Summary
Wnt pathways regulate many developmental processes, including cell-fate specification, cell polarity, and cell movements during morphogenesis. The subcellular distribution of pathway mediators in specific cellular compartments might be crucial for the selection of pathway targets and signaling specificity. We find that the ankyrin-repeat protein Diversin, which functions in different Wnt signaling branches, localizes to the centrosome in Xenopus ectoderm and mammalian cells. Upon stimulation with Wnt ligands, the centrosomal distribution of Diversin is transformed into punctate cortical localization. Also, Diversin was recruited by Frizzled receptors to non-homogeneous Dishevelled-containing cortical patches. Importantly, Diversin deletion constructs, which did not localize to the centrosome, failed to efficiently antagonize Wnt signaling. Furthermore, a C-terminal construct that interfered with Diversin localization inhibited Diversin-mediated β-catenin degradation. These observations suggest that the centrosomal localization of Diversin is crucial for its function in Wnt signaling.
Keywords: Diversin, Xenopus, Wnt, Centrosome, Frizzled, Cell polarity
Introduction
Wnt signaling pathways play crucial roles in cell proliferation, cell-fate specification and morphogenesis in early embryos. Several identified pathway branches include the β-catenin-dependent pathway, the planar cell polarity (PCP) pathway and a pathway regulating intracellular Ca2+ release (Clevers, 2006; Habas and Dawid, 2005; Semenov et al., 2007), but their conservation in diverse organisms and distinct tissues remains to be clarified. The canonical Wnt pathway is known to stabilize β-catenin, which is degraded in the absence of Wnt proteins by the protein complex that includes Axin and GSK-3 (Clevers, 2006; Klaus and Birchmeier, 2008). The PCP pathway is one of the non-canonical pathways, controlling cell polarity in the plane of epithelial tissues in Drosophila (Lawrence et al., 2007; Seifert and Mlodzik, 2007). The PCP pathway requires several core PCP proteins, including the transmembrane proteins Frizzled (Fz), Strabismus (Stbm) and Flamingo, and the intracellular proteins Dishevelled (Dsh), Prickle (Pk) and Diego, although the role for Wnt ligands is uncertain. The vertebrate homologs of the core PCP components regulate convergent extension movements that are required for neural-tube closure and lengthening of embryos along the anteroposterior axis (Keller, 2002; Wang and Nathans, 2007). Whereas some PCP proteins, such as Stbm, are exclusively involved in a particular signaling branch, others, such as Dsh or Fz, function in multiple pathways. A major challenge remains to understand how the signals are channeled to specific pathway branches.
Pathway specificity is likely to be determined by the utilization of distinct combinations of Fz receptors, low-density-lipoprotein receptor-related proteins (LRP-5 and LRP-6) or the tyrosine-kinase receptors Ror and Ryk, as well as the involvement of different intracellular mediators (Gordon and Nusse, 2006). One of the vertebrate proteins that regulates multiple signaling branches is Diversin, a distant homolog of the fly PCP mediator Diego (Moeller et al., 2006; Schwarz-Romond et al., 2002). Diversin has been reported to interact with several components of Wnt signaling. The eight N-terminal ankyrin repeats bind Dsh, the conserved middle domain associates with CK1ε and the C-terminal domain interacts with Axin (Moeller et al., 2006; Schwarz-Romond et al., 2002). Although Diversin has been shown to inhibit the Wnt–β-catenin pathway and stimulate convergent extension, how Wnt signals regulate Diversin function remains unclear.
Because localization of signaling proteins to a particular cellular compartment might be crucial for signaling (Bilic et al., 2007; Ciruna et al., 2006; Cliffe et al., 2003; Witzel et al., 2006; Yin et al., 2008), we examined the regulation of the subcellular localization of Diversin by Wnt- and PCP-signaling components in Xenopus ectoderm cells. We report that Diversin localizes to the centrosome in both Xenopus embryonic ectoderm and mammalian cultured cells. Recent studies have suggested an important role for this unique cellular organelle and its derivative cilium in a number of signaling pathways, including Hedgehog, Wnt, PDGF and FGF (Badano et al., 2005; Bisgrove and Yost, 2006; Eggenschwiler and Anderson, 2007; Neugebauer et al., 2009). After Wnt stimulation, Diversin translocated to specific puncta in the cytoplasm and cell cortex, and overexpression of Fz recruited Diversin to specific cortical patches at the cell membrane. Moreover, our structure-function analysis of Diversin revealed an association between the centrosomal localization of Diversin and its inhibitory activity in the Wnt–β-catenin pathway.
Results
Centrosomal localization of Diversin
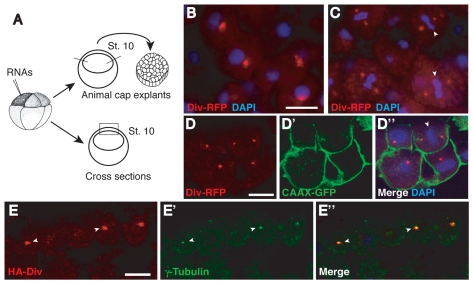
To study the distribution of Diversin in the cell, mRNA encoding mouse Diversin fused to red fluorescent protein (RFP) was injected into the animal pole region of Xenopus eight-cell embryos and embryos were cultured until they reached early gastrula stages. Fluorescence of Diversin-RFP was examined in ectodermal explants and on cross-sections of the injected embryos (Fig. 1). At high doses of Diversin RNA (1-2 ng), Diversin-RFP was detected in the nucleus and cytoplasmic puncta, suggesting that it forms aggregates in the cytoplasm. At lower doses (0.2-0.5 ng), Diversin-RFP was detected in animal cap explants or cross-sections as one or two bright puncta per cell (Fig. 1A-E). During mitosis, Diversin-RFP was detected on both sides of the metaphase plate (Fig. 1C,D), suggesting that Diversin is localized to spindle poles. This possibility was confirmed by co-staining centrosomes with antibodies to γ-tubulin. Colocalization of Diversin carrying one of two unrelated tags (Diversin-RFP or HA-Diversin) with endogenous γ-tubulin, a marker of the pericentriolar material, revealed that Diversin is indeed present at or near the centrosome (Fig. 1E; Fig. 2A).
Fig. 1.
Overexpressed Diversin localizes to the centrosome in Xenopus ectoderm. (A) Experimental scheme. (B-E) Two animal blastomeres of eight-cell embryos were injected with 0.5 ng of Diversin-RFP (Div-RFP; B-D) or HA-Diversin (HA-Div; E) RNA alone or with 0.15 ng of CAAX-GFP RNA. At stage 10, the injected embryos were fixed for immunostaining on cryosections or used to prepare ectodermal explants. (B,C) In ectodermal explants, Div-RFP was distributed as a single dot in interphase cells (B) or two dots in mitotic cells (C). (D,E) In cryosections, Div-RFP was distributed in ectodermal cells at early gastrula stage as one or two dots per cell and colocalized with γ-tubulin (E). (D) CAAX-GFP stained with anti-GFP antibodies marks the cell membrane. RFP and DAPI co-staining is shown (B,C) or a merged image (D″). Metaphase plates (C,D″) or centrosomes (E) are indicated by arrowheads. Scale bars: 20 μm. Scale bar in B also applies to C.
Fig. 2.
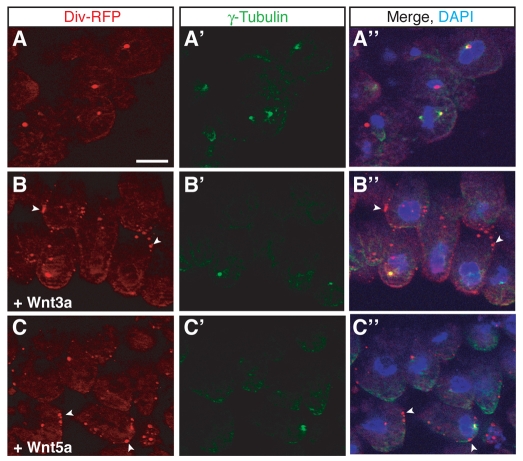
Diversin is translocated into cortical puncta in response to Wnt proteins. Embryos were co-injected with Diversin-RFP RNA (0.5 ng) and either Wnt3a or Wnt5a RNA (1 ng each) as described in Fig. 1A. Cryosections were co-stained with anti-γ-tubulin. Diversin-RFP overlaps, but does not completely colocalize with γ-tubulin, a centrosome marker (A-A″). Upon coexpression with Wnt3a (B) or Wnt5a (C), Diversin fluorescence is redistributed to cytoplasmic and cortical puncta (arrowheads in B,B″,C,C″), whereas γ-tubulin staining is not affected. DAPI co-staining is shown in merged images on the right. Scale bar: 20 μm.
We next generated specific anti-Diversin antibodies and studied the localization of endogenous Diversin at the centrosome. Western analysis revealed a single major protein band of about 75 kD in HeLa-cell lysates (supplementary material Fig. S1A), which is lower than the predicted size, possibly owing to alternative splicing. Staining of HeLa cells with anti-Diversin and anti-γ-tubulin antibodies (supplementary material Fig. S1B-D) revealed a speckled pattern throughout the cell, as well as significant colocalization of endogenous Diversin with the centrosome (supplementary material Fig. S1B-D). In dividing cells, anti-Diversin staining was often asymmetrically distributed to the two spindle poles (supplementary material Fig. S1D). These observations support our experiments with exogenous Diversin and suggest that Diversin plays a role in asymmetric cell division.
Diversin centrosomal localization is regulated by Wnt signaling
Because Diversin functions in both β-catenin-dependent and -independent Wnt pathways (Moeller et al., 2006; Schwarz-Romond et al., 2002), we examined whether its subcellular localization is affected by Wnt proteins. Wnt3a, Wnt5a and Wnt11 RNAs were coexpressed with Diversin-RFP RNA in early embryos to assess Diversin localization on embryo cross-sections. Because Wnt3a, Wnt5a and Wnt11 can stimulate different pathway branches during early development (Gordon and Nusse, 2006; Kilian et al., 2003; Schambony and Wedlich, 2007; Tada and Smith, 2000), one might expect distinct effects of these ligands on Diversin localization. Upon expression of these Wnt proteins, the localization of Diversin at the centrosome transformed into a punctate staining pattern, whereas staining for γ-tubulin, a centrosome marker, was unaffected (Fig. 2). Most of the puncta are detected at the cell cortex at or adjacent to the cell membrane, marked with CAAX-GFP (supplementary material Fig. S2A-C), whereas the other puncta are found in the cytoplasm. No pronounced differences between the effects of different Wnt ligands were detected. These findings demonstrate that Wnt proteins alter the subcellular localization of Diversin and displace it from the centrosome.
Non-uniform cortical localization of Diversin in the presence of Fz8
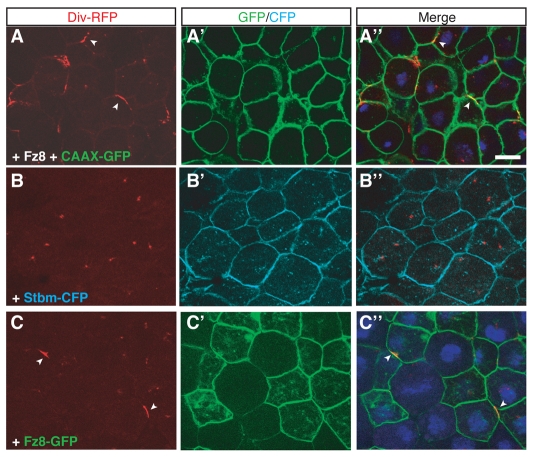
In zebrafish embryos, Diversin has been reported to regulate convergent extension movements (Moeller et al., 2006), which is a process similar to the establishment of fly PCP (Sokol, 2000; Wallingford et al., 2002). Therefore, we wanted to examine the effects of the PCP components Fz and Stbm on Diversin localization. Coexpression of Frizzled 8 (Fz8) and Diversin-RFP resulted in the recruitment of Diversin to the cell membrane (Fig. 3). Coexpression of CAAX-GFP, a uniform cell-membrane marker, indicated that Diversin localized to cortical patches at the cell membrane (Fig. 3A). We next compared the effect of Fz to the effect of Stbm, a known antagonist of Fz in the PCP pathway (Seifert and Mlodzik, 2007). Unlike Fz8, Stbm did not affect Diversin localization (Fig. 3B). We conclude that Fz8 triggers the redistribution of Diversin to the cell membrane in a non-uniform manner.
Fig. 3.
Non-uniform membrane recruitment of Diversin by Fz. (A-C) Animal cap explants were prepared from stage-10 embryos injected as described in Fig. 1A with the following RNAs: Div-RFP (0.5 ng), Fz8 (1 ng), Stbm-CFP (0.5 ng), CAAX-GFP (0.1 ng) and Fz8-GFP (1 ng). Diversin was recruited to the cell membrane in a non-uniform manner by Fz8 (A,C; arrowheads), but not by Stbm (B). The distribution of Fz8-GFP differed from the localization of Diversin (C). Note the uniform membrane localization of CAAX-GFP, Stbm-CFP and Fz8-GFP (A-C). Scale bar: 20 μm.
One possible explanation of the observed non-uniform protein distribution is that Fz8 is present at the cell membrane in patches and directly recruits Diversin to these patches. To test this possibility, we constructed a Fz8-GFP fusion protein and assessed its localization in the presence of Diversin. Fz8-GFP was homogeneously distributed on the cell membrane, whereas Diversin was localized in patches (Fig. 3C). This result indicates that the patchy distribution of Diversin in response to Fz8 is not directly related to the localization of Fz8 at the cell membrane. Also, the relevance of these findings to the effect of Wnt proteins on Diversin localization is presently unclear.
Colocalization of Diversin and Dsh
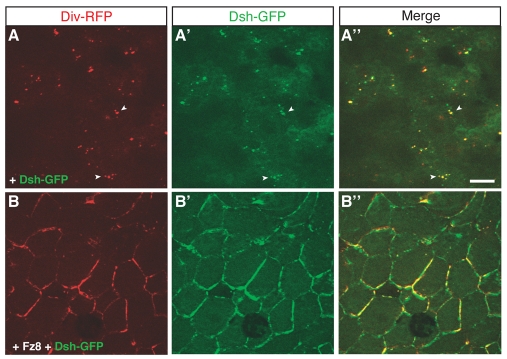
Because Dsh was reported to associate with the N-terminal ankyrin repeats of Diversin in HEK293T cells (Moeller et al., 2006), we examined the interaction of Dsh and Diversin in Xenopus embryonic ectoderm and assessed their subcellular distribution upon coexpression. When expressed separately, Dsh-GFP forms cytoplasmic aggregates (Itoh et al., 2000; Schwarz-Romond et al., 2005; Smalley et al., 2005), whereas Diversin-RFP associates with the centrosome. By contrast, coexpression of Dsh-GFP with Diversin-RFP revealed multiple puncta that contained both proteins (Fig. 4A). These puncta were mainly cytoplasmic (supplementary material Fig. S3A), and probably different from the puncta observed in cells responding to Wnt stimulation (Fig. 2B,C) that are associated with the cell membrane and/or cortex. This result demonstrates that Dsh can regulate the centrosomal localization of Diversin and stimulates its redistribution to Dsh-containing aggregates (Fig. 4A; supplementary material Fig. S3).
Fig. 4.
Colocalization of Diversin and Dsh. (A,B) Animal cap explants were prepared from stage-10 embryos injected with the following RNAs: Div-RFP (0.5 ng), Dsh-GFP (0.15 ng) and Fz8 (1 ng). Diversin colocalized with Dsh to cytoplasmic puncta (A, arrowheads), whereas Fz8 caused a non-uniform distribution of both Diversin and Dsh on the cell membrane (B). Scale bar: 20 μm.
Upon Fz8 coexpression, both Diversin and Dsh were present at the cell membrane in a non-uniform manner (Fig. 4B). Given homogeneous membrane recruitment of Dsh in response to Fz (Axelrod et al., 1998; Itoh et al., 2005; Rothbacher et al., 2000; Yang-Snyder et al., 1996), this indicates that Dsh localization is altered by Diversin. In a reciprocal experiment, Diversin was more uniformly recruited by Fz8 in the presence of increasing amounts of Dsh (supplementary material Fig. S3). These results show that the subcellular localization of Dsh and Diversin is interdependent and provide further evidence for the dose-dependent interaction of these proteins.
Diversin recruitment by Fz8 requires its N-terminal and middle domains
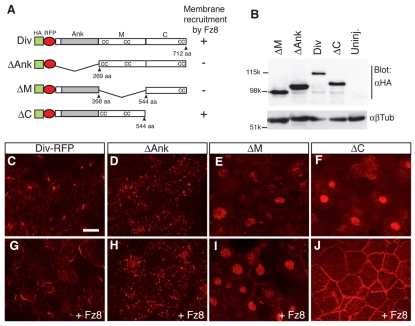
A structure-function analysis was carried out to evaluate which protein region(s) mediate Diversin localization to the centrosome and are required for membrane targeting in the presence of Fz8. To this end, we generated several deletion constructs, which retained different regions of Diversin and were well expressed in early embryos at comparable levels (Fig. 5A,B). ΔAnk-Diversin, which lacks the N-terminal ankyrin repeats, was localized to many cytoplasmic puncta (Fig. 5D). ΔM-Diversin, lacking the middle region of the protein, localized to nuclear puncta and the surrounding material (Fig. 5E), whereas ΔC-Diversin, with a C-terminal deletion, had a general nuclear localization (Fig. 5F). Despite this localization of Diversin constructs, no typical nuclear localization signals are apparent in the Diversin sequence. The three deletion constructs revealed much weaker centrosomal distribution as compared with the wild-type Diversin (Fig. 5C-F). This indicates that all three domains of Diversin are involved in the control of its centrosomal localization.
Fig. 5.
Different Diversin domains play distinct roles in Diversin subcellular localization. (A) Diversin constructs and summary of the Fz8 recruitment data. Ank, ankyrin repeats (grey bar); M, middle region; C, C-terminal region; cc, coiled-coil motif. (B) Western analysis of stage-10.5 embryo lysates demonstrates similar expression levels of Diversin constructs. Embryos were injected with the following RNAs: HA-Div-RFP (0.5 ng), HA-ΔM-RFP (0.25 ng), HA-ΔAnk-RFP (0.25 ng) and HA-ΔC-RFP (2 ng). β-tubulin (βTub) controls loading. Molecular weight markers are indicated. Uninj., un-injected. (C-J) Animal cap explants from embryos injected with Diversin RNAs as in B (C-F) or co-injected with Fz8 RNA (1 ng; G-J). In the absence of Fz8, ΔAnk is present in many puncta (D), ΔM in nucleoplasm and nuclear puncta (E), and ΔC in the nucleus (F). Upon coexpression of Fz8, ΔC and full-length Diversin were recruited to the cell cortex (G,J), whereas ΔAnk and ΔM were not (H,I). Note the uniform membrane localization of ΔC (J). Scale bar: 20 μm.
When the same deletion constructs were coexpressed with Fz8 RNA, ΔC-Diversin, but not ΔAnk-Diversin nor ΔM-Diversin, was recruited to the cell membrane (Fig. 5G-J). Therefore, the C-terminus of Diversin is not required for its membrane recruitment. Interestingly, the distribution of ΔC-Diversin on the cell membrane was uniform, contrasting the non-uniform distribution of wild-type Diversin (Fig. 5G,J). We conclude that both the ankyrin repeat and the middle regions are required for the membrane recruitment of Diversin by Fz8, whereas the C-terminal region contains a motif(s) essential for the non-uniform membrane localization of Diversin.
Centrosomal localization of Diversin is associated with β-catenin degradation
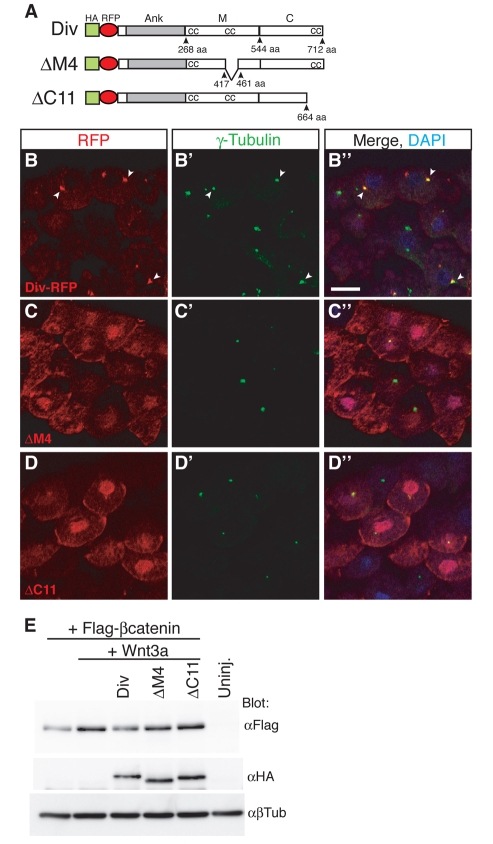
To further define the domain(s) required for the centrosomal localization of Diversin, we assessed the subcellular localization of mutated Diversin constructs carrying small deletions in the middle or the C-terminal domain. Among six constructs with deletions in the middle region, only ΔM4, which lacks 43 amino acids corresponding to a coiled-coil motif, revealed broad cytoplasmic and nuclear distribution, with only residual centrosomal localization (Fig. 6A-C). Similarly, among ten constructs with deletions in the C-terminal domain, only ΔC11, which lacks 47 amino acids in the area of the third coiled-coil motif, had lost most of its centrosomal localization (Fig. 6A,D).
Fig. 6.
Diversin constructs with a defect in centrosomal localization are unable to promote β-catenin degradation. (A) HA-Diversin-RFP constructs with deletions in the middle and in the C-terminal region. (B-D) Cryosections prepared from embryos injected with the following RNA: HA-Diversin-RFP (0.3 ng), HA-ΔM4 (0.15 ng) or HA-ΔC11 (2 ng). Sections were immunostained with anti-γ-tubulin antibodies. ΔM4 and ΔC11 reveal mostly nuclear and peri-membrane, rather than centrosomal, localization. (B-D) Diversin colocalized with γ-tubulin (arrowheads). Scale bar: 20 μm. (E) Levels of β-catenin in embryonic animal caps injected with the following RNAs: FLAG–β-catenin (30 pg), Wnt3a (10 pg), HA-Diversin-RFP (0.5 ng), HA-ΔM4 (0.25 ng) and HA-ΔC11 (2 ng). Wnt3a-dependent β-catenin stabilization was inhibited by wild-type Diversin, but not by ΔM4 or ΔC11. Diversin constructs are expressed at similar levels, β-tubulin is a loading control. Uninj., un-injected.
To address the issue of whether centrosomal localization is crucial for Diversin function, we evaluated the ability of the two mutant Diversin constructs to degrade β-catenin. Stabilization of β-catenin induced by Wnt3a was prevented by wild-type Diversin, but not by ΔM4 or ΔC11, despite similar protein expression levels for these constructs (Fig. 6E). These results suggest that the centrosomal localization of Diversin is essential for its ability to antagonize Wnt-mediated β-catenin stabilization.
Interfering with Diversin localization reduces its ability to antagonize Wnt signaling
Our results indicate that the C-terminal region of Diversin is required for non-uniform membrane localization of the protein in the presence of Fz8 (Fig. 5). Also, we found that the C-terminal fragment (Div-C) contains one of the centrosomal-localization domains (Fig. 6A,D). We therefore wanted to test whether the C-terminal domain of Diversin interferes with the localization of full-length HA-tagged Diversin. Overexpression of Div-C altered HA-Diversin distribution from centrosomal to broad cytoplasmic (Fig. 7A-D), indicating that Div-C can serve as a potent inhibitor of Diversin localization. By contrast, the integrity of the centrosome assessed by γ-tubulin staining was not visibly affected by Div-C (supplementary material Fig. S4).
Fig. 7.
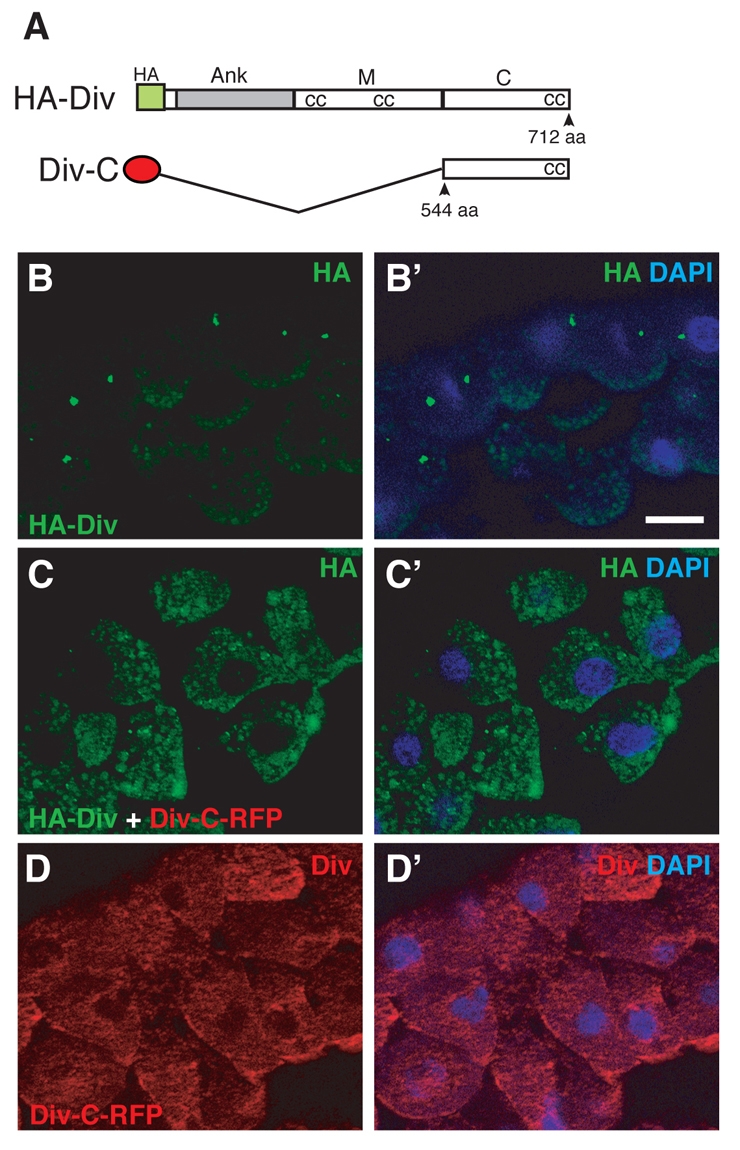
Centrosomal localization of Diversin was disrupted by the C-terminal construct. (A) Scheme showing the C-terminal construct of Diversin (Div-C). Ank, ankyrin repeats (grey bar); M, middle region; C, C-terminal region; cc, coiled-coil motif. (B-D) Cryosections from embryos injected with 0.5 ng HA-Diversin RNA and/or 1 ng RFP-Div-C RNA. Staining with anti-HA (B,C) and anti-Diversin (D) antibodies revealed the disruption of the centrosomal localization of HA-Diversin by Div-C. Scale bar: 20 μm.
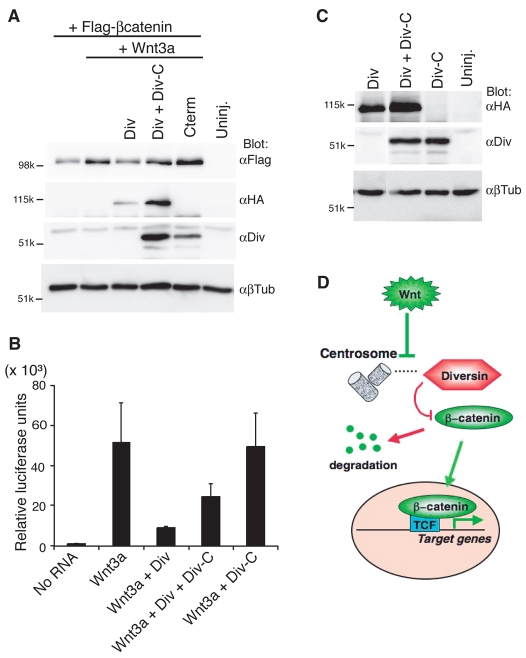
Because Div-C drastically changed the subcellular localization of HA-Diversin, we further investigated whether the ability of Diversin to inhibit Wnt signaling was also affected. To address this issue, we coexpressed Div-C with full-length Diversin-RFP and assessed β-catenin stabilization and Wnt3a-dependent activation of the Siamois-luciferase reporter (Fan et al., 1998). In both assays, Div-C reversed the inhibitory effects of Diversin (Fig. 8A-C). The activity of Diversin-RFP was lower despite the upregulation of its levels in cells coexpressing Div-C (Fig. 8A,C). These results are consistent with the idea that centrosomal localization of Diversin is crucial for its inhibitory activity in the regulation of the Wnt–β-catenin pathway.
Fig. 8.
Div-C diminishes Wnt inhibitory activity of the wild-type Diversin. (A) Div-C suppresses the inhibitory effect of wild-type Diversin on β-catenin stabilization. RNAs injected were: Wnt3a (10 pg), HA-Diversin-RFP (0.5 ng), Div-C–RFP (1 ng) and FLAG–β-catenin (30 pg). (A,C) After protein separation and transfer, transfer membranes were cut at approximately the 80-kD band level and separately probed with anti-HA antibody to detect overexpressed wild-type Diversin and with anti-Diversin antibody to detect Div-C (which has no HA tag). β-tubulin is a loading control. (B) Div-C diminishes the inhibitory effects of Diversin on Siamois reporter activation by Wnt3a. Luciferase assay with pSia-Luc DNA (20 pg) upon co-injection of 10 pg of Wnt3a RNA with 0.5 ng of HA-Div-RFP and 1 ng of Div-C-RFP into animal ventral blastomere of eight-cell embryos. Four groups of six embryos were collected at stage 10 for the reporter assay. Means ± s.d. are shown. (C) Diversin and Div-C expression levels in embryos used for B. (D) Model for Diversin function at the centrosome. Centrosomal Diversin promotes β-catenin degradation. Upon Wnt signaling, Diversin is redistributed to punctate structures at the cell cortex and the cytoplasm, resulting in stabilized β-catenin and increased target gene transcription.
Discussion
Our study demonstrates a specific localization of Diversin at the centrosome of Xenopus embryonic ectoderm and in mammalian cells. This localization is highly sensitive to signaling initiated by different components of the Wnt pathway, including Wnt, Fz8 and Dsh proteins, and suggests a role for Diversin in cell polarity. Several observations support a model in which Diversin functions at the centrosome to antagonize β-catenin stabilization in response to Wnt signaling (Fig. 8D). First, the Diversin constructs that do not localize to the centrosome are defective in their ability to promote β-catenin degradation. Second, a C-terminal fragment of Diversin that interferes with the distribution of wild-type Diversin constructs to the centrosome suppresses the inhibitory activity of Diversin in the Wnt–β-catenin pathway. Finally, the data on the redistribution of Diversin from the centrosome in response to Wnt signaling suggest that Wnt proteins promote target gene activation by altering Diversin centrosomal localization and thereby inhibiting its function in β-catenin degradation. Together, these results indicate that Diversin distribution to specific cellular compartments might be crucial for Wnt-pathway specificity.
Our data support a hypothesis that the centrosome plays a negative regulatory role in Wnt signaling. In agreement with this view, several Wnt-signaling components were reported to associate with the centrosome, including APC, Dishevelled, GSK-3, CK1, β-catenin, Axin and SCF ubiquitin ligase complex (Freed et al., 1999; Fumoto et al., 2009; Kaplan et al., 2004; Kim et al., 2009; Louie et al., 2004; Park et al., 2008; Sillibourne et al., 2002; Wakefield et al., 2003). Moreover, interference with the basal body and ciliary proteins leads to the disruption of PCP and enhances canonical Wnt responses, indicating that the centrosome is involved in the regulation of Wnt signaling (Corbit et al., 2008; Gerdes et al., 2007; Jones et al., 2008; Ross et al., 2005). Both phosphorylated β-catenin and the proteasomal machinery have been shown to associate with the centrosome (Badano et al., 2005; Bahmanyar et al., 2008; Gerdes et al., 2007; Wigley et al., 1999). Thus, it is possible that one role of the centrosome is to promote proteasome-dependent β-catenin degradation either near the centrosome or in the cytoplasm. These reports further support our model that Diversin functions at the centrosome to stimulate β-catenin degradation.
The observed relocalization of Diversin to cytoplasmic and cortical puncta in response to Wnt ligands might reflect normal regulation of Diversin function during signal transduction, resulting in β-catenin stabilization. We also observed that Fz8, but not Stbm, recruited Diversin to the cell membrane, revealing a possible intermediate signaling protein complex, reminiscent of the proposed signalosome (Bilic et al., 2007). Other components of this protein complex might include Axin and Dsh (Bilic et al., 2007; Cliffe et al., 2003; Itoh et al., 2000; Zeng et al., 2008), because we found that Diversin colocalized with Dsh. Of interest, Fz8-mediated recruitment of Diversin leads to its non-uniform distribution at the cell membrane, indicating cell polarization. This polarization required the C-terminal domain of Diversin, but was independent of the distribution of the Fz receptor, indicating a complex mechanism, possibly related to PCP determination and/or β-catenin-independent Wnt signaling. Because the Fz8 receptor itself was distributed relatively uniformly at the cell membrane, it is likely that the Diversin C-terminal domain associates with yet-unknown polarized cell components. Given that ectoderm cells are usually considered to have very low background levels of Wnt signaling based on lack of reporter activation, the recruitment of Diversin-RFP by Fz8 is unlikely to reflect signaling by endogenous Wnt ligands.
Non-uniform membrane localization has been described in Xenopus ectodermal explants expressing the core PCP proteins Stbm and Pk (Jenny et al., 2003), whereas the protein complex of Diego, Dsh and Fz was reported to segregate from the Stbm-Pk complex in Drosophila wing epithelial cells (Axelrod, 2001; Das et al., 2004; Strutt, 2001; Tree et al., 2002; Wu et al., 2008). At present, it is unclear how these findings relate to the Fz-Diversin cortical patches observed in animal pole ectoderm in our experiments. Together with other reports (Cliffe et al., 2003; Itoh et al., 2000; Jenny et al., 2003; Witzel et al., 2006; Zeng et al., 2008), our study emphasizes the complexity of the mechanisms operating to regulate cell polarity by Wnt and PCP core components. Given inherent limitations of overexpression studies, further analysis of endogenous proteins in polarized tissues is needed to fully understand the interactions of core PCP proteins during convergent extension movements in vertebrate embryos and PCP determination in Drosophila.
Materials and Methods
DNA constructs
Diversin-RFP-pCS105 was generated by in-frame fusion of mouse Diversin cDNA (Schwarz-Romond et al., 2002) to the coding sequence of mRFP at the 5′ terminus. Other Diversin constructs, including ΔAnk-RFP, ΔC-RFP, Div-C–RFP, HA-Diversin-RFP and HA-Diversin in pCS105, were synthesized by PfuI DNA polymerase from the Diversin-RFP-pCS105 template with specific primers, followed by DpnI digestion of the template (Makarova et al., 2000). HA-ΔM-RFP, HA-ΔM4-RFP and HA-ΔC11-RFP were generated using HA-Diversin-RFP as template. The primers used were: 5′-CAGGATGCGGTCGCTAGTCGTGGGCGAAGCCTG-3′ for ΔAnk-RFP; 5′-CAACCAGTGGTAGCTTGAGGGCCAGGAGCAGCTTC-3′ for ΔC-RFP; 5′-CGATTTAAAGCTATGTACCCATACGATGTTCCAGATTACGCTGCCTCCTCCGAGGACGTC-3′ for HA-Diversin-RFP; 5′-CAGATCTTGCGCTTCGCTGCAGGGCCAGGAGCA-3′ for HA-ΔM-RFP; 5′-TGTGAACCTCTAATCGATAACTGATGGTGGAACGG-3′ for ΔM4; 5′-CATACCACTTTTCCACTTAAGCAGAAACGGCCTCAAAA-3′ for ΔC11; 5′-CAGGATGCGGTCGCTGCTGCAGGGCCAGGAGCA-3′ for Div-C; and 5′-TACCCATACGATGTTCCAGATTACGCT-3′ for HA-Diversin.
HA-ΔAnk-RFP and HA-ΔC-RFP were generated by swapping deleted regions from ΔAnk-RFP and ΔC-RFP with HA-Diversin-RFP. CFP-Stbm in pCS105 was made by fusing Xenopus Stbm cDNA (Darken et al., 2002) to the 3′ terminus of the coding sequence of CFP in frame. Fz8-GFP in pXT7 was constructed as an in-frame fusion of Xenopus Fz8 (Xfz8) lacking the C-terminal 17 amino acids to eGFP (at the C-terminus). Details of plasmid construction are available on request.
Embryo culture, microinjection and explantation for localization studies
Eggs and embryos were obtained from Xenopus laevis and cultured in 0.1× Marc's modified Ringer's solution (MMR) (Newport and Kirschner, 1982) as described previously (Itoh et al., 2005). Staging was according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967). For microinjection, embryos were transferred to 3% Ficoll in 0.5× MMR and injected at the four- to eight-cell stage with 10 nl of a solution containing RNAs. Capped synthetic RNAs were generated by in vitro transcription with SP6, T7 or T3 RNA polymerase using the mMessage mMachine kit (Ambion). Injections were carried out into two animal blastomeres of four- to eight-cell embryos. When the injected embryos reached stage 10, ectodermal explants (animal caps) were excised to examine subcellular localization of fluorescent proteins. The animal caps were fixed in 3.7% formaldehyde in phosphate buffered saline (PBS) for 30 minutes, washed with PBS, stained with 1 μg/ml 4,6-diamidino-2-phenylindole (DAPI) and mounted in 70% glycerol-PBS containing 25 mg of diazacyclo(2,2,2)-octane (DABCO, Sigma) per ml. Fluorescence was visualized using Apotome optical sections with a Zeiss Axio Imager.
The injected mRNAs encode Xfz8 (Itoh et al., 1998), Xdsh-GFP (Itoh et al., 2005), CAAX-GFP (Ossipova et al., 2007), Xfz8-GFP, Xenopus Stbm-CFP, mouse Diversin-RFP, HA-Diversin-RFP and deletion constructs of Diversin (ΔAnk, ΔM and ΔC). For each group, 10-15 animal caps from the same batch of embryos were excised for observation. Approximately 80-90% of the animal caps showed clear fluorescence with reproducible localization patterns. Each result was reproduced in two to five independent experiments.
Western analysis and antibodies
Western analysis was carried out using standard techniques as previously described (Itoh et al., 2000). Four animal blastomeres were injected with RNAs encoding HA-Diversin-RFP, ΔAnk-RFP, ΔM-RFP, ΔC-RFP, HA-ΔM4-RFP, HA-ΔC11-RFP, Div-C-RFP, Wnt3a and FLAG–β-catenin. Cellular lysates from the injected embryos were prepared at stage 10.5 and used for western analysis. For the analysis of β-catenin stabilization, animal caps were excised from injected embryos at stage 9 and the explants were cultured until stage 10.5. Other antibodies were anti-HA 12CA5 (Roche, 1/200), anti-β-tubulin (BioGenex, 1/200) and anti-FLAG M2 (Sigma). For the generation of anti-Diversin antibodies, a fragment corresponding to amino acids 573-649 of mouse Diversin was fused with glutathione-S-transferase (GST) and purified from Escherichia coli BL21 cells following standard techniques. Sera from rabbits immunized with this protein were affinity purified and used for western analysis (1/1000) and immunofluorescence (1/200).
Immunostaining and tissue culture
For cryosectioning, embryos injected with RNAs were devitellinized at stage 10, fixed in Dent's fixative for 1-2 hours, washed with PBS and embedded in 15% fish gelatin/15% sucrose solution. The embedded embryos were frozen in dry ice and sectioned at 10 μm with Leica Cryostat. Sagittal sections were stained with goat (Santa Cruz Biotechnology, 1/500) or mouse (Sigma, 1/500) anti-γ-tubulin, mouse-anti-HA (1/200), rabbit-anti-GFP (1/1000), or rabbit anti-Diversin antibodies (1/100) and Alexa-Fluor-488-conjugated (Invitrogen, 1/200) or Cy3-conjugated (Jackson ImmunoResearch, 1/200) secondary antibodies. Diversin-RFP was visualized without immunostaining. HA-Diversin was stained with anti-HA and Div-C–RFP was stained with anti-Diversin antibodies. Fluorescence was visualized as described above. The following proteins were supplied by RNA microinjection: Xwnt3a (Wolda et al., 1993), Xwnt5a (Moon et al., 1993), Xwnt11 (Tada and Smith, 2000), Xfz8 (Itoh et al., 1998), Myc-Dsh (Sokol, 1996), HA-Diversin, HA-Diversin-RFP constructs, Div-C–RFP and CAAX-GFP. Nine to ten embryos were examined in cross-sections for each group. RFP fluorescence and γ-tubulin staining were identified in more than 80% of injected cells in the sectioned embryos. Reproducible localization patterns of Diversin were observed in two to four independent experiments for each construct.
HeLa cells were cultured as described (Itoh et al., 2005). For immunostaining, cells were treated with extraction buffer, containing 0.5% Triton X-100, 80 mM PIPES (pH 6.8), 1 mM magnesium chloride and 1 mM EGTA, for 30 seconds and fixed in methanol at –20°C for 10 minutes as described in Bahmanyar et al. (Bahmanyar et al., 2008). The cells were co-stained with anti-Diversin and anti-γ-tubulin antibodies and CyIII-conjugated anti-rabbit and Alexa-Fluor-488-conjugated anti-goat secondary antibodies.
Luciferase assay
For luciferase assays, pSia-Luc reporter DNA (20 pg) was co-injected with RNAs encoding Wnt3a and different Diversin constructs into one animal-ventral blastomere at the four- to eight-cell stage. Luciferase activity was measured as described (Fan et al., 1998).
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/20/3791/DC1
We thank Claudia Sievers and members of the Sokol laboratory for helpful discussions and critical comments on the manuscript. We also thank Jane Babiarz for participation in HeLa-cell culture experiments and Takayuki Yasunaga for help in purifying anti-Diversin antibody. This study was supported by NIH grants to S.Y.S. and M.M. Deposited in PMC for release after 12 months.
References
- Axelrod, J. D. (2001). Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 15, 1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod, J. D., Miller, J. R., Shulman, J. M., Moon, R. T. and Perrimon, N. (1998). Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 12, 2610-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano, J. L., Teslovich, T. M. and Katsanis, N. (2005). The centrosome in human genetic disease. Nat. Rev. Genet. 6, 194-205. [DOI] [PubMed] [Google Scholar]
- Bahmanyar, S., Kaplan, D. D., Deluca, J. G., Giddings, T. H., Jr, O'Toole, E. T., Winey, M., Salmon, E. D., Casey, P. J., Nelson, W. J. and Barth, A. I. (2008). beta-Catenin is a Nek2 substrate involved in centrosome separation. Genes Dev. 22, 91-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilic, J., Huang, Y. L., Davidson, G., Zimmermann, T., Cruciat, C. M., Bienz, M. and Niehrs, C. (2007). Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316, 1619-1622. [DOI] [PubMed] [Google Scholar]
- Bisgrove, B. W. and Yost, H. J. (2006). The roles of cilia in developmental disorders and disease. Development 133, 4131-4143. [DOI] [PubMed] [Google Scholar]
- Ciruna, B., Jenny, A., Lee, D., Mlodzik, M. and Schier, A. F. (2006). Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439, 220-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers, H. (2006). Wnt/beta-catenin signaling in development and disease. Cell 127, 469-480. [DOI] [PubMed] [Google Scholar]
- Cliffe, A., Hamada, F. and Bienz, M. (2003). A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr. Biol. 13, 960-966. [DOI] [PubMed] [Google Scholar]
- Corbit, K. C., Shyer, A. E., Dowdle, W. E., Gaulden, J., Singla, V., Chen, M. H., Chuang, P. T. and Reiter, J. F. (2008). Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat. Cell Biol. 10, 70-76. [DOI] [PubMed] [Google Scholar]
- Darken, R. S., Scola, A. M., Rakeman, A. S., Das, G., Mlodzik, M. and Wilson, P. A. (2002). The planar polarity gene strabismus regulates convergent extension movements in Xenopus. EMBO J. 21, 976-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, G., Jenny, A., Klein, T. J., Eaton, S. and Mlodzik, M. (2004). Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development 131, 4467-4476. [DOI] [PubMed] [Google Scholar]
- Eggenschwiler, J. T. and Anderson, K. V. (2007). Cilia and developmental signaling. Annu. Rev. Cell Dev. Biol. 23, 345-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, M. J., Gruning, W., Walz, G. and Sokol, S. Y. (1998). Wnt signaling and transcriptional control of Siamois in Xenopus embryos. Proc. Natl. Acad. Sci. USA 95, 5626-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed, E., Lacey, K. R., Huie, P., Lyapina, S. A., Deshaies, R. J., Stearns, T. and Jackson, P. K. (1999). Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 13, 2242-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumoto, K., Kadono, M., Izumi, N. and Kikuchi, A. (2009). Axin localizes to the centrosome and is involved in microtubule nucleation. EMBO Rep. 10, 606-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes, J. M., Liu, Y., Zaghloul, N. A., Leitch, C. C., Lawson, S. S., Kato, M., Beachy, P. A., Beales, P. L., DeMartino, G. N., Fisher, S. et al. (2007). Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nat. Genet. 39, 1350-1360. [DOI] [PubMed] [Google Scholar]
- Gordon, M. D. and Nusse, R. (2006). Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol. Chem 281, 22429-22433. [DOI] [PubMed] [Google Scholar]
- Habas, R. and Dawid, I. B. (2005). Dishevelled and Wnt signaling: is the nucleus the final frontier? J. Biol. 4, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, K., Jacob, J. and Sokol, Y. S. (1998). A role for Xenopus Frizzled 8 in dorsal development. Mech. Dev. 74, 145-157. [DOI] [PubMed] [Google Scholar]
- Itoh, K., Antipova, A., Ratcliffe, M. J. and Sokol, S. (2000). Interaction of dishevelled and Xenopus axin-related protein is required for wnt signal transduction. Mol. Cell. Biol. 20, 2228-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, K., Brott, B. K., Bae, G. U., Ratcliffe, M. J. and Sokol, S. Y. (2005). Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J. Biol. 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenny, A., Darken, R. S., Wilson, P. A. and Mlodzik, M. (2003). Prickle and Strabismus form a functional complex to generate a correct axis during planar cell polarity signaling. EMBO J. 22, 4409-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, C., Roper, V. C., Foucher, I., Qian, D., Banizs, B., Petit, C., Yoder, B. K. and Chen, P. (2008). Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat. Genet. 40, 69-77. [DOI] [PubMed] [Google Scholar]
- Kaplan, D. D., Meigs, T. E., Kelly, P. and Casey, P. J. (2004). Identification of a role for beta-catenin in the establishment of a bipolar mitotic spindle. J. Biol. Chem. 279, 10829-10832. [DOI] [PubMed] [Google Scholar]
- Keller, R. (2002). Shaping the vertebrate body plan by polarized embryonic cell movements. Science 298, 1950-1954. [DOI] [PubMed] [Google Scholar]
- Kilian, B., Mansukoski, H., Barbosa, F. C., Ulrich, F., Tada, M. and Heisenberg, C. P. (2003). The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech. Dev. 120, 467-476. [DOI] [PubMed] [Google Scholar]
- Kim, S. M., Choi, E. J., Song, K. J., Kim, S., Seo, E., Jho, E. H. and Kee, S. H. (2009). Axin localizes to mitotic spindles and centrosomes in mitotic cells. Exp. Cell Res. 315, 943-954. [DOI] [PubMed] [Google Scholar]
- Klaus, A. and Birchmeier, W. (2008). Wnt signalling and its impact on development and cancer. Nat. Rev. Cancer 8, 387-398. [DOI] [PubMed] [Google Scholar]
- Lawrence, P. A., Struhl, G. and Casal, J. (2007). Planar cell polarity: one or two pathways? Nat. Rev. Genet. 8, 555-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie, R. K., Bahmanyar, S., Siemers, K. A., Votin, V., Chang, P., Stearns, T., Nelson, W. J. and Barth, A. I. (2004). Adenomatous polyposis coli and EB1 localize in close proximity of the mother centriole and EB1 is a functional component of centrosomes. J. Cell Sci. 117, 1117-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova, O., Kamberov, E. and Margolis, B. (2000). Generation of deletion and point mutations with one primer in a single cloning step. Biotechniques 29, 970-972. [DOI] [PubMed] [Google Scholar]
- Moeller, H., Jenny, A., Schaeffer, H. J., Schwarz-Romond, T., Mlodzik, M., Hammerschmidt, M. and Birchmeier, W. (2006). Diversin regulates heart formation and gastrulation movements in development. Proc. Natl. Acad. Sci. USA 103, 15900-15905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, R. T., Campbell, R. M., Christian, J. L., McGrew, L. L., Shih, J. and Fraser, S. (1993). Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development 119, 97-111. [DOI] [PubMed] [Google Scholar]
- Neugebauer, J. M., Amack, J. D., Peterson, A. G., Bisgrove, B. W. and Yost, H. J. (2009). FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature 458, 651-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport, J. and Kirschner, M. (1982). A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell 30, 675-686. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop, P. D. and Faber, J. (1967). Normal table of Xenopus laevis (Daudin). Amsterdam: North-Holland Publishing.
- Ossipova, O., Tabler, J., Green, J. B. and Sokol, S. Y. (2007). PAR1 specifies ciliated cells in vertebrate ectoderm downstream of aPKC. Development 134, 4297-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, T. J., Mitchell, B. J., Abitua, P. B., Kintner, C. and Wallingford, J. B. (2008). Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat. Genet. 40, 871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, A. J., May-Simera, H., Eichers, E. R., Kai, M., Hill, J., Jagger, D. J., Leitch, C. C., Chapple, J. P., Munro, P. M., Fisher, S. et al. (2005). Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat. Genet. 37, 1135-1140. [DOI] [PubMed] [Google Scholar]
- Rothbacher, U., Laurent, M. N., Deardorff, M. A., Klein, P. S., Cho, K. W. and Fraser, S. E. (2000). Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 19, 1010-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambony, A. and Wedlich, D. (2007). Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev. Cell 12, 779-792. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond, T., Asbrand, C., Bakkers, J., Kuhl, M., Schaeffer, H. J., Huelsken, J., Behrens, J., Hammerschmidt, M. and Birchmeier, W. (2002). The ankyrin repeat protein Diversin recruits Casein kinase Iepsilon to the beta-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev. 16, 2073-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Romond, T., Merrifield, C., Nichols, B. J. and Bienz, M. (2005). The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J. Cell Sci. 118, 5269-5277. [DOI] [PubMed] [Google Scholar]
- Seifert, J. R. and Mlodzik, M. (2007). Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 8, 126-138. [DOI] [PubMed] [Google Scholar]
- Semenov, M. V., Habas, R., Macdonald, B. T. and He, X. (2007). SnapShot: Noncanonical Wnt Signaling Pathways. Cell 131, 1378. [DOI] [PubMed] [Google Scholar]
- Sillibourne, J. E., Milne, D. M., Takahashi, M., Ono, Y. and Meek, D. W. (2002). Centrosomal anchoring of the protein kinase CK1delta mediated by attachment to the large, coiled-coil scaffolding protein CG-NAP/AKAP450. J. Mol. Biol. 322, 785-797. [DOI] [PubMed] [Google Scholar]
- Smalley, M. J., Signoret, N., Robertson, D., Tilley, A., Hann, A., Ewan, K., Ding, Y., Paterson, H. and Dale, T. C. (2005). Dishevelled (Dvl-2) activates canonical Wnt signalling in the absence of cytoplasmic puncta. J. Cell Sci. 118, 5279-5289. [DOI] [PubMed] [Google Scholar]
- Sokol, S. (2000). A role for Wnts in morpho-genesis and tissue polarity. Nat. Cell Biol. 2, E124-E125. [DOI] [PubMed] [Google Scholar]
- Strutt, D. I. (2001). Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol. Cell 7, 367-375. [DOI] [PubMed] [Google Scholar]
- Tada, M. and Smith, J. C. (2000). Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development 127, 2227-2238. [DOI] [PubMed] [Google Scholar]
- Tree, D. R., Shulman, J. M., Rousset, R., Scott, M. P., Gubb, D. and Axelrod, J. D. (2002). Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 109, 371-381. [DOI] [PubMed] [Google Scholar]
- Wakefield, J. G., Stephens, D. J. and Tavare, J. M. (2003). A role for glycogen synthase kinase-3 in mitotic spindle dynamics and chromosome alignment. J. Cell Sci. 116, 637-646. [DOI] [PubMed] [Google Scholar]
- Wallingford, J. B., Fraser, S. E. and Harland, R. M. (2002). Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev. Cell 2, 695-706. [DOI] [PubMed] [Google Scholar]
- Wang, Y. and Nathans, J. (2007). Tissue/planar cell polarity in vertebrates: new insights and new questions. Development 134, 647-658. [DOI] [PubMed] [Google Scholar]
- Wigley, W. C., Fabunmi, R. P., Lee, M. G., Marino, C. R., Muallem, S., DeMartino, G. N. and Thomas, P. J. (1999). Dynamic association of proteasomal machinery with the centrosome. J. Cell Biol. 145, 481-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzel, S., Zimyanin, V., Carreira-Barbosa, F., Tada, M. and Heisenberg, C. P. (2006). Wnt11 controls cell contact persistence by local accumulation of Frizzled 7 at the plasma membrane. J. Cell Biol. 175, 791-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolda, S. L., Moody, C. J. and Moon, R. T. (1993). Overlapping expression of Xwnt-3A and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev. Biol. 155, 46-57. [DOI] [PubMed] [Google Scholar]
- Wu, J., Jenny, A., Mirkovic, I. and Mlodzik, M. (2008). Frizzled-Dishevelled signaling specificity outcome can be modulated by Diego in Drosophila. Mech. Dev. 125, 30-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Snyder, J., Miller, J. R., Brown, J. D., Lai, C. J. and Moon, R. T. (1996). A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr. Biol. 6, 1302-1306. [DOI] [PubMed] [Google Scholar]
- Yin, C., Kiskowski, M., Pouille, P. A., Farge, E. and Solnica-Krezel, L. (2008). Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J. Cell Biol. 180, 221-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, X., Huang, H., Tamai, K., Zhang, X., Harada, Y., Yokota, C., Almeida, K., Wang, J., Doble, B., Woodgett, J. et al. (2008). Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135, 367-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.