Abstract
The successful elimination of pathogenic cells and microorganisms by the humoral immune system relies on effective interactions between host immunoglobulins and Fcγ receptors on effector cells, in addition to the complement system. Essential Ig motifs that direct those interactions reside within the conserved IgG lower hinge/CH2 interface. We noted that a group of tumor-related and microbial proteases cleaved human IgG1s in that region, and the “nick” of just one of the heavy chains profoundly inhibited IgG1 effector functions. We focused on IgG1 monoclonal antibodies (mAbs) since IgG1 is the most abundant human subclass and demonstrates robust Fc-mediated effector functions. The loss of Fc-mediated cell killing activities was correlated with diminished binding to the Fcγ family of receptors, but a similar decrease in affinity was not observed toward the FcRn receptor that maintains IgG in circulation. Endogenous human IgG cleavage products of comparable size to mAbs with the single cleavage were detected by Western blot analysis in synovial fluid from patients with rheumatoid arthritis and in breast carcinoma extracts. Their detection is problematic under physiological conditions, since there is no loss of structure, and antigen-binding capability is unaffected. These findings suggest that within the hostile proteolytic microenvironments associated with many diseases, key effector functions of host IgGs, or therapeutic Abs, may be compromised.
Keywords: antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity, Fc gamma Receptors, matrix metalloproteinases, monoclonal antibodies
Antibodies are key mediators of inflammatory responses that can link cell-bound antigen with immune effector cells. The structure of an IgG antibody is characterized by two antigen-binding Fab arms that are coupled to the Fc portion by the hinge region. The structural organization allows antibodies to recognize either soluble or cell-bound antigen through the Fab arms and activate (or suppress) immune cells by interactions of the Fc portion of the antibody with Fc receptor (FcR) bearing cells. Binding of antigen-engaged IgGs to FcRs activates effector functions such as phagocytosis, endocytosis of IgG-opsonized particles, release of inflammatory cytokines and chemokines, and antibody-dependent cytoxicity (ADCC) of IgG-coated cells (1). We focused on IgG1, because it is the most abundant subclass of circulating human IgG and is the most widely available of human monoclonal antibodies (mAbs). IgG1 interacts with the Fcγ family of receptors that include FcγRI (CD64), FcγRII (CD32), which has the isoforms FcγRIIa, FcγRIIb, and FcγRIIc, and FcγRIII (CD16), which has the isoforms FcγRIIIa and FcγRIIIb (1). All members of the Fcγ family of receptors bind to amino acid residues in the lower hinge region, in particular, the critical stretch in IgG1 from E233/L234/L235/G236/G237/P238 (EU numbering) (2–12). Amino acids within the lower hinge and CH2 regions are also implicated in complement C1q binding (13) and the subsequent lysis of target cells. For convenience, this domain of IgG structure will subsequently be referred to as the lower hinge. In contrast, the residues on IgG responsible for binding the MHC-class I related receptor, the neonatal Fc receptor (FcRn) that mediates the serum half-life of circulating IgGs (14–16), are located in the area between the CH2 and CH3 regions of the Fc (17–19).
Several groups previously documented that certain proteases associated with inflammation, tumor invasion, metastasis, and bacterial infections have the ability to cleave IgGs (20, 21). Several proteases preferentially cleave IgGs in the lower hinge, including the matrix metalloproteinases (MMPs) stromelysin-1 (MMP-3), metalloelastase (MMP-12) (both cleave between P232 and E233), and matrilysin (MMP-7) (between L234 and L235); cathepsin G, Staphylococcus aureus glutamyl endopeptidase I (GluV8) (both of which cleave between E233 and L234); and the IgG-degrading enzyme of Streptococcus pyogenes (IdeS) (between G236 and G237) (20–22). Proteolytic cleavage of IgGs by the latter group of proteases occurs in a step-wise process, whereby first one heavy chain is cleaved, generating an intermediate product that has a single cleavage in the lower hinge (20, 21, 23). A second, slower cleavage of the opposite heavy chain in the lower hinge separates the F(ab′)2 fragment from the Fc fragment. F(ab′)2 fragments have no Fcγ-mediated effector functions since they cannot bind to members of the FcγR or sufficiently bind C1q to mediate complement-dependent cytotoxicity (CDC). Additionally, F(ab′)2 fragments have decreased serum half-lives relative to intact IgGs since they can no longer interact with FcRn. In contrast, we are unaware of studies that have addressed the biological implications of the single-cleaved intermediate containing the Fc domain still linked through the second heavy chain.
Despite the fact that many of the above-mentioned proteases have the ability to cleave IgGs in purified in vitro systems, there are few reports of IgG cleavage products from in vivo sources. Early studies reported the presence of IgG breakdown products in abscess fluid (24, 25). We have previously shown the presence of degraded IgGs in the synovial fluids (SF) from patients with rheumatoid arthritis (21). Others have demonstrated that IdeS cleaves IgG-coated S. pyogenes with resulting inhibition of killing by phagocytic cells (26). Moreover, the existence of human autoantibodies directed against the cleaved hinge of IgGs alludes to the presence of cleaved IgGs as autoantigens (27–29). These findings led to the present examination into the presence of proteolytic intermediates from in vivo samples and the functional attributes of single-cleaved IgG.
Results and Discussion
Human and Bacterial Proteases Can Cleave Human IgG1 in the Lower Hinge/CH2 Region to Generate a Single-Cleaved Intermediate.
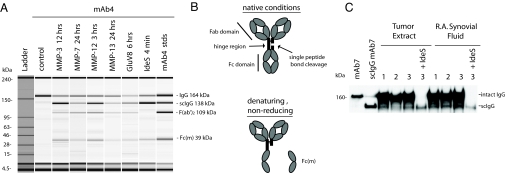
This study was prompted by repeated observations of a single-cleaved intermediate during proteolysis of humanized IgG1κ mAbs (160 kDa) by the human proteases, MMP-3, MMP-7, MMP-12, MMP-13, and the two bacterial proteases GluV8 and IdeS (21). Depending on the enzyme, cleavage occurred at slightly different positions within a closely spaced sequence of several amino acids in the lower IgG1 hinge (21) and was visualized in each case as a species of approximately 138 kDa in nonreducing SDS-PAGE or microcapillary electrophoresis (Fig. 1A). The decreased mass conforms with the separation of the Fc monomer (1/2 Fc) under the denaturing conditions as previously noted for MMP-3 and MMP-7 (20). Extended incubation led to cleavage of both heavy chains to yield F(ab′)2s in several of the protease digestions in Fig. 1A.
Fig. 1.
Accumulation of a single-cleaved proteolytic product of IgG in vitro and its detection in vivo. (A) Capillary gel electrophoresis depiction of partial digestions of mAb4 with human and bacterial proteases under denaturing, nonreducing conditions. Specific enzymes and digestion times are indicated above the lanes. The right hand lane contains a mixture of purified standards of mAb4 and its proteolytic fragments. The single-cleaved intermediate is denoted as scIgG, and the Fc monomer released under denaturing conditions is denoted Fc(m). (B) Schematic model of a human IgG1 with the position of the lower hinge cleavage indicated. Single-cleaved IgG is intact under native (nondenaturing) conditions, and the Fc monomer dissociates under denaturing conditions. (C) Detection of IgG components in protein A-purified synovial fluid (SF) from three patients with rheumatoid arthritis and from three human breast tumor extracts. Immunoblotting was performed with an anti-Fc gamma domain antibody following SDS-PAGE under denaturing, nonreducing conditions.
Under nondenaturing conditions (e.g., HPLC, SEC), the masses of intact IgG and the single-cleaved intermediate were indistinguishable because of the core hinge disulfide bridging between Fc and Fab arms and the strong noncovalent associations of the two paired Fc chains in the CH3 regions (30, 31). A putative structure for the single-cleaved IgG intermediate in nondenaturing and in denaturing conditions is depicted in Fig. 1B (see also ref. 20). Thus, analytical methodologies such as immunohistochemistry and flow cytometry that detect IgG through conventional anti-Fc or anti-heavy/light chain antibody reagents would also fail to detect the presence of the single peptide bond cleavage.
Extending previous observations of IgG breakdown products in the SF from patients with rheumatoid arthritis (21), we purified Fc-containing components from SF and invasive human breast carcinoma tissues by protein A chromatography. Immunoblotting indicated the presence of two prominent bands in denaturing, nonreducing conditions (Fig. 1C). The sizes of the two bands coincided with an intact and a single-cleaved mAb standard, respectively. Treating the SF-derived and tumor extract components with exogenous IdeS protease converted the intact IgG to the position of the endogenous single-cleaved intermediate in SF (Fig. 1C, rightmost lane). The enzymes(s) responsible for endogenous IgG cleavage in the protease-rich SF and breast tumor environments (32–34) were not identified in the present investigation. Also, the rates at which IgG cleavage products are generated or accumulate in vivo were not specifically investigated here, although such determinations are of obvious interest for future study. However, the results pointed to an accumulation of single-cleaved intermediates as observed with mAbs/purified proteases in vitro (Fig. 1A). The potential relevance of these findings for immune function in protease-rich inflammatory and tumor microenvironments (26, 35, 36) led us to investigate the functional properties of antibodies possessing the single lower hinge cleavage.
Antibodies Are Susceptible to Proteolysis While Bound to Cell Surface Antigens.
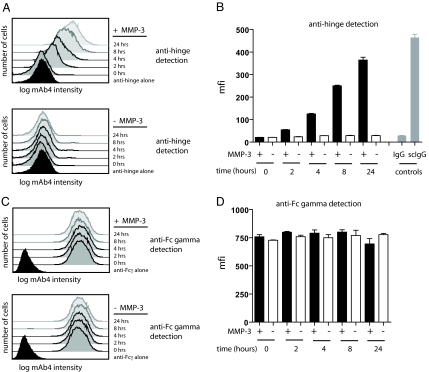
A mAb directed to CD142 on MDA-MB-231 breast carcinoma cells was prebound to the cells and then exposed to MMP-3 for varying times. The cells were processed for flow cytometry and probed for the presence of IgG1 Fc or alternatively for MMP-3-generated proteolytic derivatives of IgG1 using a specific rabbit anti-hinge antibody preparation (21). The results are presented as both comparative histograms (Fig. 2 A and C) and as bar graphs of the relative fluorescence signal over a 24-h period (Fig. 2 B and D). There was a time-dependent increase of cleaved IgG signal on MMP-3-treated cells that approached the value found for cells prebound with purified single-cleaved mAb4. There was no increase in signal with cells incubated for similar periods in the absence of MMP-3. The anti-Fc gamma domain signal was retained at a comparable level throughout this period regardless of the presence or absence of MMP-3 (Fig. 2D), indicating that the Fc component of IgG was not displaced. Thus, these results provided an example of protease-mediated generation and accumulation of the single-cleaved intermediate of prebound IgG on a cell surface.
Fig. 2.
MMP-3 addition to cells preincubated with mAb4 results in detection of cleaved IgG without appreciable loss of the Fc region. (A) Histograms for the detection of cleaved IgG hinge for MDA-MB-231 cells preincubated with mAb4, followed by addition of MMP-3 for 24, 8, 4, or 2 h. Levels of cleaved hinge were detected with a fluorescently labeled antibody specific for the MMP-3 hinge cleavage site. (B) Mean fluorescence intensities (mfi) plotted as bar graphs detected with the anti-hinge antibody are shown for each time point ± SD for three replicates. Single-cleaved IgG control is denoted as scIgG. (C) Histograms for the detection of the Fc gamma domain of mAb 4 IgG on MDA-MB-231 cells with or without the addition of MMP-3. (D) Mfi plotted as bar graphs detected with the anti-Fc gamma domain specific antibody are shown for each time point ± SD for three replicates.
Single-Cleaved Antibodies Were Ineffective at Mediating Cellular Lysis In Vitro.
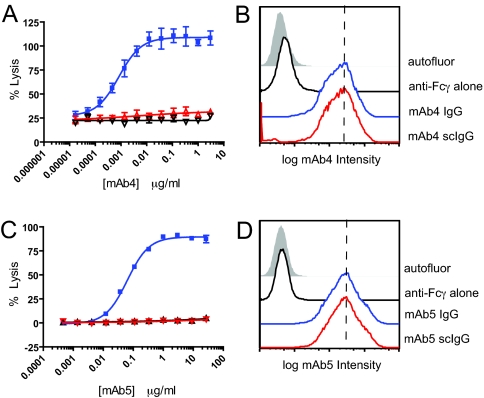
ADCC assays were performed with intact mAb4 IgG1 and its proteolytic derivatives using the MDA-MB-231 cells as targets and human PBMCs as effector cells (28). Intact mAb4 showed concentration-dependent cell lytic activity with an EC50 of approximately 3 ng/mL (Fig. 3A). The mAb4 single-cleaved IgG (via IdeS) showed no lytic activity [similar to the mAb4 F(ab′)2 fragment] at concentrations up to 3 μg/mL. However, the intact and single-cleaved mAb4 versions showed comparable antigen binding activity on the MDA-MB-231 cells, as assessed by flow cytometry (Fig. 3B). In a parallel system, complement-mediated lysis (CDC) of WIL2-S human B lymphoma cells by the anti-CD20 mAb5 IgG1 demonstrated an EC50 of approximately 100 ng/mL (Fig. 3C). The single-cleaved mAb5 derivative was inactive at up to 27 μg/mL [indistinguishable from F(ab′)2]. The intact and single-cleaved versions of mAb5 exhibited comparable antigen binding on the WIL2-S cells (Fig. 3D). In preliminary testing, an IgG3 mAb also showed a progressive, but slower, MMP-3-mediated decrease in ADCC activity, indicating that protease-mediated loss of function is not limited to IgG1. Thus, both FcγR-dependent and complement-mediated effector functions were decoupled from antigen binding function as a result of the single proteolytic cleavage in the lower hinge.
Fig. 3.
Single cleavage with IdeS inhibits ADCC and CDC activity without impacting antigen binding. (A) Concentration-dependent effects of intact mAb4 IgG (blue), its single-cleaved version (scIgG) (red), and the F(ab′)2 derivative (black) in ADCC. MDA-MB-231 were the target cells, and human PBMCs were the effector cells. Error bars correspond to the SD of triplicate determinations. (B) Surface levels of intact mAb4 (blue) and single-cleaved IgG (scIgG) of mAb4 (red) were measured by flow cytometry. The shaded area indicates autofluorescence, and the black line indicates fluorescence obtained with the anti-Fc gamma domain antibody alone. (C) CDC activity against WIL2-S cells using intact mAb5 IgG1 (blue), single-cleaved mAb5 (red), and mAb5 F(ab′)2 (black). Error bars correspond the SD of triplicate determinations. (D) Surface levels of intact mAb5 (blue) and single-cleaved mAb5 (red) were measured by flow cytometry on WIL2-S cells.
The profound loss of in vitro function resulting from the single IgG1 lower hinge cleavage was not previously noted, but carries potential in vivo implications even for high affinity Abs. If host IgGs against invasive cells undergo related single cleavages by locally expressed proteases within pathological settings, the proteolytic derivatives could retain affinity for the target antigen, remain bound to the cell, and thereby interfere with further intact IgG binding to that site. A similar hypothesis was forwarded for proteolytically cleaved IgAs in the digestive tract, allowing pathogenic organisms to colonize through evasion of host mucosal immune responses (37). A number of the proteases that we have identified to cleave IgG in vitro are expressed by invasive cells that would potentially gain advantage from localized and persistent impairment of in vivo host immune function (e.g., tumors, bacteria) (38–40). Based on the in vitro functional studies presented above, we predicted that a similar disjunction between antigen binding and effector functions should occur in vivo.
Single-Cleaved Antibodies Did Not Induce Specific Cell Clearance in Two Independent In Vivo Models.
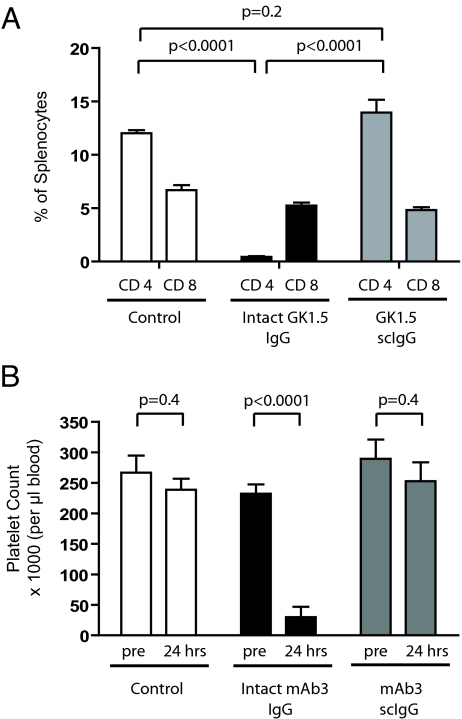
The first study assessed the survival/clearance of murine splenic CD4+ T cells by the rat GK1.5 anti-CD4 mAb (41–43). The single-cleaved version of GK1.5 was generated with IdeS and purified similarly to the human IgG1s as described in Materials and Methods. GK1.5 is a rat IgG2b with the same highly conserved LLGGPSV sequence in the lower hinge for which IdeS exhibits specificity in human, mouse, and rabbit IgGs (44). Both intact and single-cleaved GK1.5 showed similar frequencies of CD4+ T cells in mouse splenocyte preparations when the antibodies were added in vitro. Separately, groups of five age-matched, female mice were injected with either intact GK1.5 or the single-cleaved form of GK1.5 (0.3 mg/animal, respectively). After 4 days, the spleens were harvested and tested for the presence of CD8+ T cells and CD4+ T cells (using the RM4–4 anti-CD4 antibody that has a different epitope than GK1.5) (45). Intact GK1.5 induced a marked reduction in the frequency of CD4+ T cells, but not CD8+ T cells from the spleens of the treated animals (Fig. 4A). In contrast, single-cleaved GK1.5 did not induce cellular depletion, and the frequency of CD4+ T cells relative to CD8+ cells was similar to the profile in untreated mice.
Fig. 4.
Unlike intact IgGs, single-cleaved IgGs that target in vivo cells do not cause cell clearance. (A) Percentage of remaining murine CD4+ splenocytes detected in vivo by flow cytometry at 4 days following treatment with no antibody (control), intact GK1.5, and single-cleaved GK1.5 (scIgG). Bar heights correspond to the mean ± SD of five animals. (B) Blood platelet counts in dogs before and 24 h after treatment with no antibody (control), intact mAb3, and single-cleaved mAb3. Bar heights correspond to the mean ± SD of five animals.
In a second study, the intact vs. single-cleaved comparison was conducted with the chimeric mAb3 IgG1 that binds to the CD41/CD61 receptor on the surface of human and dog platelets (46). Platelet counts were monitored following i.v. administration of each agent at 0.05 mg/kg body weight (defined by an intact IgG pilot study). The results in each group of five animals (including a separate saline control group) are summarized in Fig. 4B. Approximately 90% of the circulating platelets at baseline were cleared within 24 h in the group receiving intact mAb3 IgG. No similar decline occurred in the group receiving the single-cleaved IgG derivative (not significantly different from the saline control group). Moreover, the profile in the single-cleaved IgG group remained unchanged for the 96-h observation period indicating the absence of delayed effect. These two unrelated in vivo models confirmed that single-cleaved, receptor-targeted mAbs were ineffective at cell clearance.
The Loss-of-Function of Single-Cleaved IgG Correlated with Decreased Affinity for Fcγ Receptors.
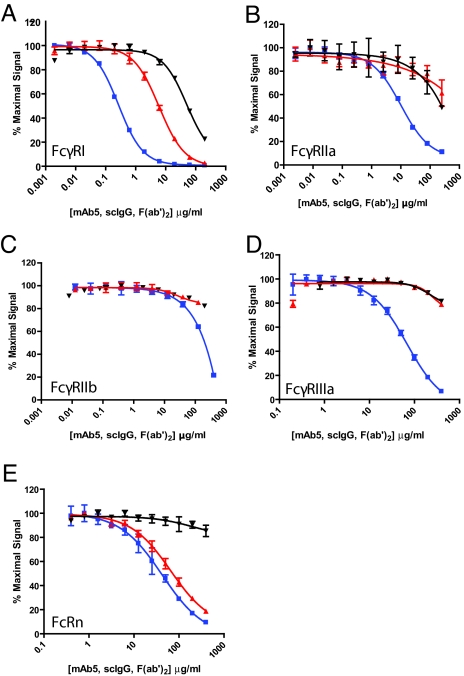
Estimates of the relative affinities of intact and single-cleaved mAb5 to the human Fcγ family of receptors; FcγRI, FcγRIIa, FcγRIIb, FcγRIIIa, and FcRn were performed with the AlphaScreen method (47), and the results are depicted as competition binding curves (Fig. 5 A–E). The F(ab′)2 fragment of mAb5 was included in each case as a control. The results indicated >20-fold losses of relative binding affinities by the single-cleaved mAb5 compared with the intact version to the various purified FcγRs. The displacement was particularly striking in regard to FcγRIIIa, the receptor on natural killer cells associated with ADCC (48, 49) (Fig. 5D). The high affinity binding to FcγRI, and to the lower affinity interactions with FcγRIIa and FcγRIIb, were also diminished compared with intact IgG. Parallel experiments using MMP-3 generated derivatives of mAb4 and mAb6 yielded similar results regarding FcγR binding (results not shown).
Fig. 5.
Single cleavage adversely impacts IgG binding avidity to purified, recombinant human Fcγ receptors without similar effect on binding to FcRn. (A) The binding of mAb5 IgG (blue), the single-cleaved (scIgG) version of mAb5 (red), or the F(ab′)2 of mAb5 (black) to FcγRI measured by competition AlphaScreen as described in the Materials and Methods. (B) Binding of mAb5 and its derivatives to FcγRIIa. (C) Binding of mAb5 and its derivatives to FcγRIIb. (D) Binding of mAb5 and its derivatives to FcγRIIIa. (E) Binding of mAb5 and its derivatives to FcRn.
The impaired interactions of single-cleaved IgGs with the Fcγ receptor family provide a mechanistic explanation for the in vitro and in vivo loss-of-function observations. Molecular structural studies had previously detailed a critical importance of the IgG1 lower hinge sequence from E233 to P238 for FcγR binding (3–12). Each of the proteases defined in the current study cleaved IgG1 within an overlapping domain from P232 through G236. The resulting loss-of-function suggests that proteolytically induced disruptions of key structural or conformational parameters in the lower hinge domain impair interactions with Fcγ receptors. This conjecture is consistent with a crystallographic analysis showing that FcγRIIIa engages Fc in an asymmetric fashion with simultaneous binding to both heavy chains at residues L234 to P238 (50). The highly conserved lower hinge sequence for FcγR binding encompasses key sites of proteolytic susceptibility—a significant challenge for any attempt to engineer protease-resistant mAbs with retention of effector functions. A similar disruption of C1q binding was suggested by the loss of complement activity in the single-cleaved IgG.
In distinct contrast to the results with the Fcγ receptors, there was little if any loss of binding affinity of mAb5 single-cleaved IgG to the FcRn receptor (Fig. 5E). FcRn engages IgGs at a different site than FcγRs (18) and extends their circulating lifespans by recycling IgGs through endothelial cells (14–16, 18). The retention of binding of single-cleaved IgG to FcRn prompted an in vivo assessment.
Single-Cleaved IgG mAbs Circulate in Mice Similarly to Intact IgGs.
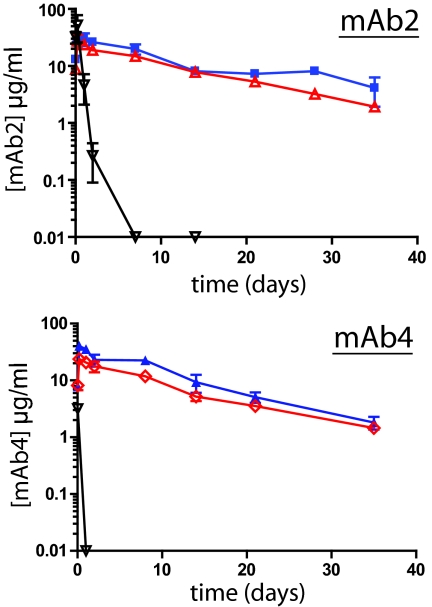
Two antibodies, mAb2 and mAb4, possessing no known binding reactivity to murine antigens, were evaluated independently in mice. Previous reports demonstrated that human IgGs bound to murine FcRn (51). Groups of mice (n = 3) received 2 mg/kg i.p. doses of each intact IgG, single-cleaved IgG, or F(ab′)2 (the proteolytic derivatives were generated with MMP-3). The results of 5-week comparisons of the serum concentrations of mAb2 and mAb4 are shown in Fig. 6. All mAb derivatives demonstrated maximum detectable levels at 1 h post-treatment. The F(ab′)2 fragments in each case disappeared rapidly from circulation. In contrast, intact IgGs and their single-cleaved derivatives remained detectable at 35 days. The results confirmed that intact human IgG mAbs possessed prolonged circulation times consistent with an FcRn-mediated mechanism and that their behavior was mirrored by the respective single-cleaved derivatives. These in vivo results in mice were in agreement with the observed binding to human FcRn in vitro (Fig. 5E).
Fig. 6.
Single cleaved IgGs possess comparable circulating lifespans in mice as intact IgGs. (Upper) Pharmacokinetics of intact mAb2 (blue), its MMP-3 generated single-cleaved IgG (red), and F(ab′)2 (black) following i.p. administration to mice. Agents were injected at 2 mg/kg, and serum samples (n = 3) were obtained at times including 1 and 5 h and 2, 7, 14, 21, 28, and 35 days. The concentrations of each component were determined by quantitative ELISA. Data points correspond to the mean ± SD of three animals. (Lower) Analogous experiment to panel A using intact mAb4 (blue), its MMP-3 generated single-cleaved IgG (red) and F(ab′)2 (black).
Although speculative, it seems likely that the most relevant in vivo cleavages in terms of immune dysfunction would be to antibodies already targeting cell surfaces as occurs in membrane receptor shedding by cellular proteases (52). Solution-phase competition can also be considered, and measurements of single-cleaved IgG derivatives vs. intact counterparts were undertaken using functional, cell-killing assays. As predicted from the equivalence in binding to surface antigens (Fig. 3 B and D), competitive interference was apparent. However, in accord with a previously reported mAb/Fc receptor study (53), the amount of bound mAb required to achieve cell lysis was well below the antigen saturation point and introduced analytical complexities that point to a need for more extensive study.
Taken together, this investigation points to a previously unrecognized functional decoupling of the antigen binding activity of human IgG1 and FcγR-mediated effector actions that result from a single proteolytic cleavage in the lower hinge. The findings were replicated with multiple human IgG1 mAbs and with several human and bacterial proteases. Evidence for IgG breakdown products, including bands analogous to the single-cleaved IgGs produced in vitro, was obtained in IgG preparations from SF and extracts of human breast tumor tissue. Proteolytic cleavage also occurred with mAbs bound to the cell surface, without disruption of binding to target antigens, suggesting that an accumulation of bound, proteolytically modified IgG might mask the target from subsequent immune surveillance mediated by competent IgGs. The initiating proteolytic event described here goes undetected in most conventional localizations of IgGs in tissues or on cells, yet may be a key in vivo breakdown pathway. The findings point to an unsuspected mechanism by which pathogenic and invasive cells might combat immune surveillance.
Materials and Methods
Monoclonal Antibodies.
mAbs are designated similarly to a previous report (21) as follows: mAb2 (anti-cytokine; human/murine chimeric IgG1); mAb3 (anti-human CD41/CD61; human/murine chimeric IgG1); mAb4 (anti-human CD142; CDR-grafted humanized IgG1); mAb5 (anti-human CD20; human/murine chimeric IgG1 from Genentech, Idec): mAb6 (anti-human Epcam; human/murine chimeric IgG1); mAb7 (anti-human CD142; CDR-grafted humanized IgG4); GK1.5 (anti-murine CD4; rat IgG2b from Harlan Bioproducts).
Proteases and IgG Digestions.
Human matrix metalloproteinases MMP-2, MMP-3, MMP-7, MMP-9, MMP-12, and MMP-13, and GluV8 and IdeS were obtained, expressed, or activated from recombinant proenzymes as previously described (21). Further details for both purified in vitro digestions and cell bound digestions can be found in the SI Text.
In Vivo Depletion Studies with Monoclonal Antibodies.
Female, age-matched C57/B6 mice were used for the CD4+ T cell in vivo depletion study. Groups of five mice were injected i.p. at a dose of 0.3 mg/animal with intact GK1.5 or single-cleaved GK1.5 (generated with IdeS) or were untreated. Fours days after injection, spleens were harvested for flow cytometry analysis using anti-CD4 (RM4–4 that binds to a site distinct from GK1.5) (45), anti-CD8α, anti-CD3, and anti-B220 (all from Becton Dickinson).
An anti-platelet antibody (mAb3) and its single-cleaved derivative were examined in male beagle dogs for immune-mediated platelet clearance (mAb3 binds to the CD41/CD61 receptor on canine platelets). Three groups of five animals received either intact mAb3 IgG (0.05 mg/kg), purified single-cleaved mAb3 generated with IdeS (0.05 mg/kg), or saline, respectively. All test materials were injected by a 20-min infusion in a total volume of 20 mL at Covance, and platelet counts were quantified by automated cell counting (Quest Diagnostics). Statistics for both in vivo depletion studies were performed using Student's t test, and calculated P values of <0.05 were considered significant.
ADCC and CDC Assays.
ADCC assays were performed as previously described using PBMCs purified from human blood and used as effector cells. MDA-MB-231 human breast carcinoma cells (ATCC) were used as target cells with a ratio of one target cell to 50 effector cells (28). CDC assays were performed with WIL2-S cells (ATCC) as previously described (28). Data for ADCC and CDC were fit to a sigmoidal dose-response model using GraphPad Prism v5.
AlphaScreen Competition Binding Assays.
AlphaScreen (PerkinElmer) competition binding assays were adapted from previously reported conditions (47) (see SI Text).
Pharmacokinetic Studies.
PK studies were performed in BALB/c mice using intact IgGs, single-cleaved IgG, and F(ab′)2 fragments over the course of 35 days to monitor their circulating life-time (see SI Text).
Detection of IgG in Synovial Fluid from Rheumatoid Arthritis Patients and Human Breast Tumor Extracts.
IgG was protein A-purified from the synovial fluid (SF) of patients with rheumatoid arthritis and human breast tumor extracts, and denaturing SDS-PAGE was performed on the resultant IgGs (see SI Text).
Supplementary Material
Acknowledgments.
We thank Eva Emmell of Centocor, Inc. for oversight of the animal studies, Stanley Prince and Rangappa Ramachandra of Covance for the conduct of the canine study, and Kerry Brosnan of the Centocor, Inc. vivarium for performing the murine studies.
Footnotes
Conflict of interest statement: All authors are employees of Centocor R&D, Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0904174106/DCSupplemental.
References
- 1.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 2.Edelman GM, et al. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci USA. 1969;63:78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton DR, Jefferis R, Partridge LJ, Woof JM. Molecular recognition of antibody (IgG) by cellular Fc receptor (FcRI) Mol Immunol. 1988;25:1175–1181. doi: 10.1016/0161-5890(88)90153-8. [DOI] [PubMed] [Google Scholar]
- 4.Canfield SM, Morrison SL. The binding affinity of human IgG for its high affinity Fc receptor is determined by multiple amino acids in the CH2 domain and is modulated by the hinge region. J Exp Med. 1991;173:1483–1491. doi: 10.1084/jem.173.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan AR, Woof JM, Partridge LJ, Burton DR, Winter G. Localization of the binding site for the human high-affinity Fc receptor on IgG. Nature. 1988;332:563–564. doi: 10.1038/332563a0. [DOI] [PubMed] [Google Scholar]
- 6.Hulett MD, Witort E, Brinkworth RI, McKenzie IF, Hogarth PM. Identification of the IgG binding site of the human low affinity receptor for IgG Fc gamma RII. Enhancement and ablation of binding by site-directed mutagenesis. J Biol Chem. 1994;269:15287–15293. [PubMed] [Google Scholar]
- 7.Jefferis R, Lund J, Pound J. Molecular definition of interaction sites on human IgG for Fc receptors (huFc gamma R) Mol Immunol. 1990;27:1237–1240. doi: 10.1016/0161-5890(90)90027-w. [DOI] [PubMed] [Google Scholar]
- 8.Partridge LJ, Woof JM, Jefferis R, Burton DR. The use of anti-IgG monoclonal antibodies in mapping the monocyte receptor site on IgG. Mol Immunol. 1986;23:1365–1372. doi: 10.1016/0161-5890(86)90022-2. [DOI] [PubMed] [Google Scholar]
- 9.Shields RL, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 10.Tamm A, Kister A, Nolte KU, Gessner JE, Schmidt RE. The IgG binding site of human FcgammaRIIIB receptor involves CC′ and FG loops of the membrane-proximal domain. J Biol Chem. 1996;271:3659–3666. doi: 10.1074/jbc.271.7.3659. [DOI] [PubMed] [Google Scholar]
- 11.Wines BD, Powell MS, Parren PW, Barnes N, Hogarth PM. The IgG Fc contains distinct Fc receptor (FcR) binding sites: The leukocyte receptors Fc gamma RI and Fc gamma RIIa bind to a region in the Fc distinct from that recognized by neonatal FcR and protein A. J Immunol. 2000;164:5313–5318. doi: 10.4049/jimmunol.164.10.5313. [DOI] [PubMed] [Google Scholar]
- 12.Woof JM, Partridge LJ, Jefferis R, Burton DR. Localisation of the monocyte-binding region on human immunoglobulin G. Mol Immunol. 1986;23:319–330. doi: 10.1016/0161-5890(86)90059-3. [DOI] [PubMed] [Google Scholar]
- 13.Duncan AR, Winter G. The binding site for C1q on IgG. Nature. 1988;332:738–740. doi: 10.1038/332738a0. [DOI] [PubMed] [Google Scholar]
- 14.Ghetie V, et al. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996;26:690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- 15.Israel EJ, Wilsker DF, Hayes KC, Schoenfeld D, Simister NE. Increased clearance of IgG in mice that lack beta 2-microglobulin: Possible protective role of FcRn. Immunology. 1996;89:573–578. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burmeister WP, Huber AH, Bjorkman PJ. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature. 1994;372:379–383. doi: 10.1038/372379a0. [DOI] [PubMed] [Google Scholar]
- 18.Roopenian DC, Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 19.Kim JK, et al. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur J Immunol. 1999;29:2819–2825. doi: 10.1002/(SICI)1521-4141(199909)29:09<2819::AID-IMMU2819>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Gearing AJH, et al. Selective cleavage of human IgG by matrix metalloproteinases, matrilysin and stromelysin. Immunol Lett. 2002;81:41–48. doi: 10.1016/s0165-2478(01)00333-9. [DOI] [PubMed] [Google Scholar]
- 21.Ryan MH, et al. Proteolysis of purified IgGs by human and bacterial enzymes in vitro and the detection of specific proteolytic fragments of endogenous IgG in rheumatoid synovial fluid. Mol Immunol. 2008;45:1837–1846. doi: 10.1016/j.molimm.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Diemel RV, ter Hart HG, Derksen GJ, Koenderman AH, Aalberse RC. Characterization of immunoglobulin G fragments in liquid intravenous immunoglobulin products. Transfusion. 2005;45:1601–1609. doi: 10.1111/j.1537-2995.2005.00549.x. [DOI] [PubMed] [Google Scholar]
- 23.Vincents B, von Pawel-Rammingen U, Björck L, Abrahamson M. Enzymatic characterization of the streptococcal endopeptidase, IdeS, reveals that it is a cysteine protease with strict specificity for IgG cleavage due to exosite binding. Biochemistry. 2004;43:15540–15549. doi: 10.1021/bi048284d. [DOI] [PubMed] [Google Scholar]
- 24.Waller M. IgG hydrolysis in abscesses. I. A study of the IgG in human abscess fluid. Immunol Lett. 1974;26:725–733. [PMC free article] [PubMed] [Google Scholar]
- 25.Waller M. IgG hydrolysis in abscesses. II. Hydrolysis of Ripley IgG by enzymes from abscess leucocytes. Immunology. 1974;26:735–741. [PMC free article] [PubMed] [Google Scholar]
- 26.von Pawel-Rammingen U, Johansson BP, Bjorck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002;21:1607–1615. doi: 10.1093/emboj/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terness P, Navolan D, Dufter C, Welschof M, Opelz G. Immunosuppressive anti-immunoglobulin autoantibodies: Specificity, gene structure and function in health and disease. Cell Mol Biol. 2002;48:271–278. [PubMed] [Google Scholar]
- 28.Brezski RJ, et al. Human anti-IgG1 hinge autoantibodies reconstitute the effector functions of proteolytically inactivated IgGs. J Immunol. 2008;181:3183–3192. doi: 10.4049/jimmunol.181.5.3183. [DOI] [PubMed] [Google Scholar]
- 29.Fumia S, et al. Human F(ab′)(2)-containing immune complexes together with anti-hinge natural antibodies stimulate complement amplification in vitro and in vivo. Mol Immunol. 2008;45:2951–2961. doi: 10.1016/j.molimm.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Dall'Acqua W, Simon AL, Mulkerrin MG, Carter P. Contribution of domain interface residues to the stability of antibody CH3 domain homodimers. Biochemistry. 1998;37:9266–9273. doi: 10.1021/bi980270i. [DOI] [PubMed] [Google Scholar]
- 31.van der Neut Kolfschoten M, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317:1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 32.Ribbens C, et al. Increased matrix metalloproteinase-3 serum levels in rheumatoid diseases: Relationship with synovitis and steroid treatment. Ann Rheum Dis. 2002;61:161–166. doi: 10.1136/ard.61.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tchetverikov I, et al. MMP Profile in paired serum and synovial fluid samples of patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:881–883. doi: 10.1136/ard.2003.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kageyama Y, et al. Levels of rheumatoid factor isotypes, metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 in synovial fluid from various athritides. Clin Rheumatol. 2000;19:14–20. doi: 10.1007/s100670050004. [DOI] [PubMed] [Google Scholar]
- 35.Dollery CM, et al. Neutrophil elastase in human arthrosclerotic plaques. Production by macrophages. Circulation. 2003;107:2829–2836. doi: 10.1161/01.CIR.0000072792.65250.4A. [DOI] [PubMed] [Google Scholar]
- 36.Vandivier RW, et al. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plaut AG. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- 38.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: Biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 39.van Kempen LC, de Visser KE, Coussens LM. Inflammation, proteases and cancer. Eur J Cancer. 2006;42:728–734. doi: 10.1016/j.ejca.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 40.von Pawel-Rammingen U, Johansson BP, Björck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002;21:1607–1615. doi: 10.1093/emboj/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benjamin RJ, Qin SX, Wise MP, Cobbold SP, Waldmann H. Mechanisms of monoclonal antibody-facilitated tolerance induction: A possible role for the CD4 (L3T4) and CD11a (LFA-1) molecules in self-non-self discrimination. Eur J Immunol. 1988;18:1079–1088. doi: 10.1002/eji.1830180717. [DOI] [PubMed] [Google Scholar]
- 42.Goronzy J, Weyand CM, Fathman CG. Long-term humoral unresponsiveness in vivo, induced by treatment with monoclonal antibody against L3T4. J Exp Med. 1986;164:911–925. doi: 10.1084/jem.164.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dialynas DP, et al. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: Similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983;131:2445–2451. [PubMed] [Google Scholar]
- 44.Wenig K, et al. Structure of the streptococcal endopeptidase IdeS, a cysteine proteinase with strict specificity for IgG. Proc Natl Acad Sci USA. 2004;101:17371–17376. doi: 10.1073/pnas.0407965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arora S, McDonald RA, Toews GB, Huffnagle GB. Effect of a CD4-depleting antibody on the development of Cryptococcus neoformans-induced allergic bronchopulmonary mycosis in mice. Infect Immun. 2006;74:4339–4348. doi: 10.1128/IAI.01989-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coller BS, Scudder LE. Inhibition of dog platelet function by in vivo infusion of F(ab′)2 fragments of a monoclonal antibody to the platelet glycoprotein IIb/IIIa receptor. Blood. 1985;66:1456–1459. [PubMed] [Google Scholar]
- 47.Lazar GA, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang Y, et al. Regulation of antibody-dependent cellular cytotoxicity by IgG intrinsic and apparent affinity for target antigen. J Immunol. 2007;179:2815–2823. doi: 10.4049/jimmunol.179.5.2815. [DOI] [PubMed] [Google Scholar]
- 49.Weiner LM. Building better magic bullets—improving unconjugated monoclonal antibody therapy for cancer. Nat Rev Cancer. 2007;7:701–706. doi: 10.1038/nrc2209. [DOI] [PubMed] [Google Scholar]
- 50.Radaev S, Motyka S, Fridman WH, Sautes-Fridman C, Sun PD. The structure of a human type III Fcgamma receptor in complex with Fc. J Biol Chem. 2001;276:16469–16477. doi: 10.1074/jbc.M100350200. [DOI] [PubMed] [Google Scholar]
- 51.Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: Implications for therapeutic antibodies. Int Immunol. 2001;13:1551–1559. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- 52.Murphy G, Murthy A, Khokha R. Clipping, shedding and RIPping keep immunity on cue. Trends Immunol. 2008;29:75–82. doi: 10.1016/j.it.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Leidi M, et al. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol. 2009;182:4415–4422. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.