studies that have demonstrated depressed theta frequency bands in patient groups with poorer performance on fluency tasks (Le Roc’h et al., 1993). It is important to note, however, that EEG recordings in the current study were not conducted during task performance and, therefore, may have been vulnerable to many factors (e.g., drowsiness). The sample size and controlled experimental methodology in the current study would argue against the possibility of Type I errors. As there have been limited studies that have examined the relationship between resting EEG measures and cognitive task performance, discrepant findings are most likely attributable to methodological differences among experiments.
Resting theta power was moderately related to category but not letter fluency task performance. The finding is similar to that of Hoptman and Davidson (1998) who did not find a relationship between theta power and performance on a written test of letter fluency. While theta has been implicated in a number cognitive processes (Kahana et al., 2001), our findings suggest some specificity in cognitive processes associated with it. Theta may be particularly linked to both memory encoding and retrieval (Ward, 2003). The significant association between theta power and category, but not letter, fluency performance in our study is consistent with this idea, given the greater load of associative information retrieval required to complete the task successfully.
Like in other investigations (e.g., Crossley et al., 1997), a significant association between fluency tasks and education level was found. Education is a common surrogate measure of cognitive reserve (Stern, 2002; Stern, 2003). Consistent with this notion, we hypothesized that greater education would be somewhat protective against the effect of age. However, education level did not appear to impact rate of fluency decline across the lifespan. Despite the fact that there was a range of educational achievement, the sample was generally well educated, and this may have impacted the findings. That is, inclusion of individuals with much lower education may have yielded significant interactions involving education. The findings, nonetheless, suggest that education may be less protective against normal aging effects on tasks of fluency.
There was a significant decline in education level across the age groups. This finding most likely represents a cohort bias secondary to random sampling from the population; older individuals, in general, may be less likely to have pursued advanced levels of education. It is important to note that despite the fact that education level was related to performance on both tasks of verbal fluency, the pattern of age-related decline was not due to this relationship. That is, education level was similarly related to FAS performance as it was to Animal Naming performance. That elderly individuals tend to have lower levels of education has important clinical implications. Although education and age do not interact with fluency performance, both demographic variables contribute independently to performance. Thus, both factors should be taken into consideration during clinical evaluation.
Whether there are sex differences in fluency performance remains somewhat elusive. We did not find the female advantage that has been reported by other investigators (Bolla et al., 1990; Capitani et al., 1998), though it is important to note that the sex distribution was not consistent across the age groups in our sample. Given the size of the sample in the current study, it is unlikely that our finding was due to Type II statistical error, even if the reported sex effect is of small magnitude, as suggested by Capitani and colleagues (1998). Furthermore, sex did not modify rate of age-related decline for either the category or letter task, as has been reported by some authors (Capitani et al., 1998). Differential performance across the lifespan as a function of sex may be detectable with more qualitative analyses of verbal fluency, as suggested by Tabert and colleagues (2001), who demonstrated sex differences in the accuracy of verbal output.
A particular strength of this study is the large sample size and the representation of subjects from several locales. Test administration was computer-based, which may limit the generalizability of our findings. However, performance on the two tasks of verbal fluency was similar to what has been reported previously. Furthermore, computer administered protocols ensure reliable test administration across all subjects and laboratory sites. Future work in this area should focus on functional neuroanatomical correlates of performance on such tasks across the lifespan. Ultimately, the sensitivity of clinical instruments to detect pathology can be refined by better understanding specific changes in normal aging, as well as factors that impact these changes.
References
- Abrahams S, Goldstein LH, Simmons A, Brammer MJ, Williams SC, Giampietro VP, et al. Functional magnetic resonance imaging of verbal fluency and confrontation naming using compressed image acquisition to permit over responses. Human Brain Mapping. 2003;20:29–40. doi: 10.1002/hbm.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo A, Loewenstein DA, Barker WW, Harwood DG, Luis C, Bravo M, et al. Category Fluency Test: Normative data for English- and Spanish-speaking elderly. Journal of the International Neuropsychological Society. 2000;6:760–769. doi: 10.1017/s1355617700677032. [DOI] [PubMed] [Google Scholar]
- Barr A, Brandt J. Word-list generation deficits in dementia. Journal of Clinical and Experimental Neuropsychology. 1996;18:810–822. doi: 10.1080/01688639608408304. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, van Berkum JJ, Hagoort P. Event-related theta power increases in the human EEG during online sentence processing. Neuroscience Letters. 2002a;323:13–16. doi: 10.1016/s0304-3940(01)02535-6. [DOI] [PubMed] [Google Scholar]
- Bastiaansen MC, van Berkum JJ, Hagoort P. Syntactic processing modulates the theta rhythm of the human EEG. Neuroimage. 2002b;17:1479–1492. doi: 10.1006/nimg.2002.1275. [DOI] [PubMed] [Google Scholar]
- Benton AL. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 1968;6:53–60. [Google Scholar]
- Benton AL, Hamsher K. Multilingual aphasia examination manual. Iowa City, IA: University of Iowa; 1989. [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual aphasia examination. Iowa City, IA: AJA; 1976. [Google Scholar]
- Bolla KI, Gray S, Resnick SM, Galante R, Kawas C. Category and letter fluency in highly educated older adults. The Clinical Neuropsychologist. 1998;12:330–338. [Google Scholar]
- Bolla KI, Lindgren KN, Bonaccorsy C, Bleecker ML. Predictors of verbal fluency (FAS) in the healthy elderly. Journal of Clinical Psychology. 1990;46:623–628. doi: 10.1002/1097-4679(199009)46:5<623::aid-jclp2270460513>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Borod J, Goodglass H, Kaplan E. Normative data on the Boston diagnostic aphasia examination, parietal lobe battery, and Boston naming test. Journal of Clinical Neuropsychology. 1980;2:209–216. [Google Scholar]
- Brickman AM, Buchsbaum MS, Shihabuddin L, Hazlett EA, Borod JC, Mohs RC. Striatal size, glucose metabolic rate, and verbal learning in normal aging. Cognitive Brain Research. 2003;17:106–116. doi: 10.1016/s0926-6410(03)00085-5. [DOI] [PubMed] [Google Scholar]
- Butters N, Granholm E, Salmon DP, Grant I, Wolfe J. Episodic and semantic memory: A comparison of amnestic and demented patients. Journal of Clinical and Experimental Neuropsychology. 1987;9:479–497. doi: 10.1080/01688638708410764. [DOI] [PubMed] [Google Scholar]
- Capitani E, Laiacona M, Basso A. Phonetically cued word-fluency, gender differences and aging: A reappraisal. Cortex. 1998;34:779–783. doi: 10.1016/s0010-9452(08)70781-0. [DOI] [PubMed] [Google Scholar]
- Clark R, Veltmeyer MD, Hamilton RJ, Simms E, Paul R, Hermens D, et al. Spontaneous alpha peak frequency predicts working memory performance across the age span. International Journal of Psychophysiology. 2004;53:1–9. doi: 10.1016/j.ijpsycho.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Crossley M, D’Arcy C, Rawson NSB. Letter and category fluency in community-dwelling Canadian seniors: A comparison of normal participants to those with dementia of the Alzheimer or vascular type. Journal of Clinical and Experimental Neuropsychology. 1997;19:52–62. doi: 10.1080/01688639708403836. [DOI] [PubMed] [Google Scholar]
- Duffy FH, McAnulty GB, Albert MS. Temporoparietal electrophysiological differences characterize patients with Alzheimer’s disease: A split-half replication study. Cerebral Cortex. 1995;5:215–221. doi: 10.1093/cercor/5.3.215. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Shearer DE, Emmerson RY. EEG and event-related potentials in normal aging. Progress in Neurobiology. 1993;41:369–401. doi: 10.1016/0301-0082(93)90005-d. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Human Brain Mapping. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. Assessment of aphasia and related disorders. Philadelphia: Lea & Febiger; 1972. [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hickie I, Hadzi-Pavlovic D, Scott E, Davenport T, Koschera A, Naismith S. SPHERE: A national depression project. Australasian Psychiatry. 1998;6:248–250. [Google Scholar]
- Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer’s disease: Failure of access or degraded knowledge? Neuropsychologia. 1992;30:301–314. doi: 10.1016/0028-3932(92)90104-t. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Davidson RJ. Baseline EEG asymmetries and performance on neuropsychological tasks. Neuropsychologia. 1998;36:1343–1353. doi: 10.1016/s0028-3932(98)00023-2. [DOI] [PubMed] [Google Scholar]
- Huang C, Wahlund LO, Dierks T, Julin P, Winblad B, Jelic V. Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: A cross-sectional and longitudinal study. Clinical Neurophysiology. 2000;111:1961–1967. doi: 10.1016/s1388-2457(00)00454-5. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Kritchevsky M, Squire LR. Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behavioral Neuroscience. 1989;103:548–560. doi: 10.1037//0735-7044.103.3.548. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Current Opinion in Neurobiology. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kempler D, Teng EL, Dick M, Taussig IM, Davis DS. The effects of age, education, and ethnicity on verbal fluency. Journal of the International Neuropsychological Society. 1998;4:531–538. doi: 10.1017/s1355617798466013. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Wimmer H, Schwaigler J, Rohm D, Gruber W, et al. Theta band power changes in normal and dyslexic children. Clinical Neurophysiology. 2001;112:1174–1185. doi: 10.1016/s1388-2457(01)00545-4. [DOI] [PubMed] [Google Scholar]
- Kozora E, Cullum MC. Generative naming in normal aging: Total output and qualitative changes using letter and semantic constraints. The Clinical Neuropsychologist. 1995;9:313–320. [Google Scholar]
- Le Roc’h K, Rancurel G, Poitrenaud J, Bourgin P, Sebban C. Verbal fluency and EEG coherence in Alzheimer’s disease. Neurophysiologie Clinique. 1993;23:422–433. doi: 10.1016/s0987-7053(05)80392-2. [DOI] [PubMed] [Google Scholar]
- Lezak M. Neuropsychologial assessment. 3rd ed. New York: Oxford University Press; 1995. [Google Scholar]
- Loonstra AS, Tarlow AR, Sellers AH. COWAT metanorms across age, education, and gender. Applied Neuropsychology. 2001;8:161–166. doi: 10.1207/S15324826AN0803_5. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Archives of Neurology. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Moskovitch M. Cognitive resources and dual-task interference effects at retrieval in normal people: The role of the frontal lobes and medial temporal cortex. Neuropsychology. 1995;8:524–534. [Google Scholar]
- Ober BA, Dronkers NF, Koss E, Delis DC, Friedland RP. Retrieval from semantic memory in Alzheimer-type dementia. Journal of Clinical and Experimental Neuropsychology. 1986;8:75–92. doi: 10.1080/01688638608401298. [DOI] [PubMed] [Google Scholar]
- Perlmuter LC, Tun P. Age and diabetes related changes in verbal fluency. Experimental Aging Research. 1987;13:9–14. doi: 10.1080/03610738708259294. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Hyder F, Blamire AM, Shulman RG. FMRI of the prefrontal cortex during overt verbal fluency. Neuroreport. 1997;8:561–565. doi: 10.1097/00001756-199701200-00036. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Laakso M, Partanen K, et al. Verbal fluency activates the left medial temporal lobe: A functional magnetic resonance imaging study. Annals of Neurology. 2000;47:470–476. [PubMed] [Google Scholar]
- Rosen WG. Verbal fluency in aging and dementia. Journal of Clinical Neuropsychology. 1980;2:135–146. [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y. The concept of cognitive reserve: A catalyst for research. Journal of Clinical and Experimental Neuropsychology. 2003;25:589–593. doi: 10.1076/jcen.25.5.589.14571. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, et al. The effects of focal anterior and posterior brain lesions on verbal fluency. Journal of the International Neuropsychological Society. 1998;4:265–278. [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 4th ed. Boston, MA: Allyn and Bacon; 2001. [Google Scholar]
- Tabert MH, Peery S, Borod JC, Schmidt JM, Grunwald I, Sliwinski M. Lexical emotional expression across the life span: Quantitative and Qualitative analyses of word list generation tasks. The Clinical Neuropsychologist. 2001;15:531–550. doi: 10.1076/clin.15.4.531.1876. [DOI] [PubMed] [Google Scholar]
- Thurstone LL. Primary mental abilities. Psychometric Monographs. 1938;1 [Google Scholar]
- Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and Animal Naming. Archives of Clinical Neuropsychology. 1999;14:167–177. [PubMed] [Google Scholar]
- Tomer R, Levin BE. Differential effects of aging on two verbal fluency tasks. Perceptual and Motor Skills. 1993;76:465–466. doi: 10.2466/pms.1993.76.2.465. [DOI] [PubMed] [Google Scholar]
- Uclés P, Almárcegui C, Lorente S, Romero F, Marco M. Evaluation of cerebral function after carotid endarterectomy. Journal of Clinical Neurophysiology. 1997;14:242–249. doi: 10.1097/00004691-199705000-00010. [DOI] [PubMed] [Google Scholar]
- Ward LM. Synchronous neural oscillations and cognitive processes. Trends in Cognitive Sciences. 2003;12:553–559. doi: 10.1016/j.tics.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Weiss S, Rappelsberger P. Left frontal EEG coherence reflects modality independent language processes. Brain Topography. 1998;11:33–42. doi: 10.1023/a:1022266419488. [DOI] [PubMed] [Google Scholar]
- Whelihan WM, Lesher EL. Neuropsychological changes in frontal functions with aging. Developmental Neuropsychology. 1985;1:371–380. [Google Scholar]
- Yeudall LT, Fromm D, Reddon jR, Stefanyk WO. Normative data stratified by age and sex for 12 neuropsychological tests. Journal of Clinical Psychology. 1986;42:918–946. doi: 10.1002/1097-4679(198705)43:3<346::aid-jclp2270430308>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]