Abstract
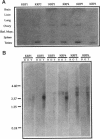
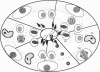
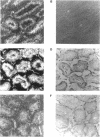
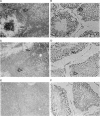
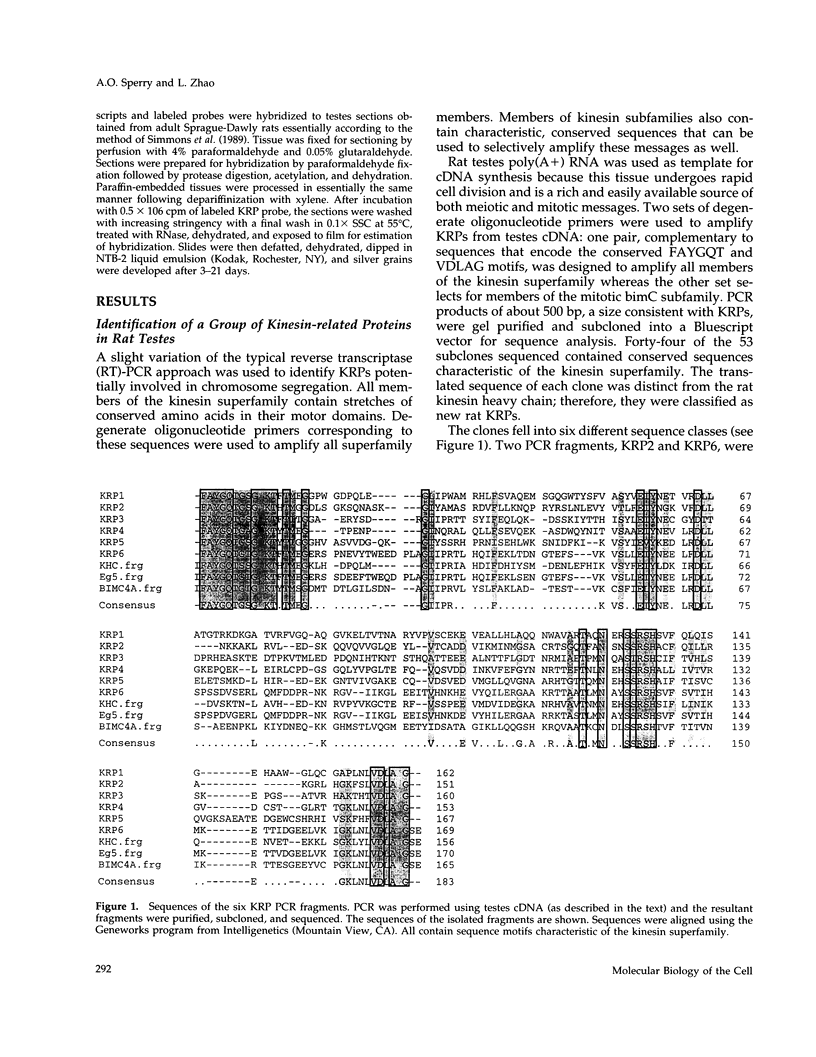
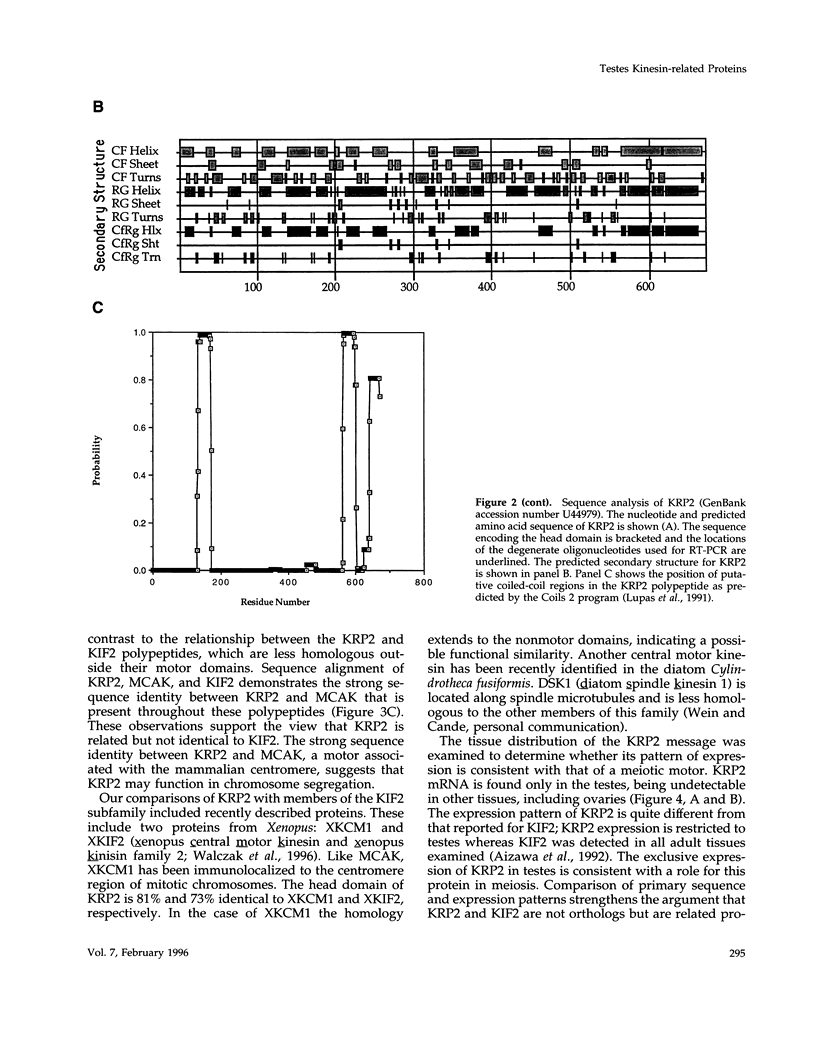
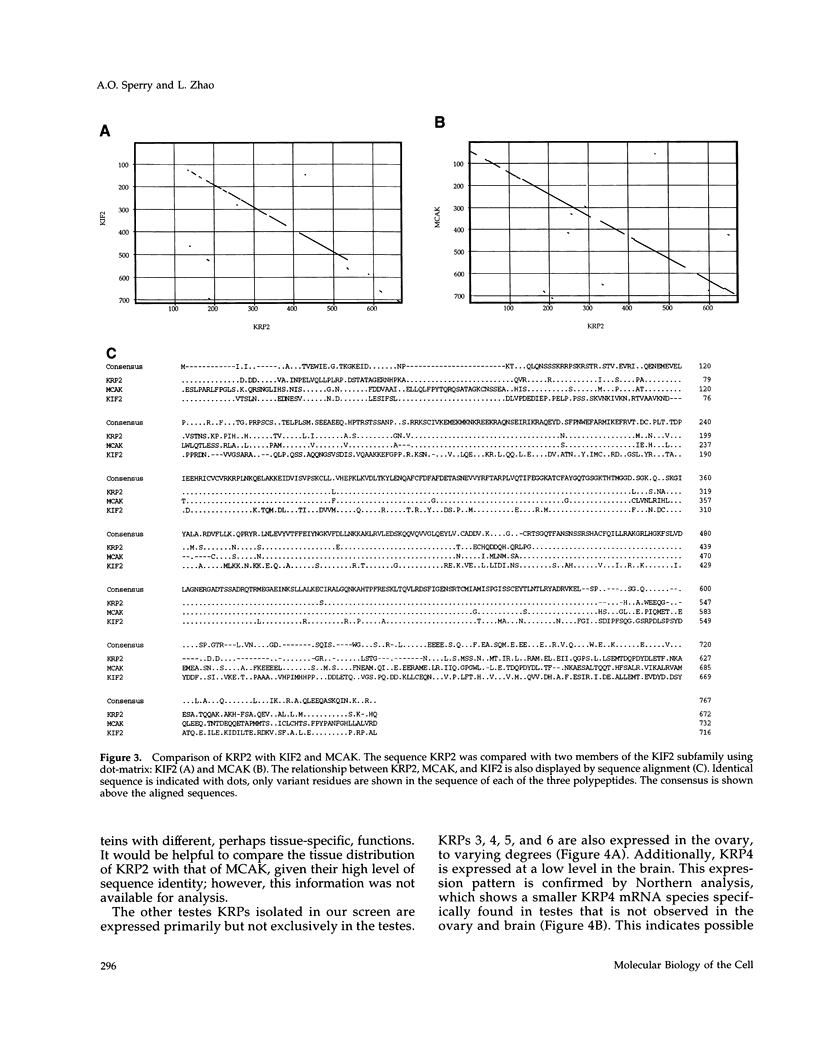
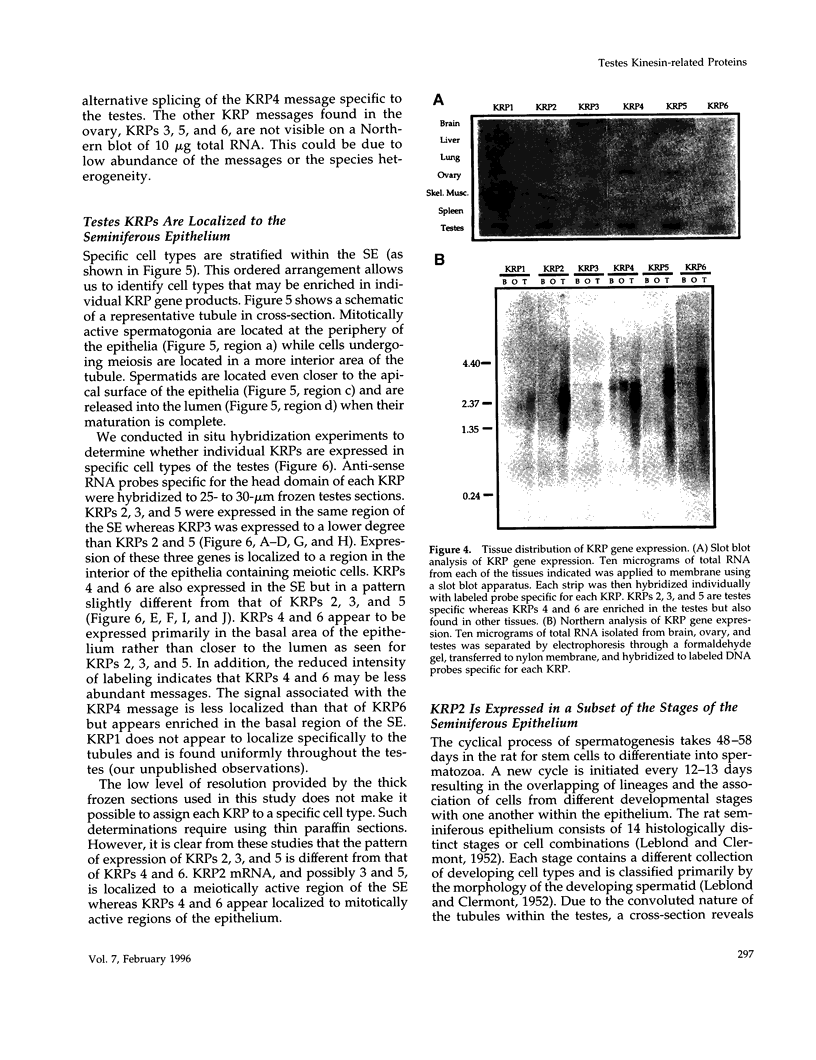
The kinesin superfamily of molecular motors comprises proteins that participate in a wide variety of motile events within the cell. Members of this family share a highly homologous head domain responsible for force generation attached to a divergent tail domain thought to couple the motor domain to its target cargo. Many kinesin-related proteins (KRPs) participate in spindle morphogenesis and chromosome movement in cell division. Genetic analysis of mitotic KRPs in yeast and Drosophila, as well as biochemical experiments in other species, have suggested models for the function of KRPs in cell division, including both mitosis and meiosis. Although many mitotic KRPs have been identified, the relationship between mitotic motors and meiotic function is not clearly understood. We have used sequence similarity between mitotic KRPs to identify candidates for meiotic and/or mitotic motors in a vertebrate. We have identified a group of kinesin-related proteins from rat testes (termed here testes KRP1 through KRP6) that includes new members of the bimC and KIF2 subfamilies as well as proteins that may define new kinesin subfamilies. Five of the six testes KRPs identified are expressed primarily in testes. Three of these are expressed in a region of the seminiferous epithelia (SE) rich in meiotically active cells. Further characterization of one of these KRPs, KRP2, showed it to be a promising candidate for a motor in meiosis: it is localized to a meiotically active region of the SE and is homologous to motor proteins associated with the mitotic apparatus. Testes-specific genes provide the necessary probes to investigate whether the motor proteins that function in mammalian meiosis overlap with those of mitosis and whether motor proteins exist with functions unique to meiosis. Our search for meiotic motors in a vertebrate testes has successfully identified proteins with properties consistent with those of meiotic motors in addition to uncovering proteins that may function in other unique motile events of the SE.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa H., Sekine Y., Takemura R., Zhang Z., Nangaku M., Hirokawa N. Kinesin family in murine central nervous system. J Cell Biol. 1992 Dec;119(5):1287–1296. doi: 10.1083/jcb.119.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando A., Kikuti Y. Y., Kawata H., Okamoto N., Imai T., Eki T., Yokoyama K., Soeda E., Ikemura T., Abe K. Cloning of a new kinesin-related gene located at the centromeric end of the human MHC region. Immunogenetics. 1994;39(3):194–200. doi: 10.1007/BF00241260. [DOI] [PubMed] [Google Scholar]
- Bernstein M., Beech P. L., Katz S. G., Rosenbaum J. L. A new kinesin-like protein (Klp1) localized to a single microtubule of the Chlamydomonas flagellum. J Cell Biol. 1994 Jun;125(6):1313–1326. doi: 10.1083/jcb.125.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endow S. A. Chromosome distribution, molecular motors and the claret protein. Trends Genet. 1993 Feb;9(2):52–55. doi: 10.1016/0168-9525(93)90187-M. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Henikoff S., Soler-Niedziela L. Mediation of meiotic and early mitotic chromosome segregation in Drosophila by a protein related to kinesin. Nature. 1990 May 3;345(6270):81–83. doi: 10.1038/345081a0. [DOI] [PubMed] [Google Scholar]
- Endow S. A. The emerging kinesin family of microtubule motor proteins. Trends Biochem Sci. 1991 Jun;16(6):221–225. doi: 10.1016/0968-0004(91)90089-e. [DOI] [PubMed] [Google Scholar]
- Enos A. P., Morris N. R. Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell. 1990 Mar 23;60(6):1019–1027. doi: 10.1016/0092-8674(90)90350-n. [DOI] [PubMed] [Google Scholar]
- Fuller M. T., Caulton J. H., Hutchens J. A., Kaufman T. C., Raff E. C. Mutations that encode partially functional beta 2 tubulin subunits have different effects on structurally different microtubule arrays. J Cell Biol. 1988 Jul;107(1):141–152. doi: 10.1083/jcb.107.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. S. With apologies to scheherazade: tails of 1001 kinesin motors. Annu Rev Genet. 1993;27:319–351. doi: 10.1146/annurev.ge.27.120193.001535. [DOI] [PubMed] [Google Scholar]
- Gorbsky G. J. Chromosome motion in mitosis. Bioessays. 1992 Feb;14(2):73–80. doi: 10.1002/bies.950140202. [DOI] [PubMed] [Google Scholar]
- Hagan I., Yanagida M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature. 1990 Oct 11;347(6293):563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- Hatsumi M., Endow S. A. Mutants of the microtubule motor protein, nonclaret disjunctional, affect spindle structure and chromosome movement in meiosis and mitosis. J Cell Sci. 1992 Mar;101(Pt 3):547–559. doi: 10.1242/jcs.101.3.547. [DOI] [PubMed] [Google Scholar]
- Hawley R. S., Frazier J. A., Rasooly R. Separation anxiety: the etiology of nondisjunction in flies and people. Hum Mol Genet. 1994 Sep;3(9):1521–1528. doi: 10.1093/hmg/3.9.1521. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., He L., Loo K. K., Saunders W. S. Two Saccharomyces cerevisiae kinesin-related gene products required for mitotic spindle assembly. J Cell Biol. 1992 Jul;118(1):109–120. doi: 10.1083/jcb.118.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komma D. J., Horne A. S., Endow S. A. Separation of meiotic and mitotic effects of claret non-disjunctional on chromosome segregation in Drosophila. EMBO J. 1991 Feb;10(2):419–424. doi: 10.1002/j.1460-2075.1991.tb07963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R., Kofron M., Essner R., Kato T., Dragas-Granoic S., Omoto C. K., Khodjakov A. Characterization of a minus end-directed kinesin-like motor protein from cultured mammalian cells. J Cell Biol. 1995 May;129(4):1049–1059. doi: 10.1083/jcb.129.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
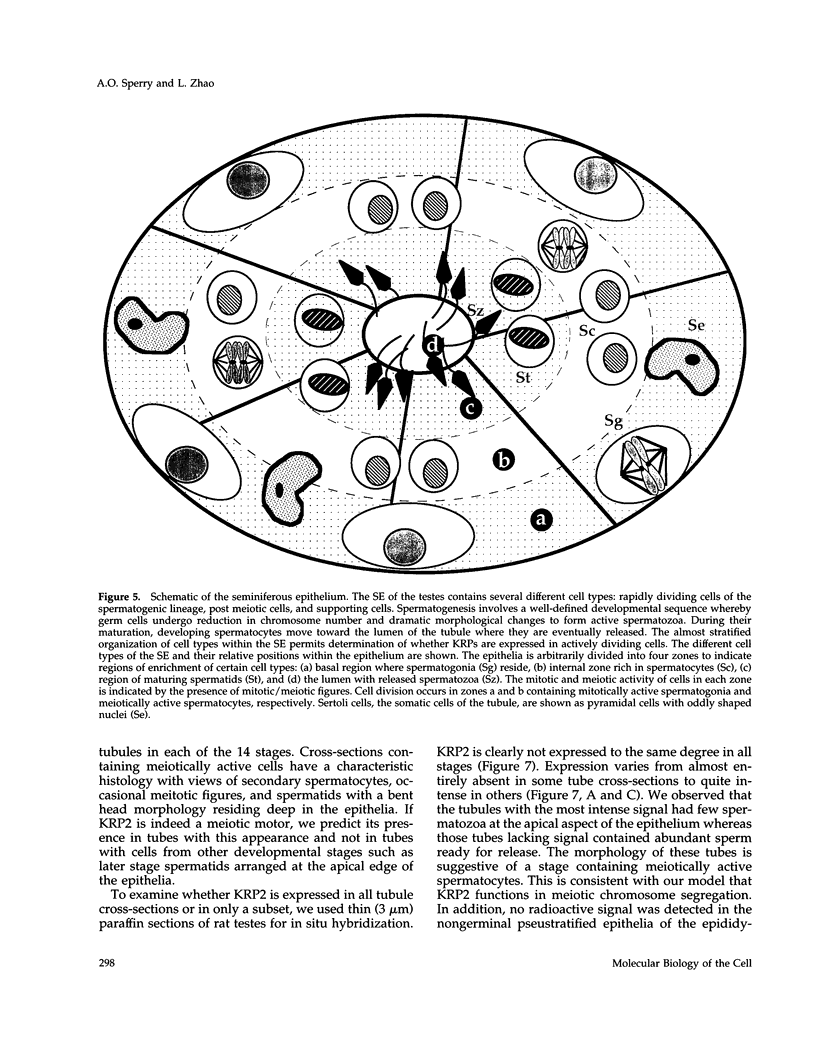
- LEBLOND C. P., CLERMONT Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952 Nov 20;55(4):548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Le Guellec R., Paris J., Couturier A., Roghi C., Philippe M. Cloning by differential screening of a Xenopus cDNA that encodes a kinesin-related protein. Mol Cell Biol. 1991 Jun;11(6):3395–3398. doi: 10.1128/mcb.11.6.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald H. B., Goldstein L. S. Identification and characterization of a gene encoding a kinesin-like protein in Drosophila. Cell. 1990 Jun 15;61(6):991–1000. doi: 10.1016/0092-8674(90)90064-l. [DOI] [PubMed] [Google Scholar]
- Redenbach D. M., Vogl A. W. Microtubule polarity in Sertoli cells: a model for microtubule-based spermatid transport. Eur J Cell Biol. 1991 Apr;54(2):277–290. [PubMed] [Google Scholar]
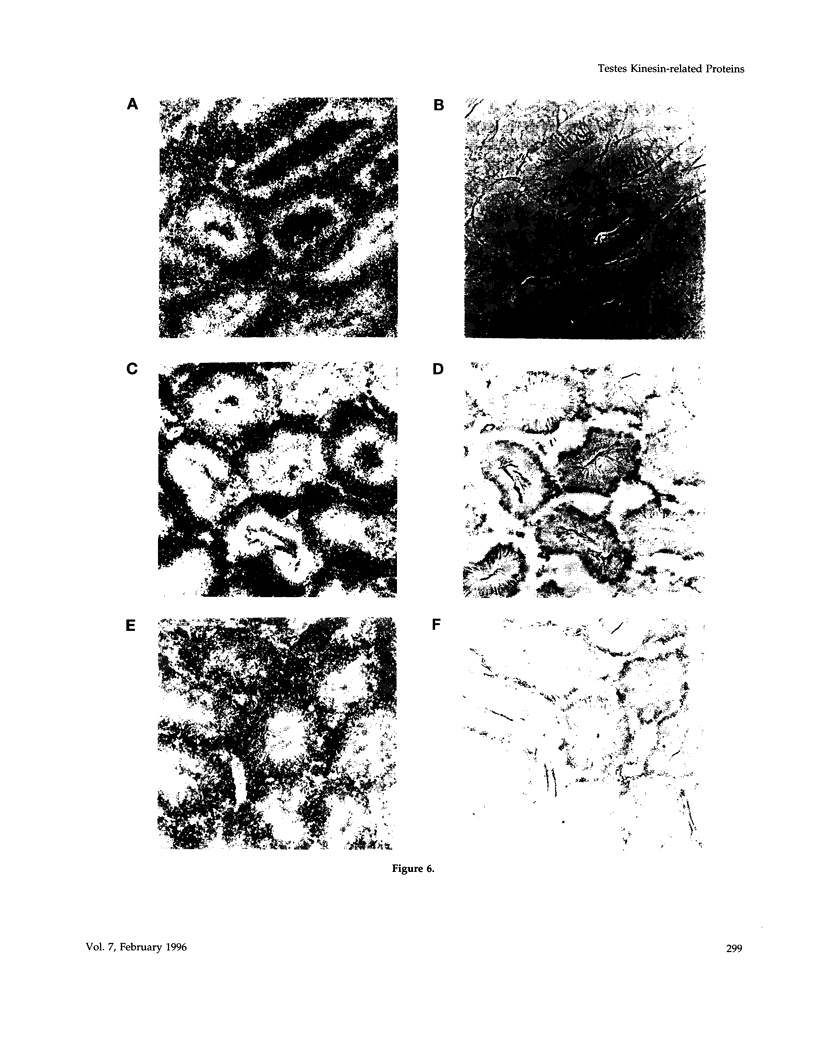
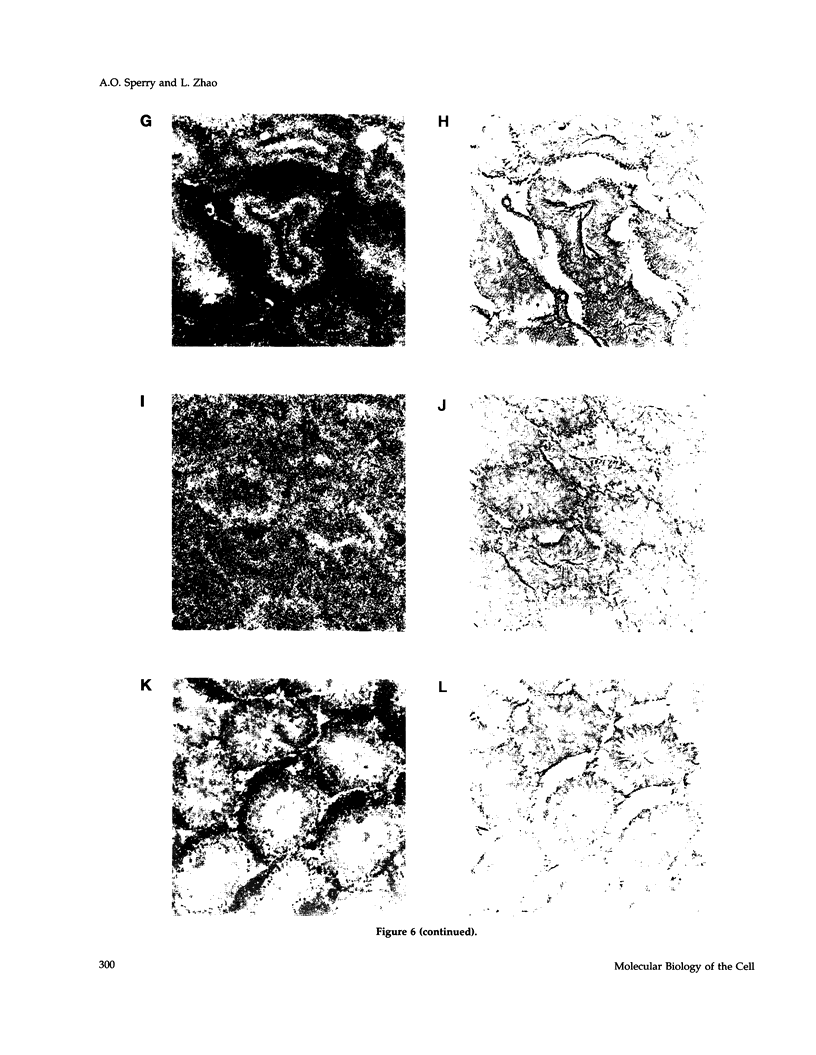
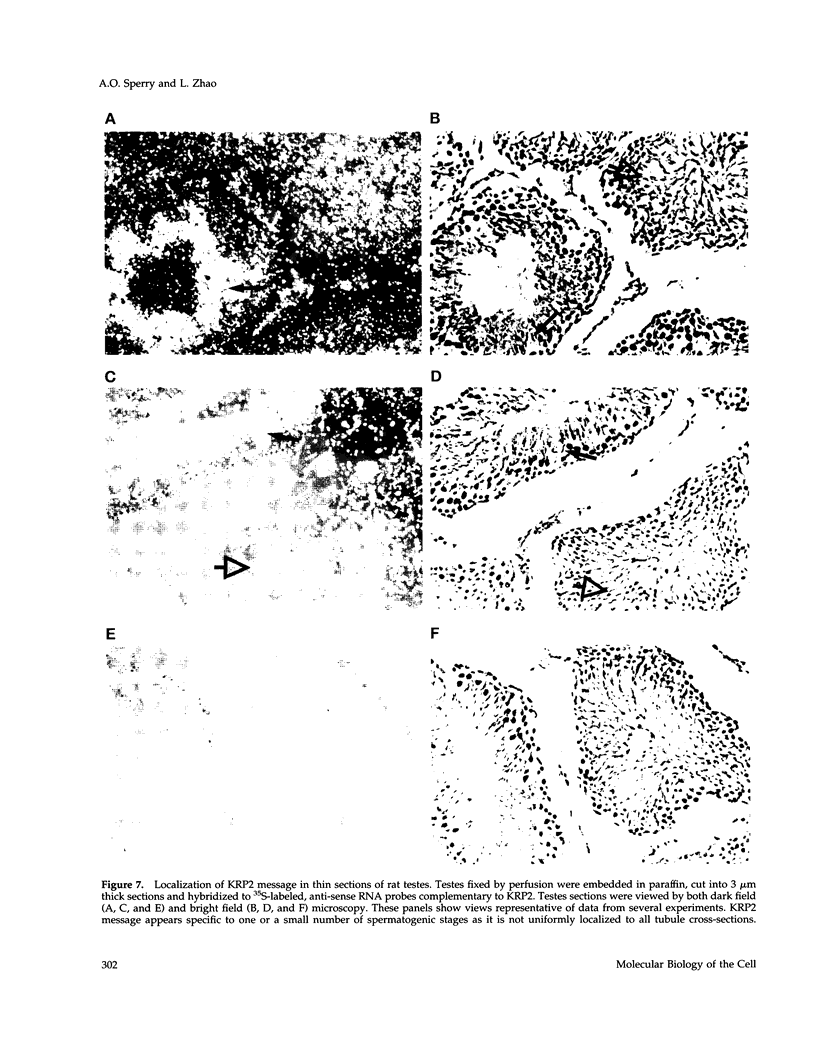
- Roof D. M., Meluh P. B., Rose M. D. Kinesin-related proteins required for assembly of the mitotic spindle. J Cell Biol. 1992 Jul;118(1):95–108. doi: 10.1083/jcb.118.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders W. S., Hoyt M. A. Kinesin-related proteins required for structural integrity of the mitotic spindle. Cell. 1992 Aug 7;70(3):451–458. doi: 10.1016/0092-8674(92)90169-d. [DOI] [PubMed] [Google Scholar]
- Sawin K. E., Endow S. A. Meiosis, mitosis and microtubule motors. Bioessays. 1993 Jun;15(6):399–407. doi: 10.1002/bies.950150606. [DOI] [PubMed] [Google Scholar]
- Sawin K. E., LeGuellec K., Philippe M., Mitchison T. J. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992 Oct 8;359(6395):540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- Theurkauf W. E., Hawley R. S. Meiotic spindle assembly in Drosophila females: behavior of nonexchange chromosomes and the effects of mutations in the nod kinesin-like protein. J Cell Biol. 1992 Mar;116(5):1167–1180. doi: 10.1083/jcb.116.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth P. Mitosis: spindle assembly and chromosome motion. Curr Opin Cell Biol. 1993 Feb;5(1):123–128. doi: 10.1016/s0955-0674(05)80017-1. [DOI] [PubMed] [Google Scholar]
- Wordeman L., Mitchison T. J. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J Cell Biol. 1995 Jan;128(1-2):95–104. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Knowles B. A., Goldstein L. S., Hawley R. S. A kinesin-like protein required for distributive chromosome segregation in Drosophila. Cell. 1990 Sep 21;62(6):1053–1062. doi: 10.1016/0092-8674(90)90383-p. [DOI] [PubMed] [Google Scholar]