Abstract
Background
Chemoprevention utilizing dietary agents is an effective means to slow the development of prostate cancer. We evaluated the potential additive and synergistic effects of genistein and resveratrol for suppressing prostate cancer in the Simian Virus-40 T-antigen (SV-40 Tag) targeted probasin promoter rat model, a transgenic model of spontaneously developing prostate cancer.
Methods
Rats were fed genistein or resveratrol (250 mg/kg AIN-76A diet) alone and in combination, and a low dose combination (83 mg genistein + 83 mg resveratrol/kg diet). Histopathology and mechanisms of action studies were conducted at 30 and 12 weeks of age, respectively.
Results
Genistein, resveratrol, and the high dose combination treatments suppressed prostate cancer. The low dose combination did not elicit protection against prostate cancer and was most likely below the effective dose for causing significant histopathological changes. Total genistein and resveratrol concentrations in the blood reached 2160 and 211 nM, respectively in rats exposed to the single treatments. Polyphenol treatments decreased cell proliferation and insulin-like growth factor-1 (IGF-1) protein expression in the prostate. In addition, genistein as a single agent induced apoptosis and decreased steroid receptor coactivator-3 (SRC-3) in the ventral prostate (VP).
Conclusions
Genistein and resveratrol, alone and in combination, suppress prostate cancer development in the SV-40 Tag model. Regulation of SRC-3 and growth factor signaling proteins are consistent with these nutritional polyphenols reducing cell proliferation and increasing apoptosis in the prostate.
Keywords: Genistein, Resveratrol, Prostate Cancer, Chemoprevention, SV-40 Tag Rat
INTRODUCTION
Prostate cancer is the most common non-skin malignancy affecting men in the United States and the second leading cause of death after lung cancer. One in six men will develop prostate cancer at some point in their life. The American Cancer Society estimates that there will be approximately 186,320 new cases of prostate cancer and 28,660 deaths in the United States in 2008 (1). Epidemiological and laboratory data suggest that cancer is linked not only to genetics but also lifestyle, including dietary and environmental exposure. In fact, cancer is largely a preventable disease with as much as two-thirds of all cancers being attributable to lifestyle (2). Chemoprevention via the use of natural compounds is an attractive option in an effort to inhibit, delay, or reverse the initiation, promotion, or progression of carcinogenesis.
Several dietary agents, micronutrients, and vitamins have been associated with prostate cancer chemoprevention. The consumption of soy and its major isoflavone, genistein has gained popularity in the fight against cancer within the last decade. Soy has been found to inhibit the growth of transplantable human prostate carcinomas and tumor angiogenesis in mice (3). Our laboratory has shown that genistein suppresses prostate cancer development in chemically-induced rat and transgenic mouse models (4,5). Resveratrol, a polyphenolic phytoalexin found in red wine and grape-derived products has also recently received much attention in regards to cancer prevention. Although red wine consumption has been linked to the French paradox and the reduction in the risk of heart disease, it is still unclear whether or not a correlation exists between increased red wine intake and decreased prostate cancer risk. In 1997, Jang et al. demonstrated resveratrol’s ability to inhibit the initiation, promotion, and progression of cancer in leukemia cells (6). Three reports have shown that resveratrol suppressed chemically-induced mammary cancer in Sprague Dawley rats (7–9). More recently, we have demonstrated the ability of resveratrol to suppress late-stage prostate cancer in TRAMP mice and SV-40-Tag rats (10, 11).
The etiology of prostate cancer is not completely understood. However, it is speculated that sex steroid receptor and growth factor signaling play a major role in the progression of prostate cancer. The androgen receptor (AR) has been reported to participate in prostate cancer progression by its activation and up-regulation, point mutations, and ligand-independent activation (12–14). On the other hand, the AR has been postulated as a tumor suppressor to inhibit uncontrolled proliferation in normal prostate epithelium (15). Then, upon malignant conversion, AR undergoes a molecular switch to act as an oncogene and stimulate the growth of prostate cancer cells. Likewise, it is known that abnormalities in AR coactivator expression, including steroid receptor coactivator-3 (SRC-3), are associated with prostate cancer progression (16) and may contribute to the switch in the role of AR from that of a tumor suppressor to that of an oncogene. Moreover, SRC-3 is an important regulator of prostate cancer proliferation and survival and is essential for the progression of prostate tumorigenesis in the TRAMP model (17).
In addition to sex steroid receptor signaling, growth factor signaling pathways are thought to play a role in prostate cancer development. One of these, the insulin-like growth factor axis is a pathway that plays a critical role in the growth and development of many tissues, including the prostate. Elevated levels of the insulin-like growth factor-1 (IGF-1) in the blood have been associated with an increased risk of advanced stage prostate cancer (18). Additional support in regards to the role of IGF-1 and prostate growth can be found in IGF-1 deficient mice that exhibit decreased prostate size (19) and increased rat prostate growth with systemic administration of IGF-1 (20). Therefore, agents with the ability to modulate sex steroid receptor and growth factor pathways and little or no toxicity make attractive candidates for prevention and therapy.
Having shown that genistein and resveratrol independently suppressed prostate cancer development in rodent models (4,5,10,11), it became our goal to investigate the potential of combinational use of genistein and resveratrol to exert additive or synergistic chemoprevention against spontaneously developing prostate cancer. For this study, we selected the novel SV-40 Tag rat model developed by Asamoto et al. (21). The SV-40 Tag rat is similar to the TRAMP model developed in 1996 by Greenberg et al. (22) that has been used extensively over the past decade in prostate cancer therapeutic and chemoprevention studies. In both models, the Simian Virus-40 large T antigen is under control of the rat probasin promoter, allowing androgen-regulated protein expression specific to the epithelium of the prostate. In contrast to the TRAMP model where prostate cancer develops first in the dorsolateral prostate (DLP) (23), it develops first and most extensively in the VP of SV-40 Tag rats (24, 25). However, it is inconclusive as to which prostate lobe in rodents is homologous to the peripheral zone in the human prostate (26, 27). The rapid development of PIN and prostate cancer in conjunction with the large prostate size makes the SV-40 Tag rat an attractive model for studying prostate cancer.
The idea of administering pure dietary agents in combination to evaluate potential additive, synergistic, or antagonistic effects in prostate cancer chemoprevention is relatively unexplored. In this study, we have investigated the potential of genistein, and resveratrol to protect against spontaneously developing prostate cancer in SV-40 Tag rats. We tested the hypothesis that genistein and resveratrol, alone and in combination, can protect against prostate cancer by regulating cell proliferation, apoptosis, hormone levels, sex steroid receptors, and growth factor signaling.
Treatment of Animals
Animal care and use were conducted according to established guidelines approved by the National Institutes of Health (NIH) and the Institutional Animal Care and Use Committee (IACUC) at the University of Alabama at Birmingham (UAB). Rodents were housed in rooms maintained at 24 ± 1°C with a 12 hr light-dark cycle. SV-40 transgenic Sprague Dawley rat breeders were provided to us by Dr. Tomoyuki Shirai of Nagoya City University Medical School via Drs. Gail Prins and Steve Swanson of the University of Illinois at Chicago. Heterozygous transgenic (SV-40 Tag) females were crossed with non-transgenic Sprague Dawley males to generate heterozygous transgenic (SV-40 Tag) male offspring (SV-40 Tag SD females X SD male breeders). At five weeks of age, the offspring were weaned and tails were clipped. DNA was extracted using a DNeasy Tissue Kit (Qiagen, Valencia, CA), and a PCR-based screening assay was performed to evaluate transgene incorporation (22,25).
Body weights were monitored starting at five weeks of age, at five-week intervals until the animal was sacrificed. At time of dissection, prostates, testes and seminal vesicles were excised, weighed, and flash frozen in liquid nitrogen. Food and water consumptions were monitored to evaluate treatment tolerability. Blood serum was collected at the time that animals were killed and stored at −80°C until time of analysis.
All polyphenol containing diets were mixed in the laboratory of Dr. Clinton Grubbs (UAB Chemoprevention Center). All control animals received powdered phytoestrogen-free AIN-76A diet (Harlan Teklad Global Diets, Wilmington, DE). Our laboratory has previously determined that 250 mg genistein or resveratrol/kg AIN-76A diet results in a chemopreventive effect and no toxicity in chemically-induced prostate cancer in Lobund-Wistar rats (4) and in TRAMP mice (5,10). Therefore, 250 mg genistein and resveratrol/kg diets were used alone and/or in combination. A low dose combination treatment (83 mg genistein + 83 mg resveratrol/kg diet) was also implemented.
SV-40 Tag rats were exposed to the polyphenol containing diets starting at birth in order to evaluate lifetime exposure. Treatments continued until the animals were killed at either 12 or 30 weeks of age for mechanisms of action or chemoprevention studies, respectively. Twelve weeks of age was selected to evaluate the influence of polyphenols on early-stage prostate cancer, specifically the transition from high-grade PIN to well-differentiated carcinoma (25). At this age, SV-40 Tag animals display pre-malignant lesions but have not developed tumors that have the capacity to compromise oxygen and nutrients to the tissue. Thirty weeks of age was selected to evaluate the ability of polyphenols to prevent late-stage poorly differentiated prostate cancer in SV-40 Tag rats (25).
Genistein was provided as a gift from the former Vitamins Division of F. Hoffmann-La Roche Ltd, now DSM Nutritional Products Ltd, Switzerland. Resveratrol was extracted from Rhizoma Polygoni Cuspidati (Xi’an Sino-Dragon Import & Export Co., China). Both chemicals were confirmed to be > 98.5% pure by mass spectrometry.
Histopathology
At necropsy, organs were examined for gross abnormalities in all animals. Macro-metastasis to the bone, abdominal wall, lymph nodes, liver, kidney, and lung was investigated. Prostate, testes, seminal vesicle, and tumor weights were recorded. The prostate and organs of frequent micro-metastasis (lymph nodes, lung, liver, kidney) (28) were placed in cassettes, immersed in 10% formalin, dehydrated in a series of alcohol dilutions, fixed in xylene, embedded in paraffin wax, sliced into 5 μm sections, and placed on microscope slides as described by Folkvord et al. (29). Sections were stained with hematoxylin and eosin prior to histopathological examination. Dr. Isam Eltoum, a Board Certified Pathologist, blindly scored each coded sample using the following grading scale developed specifically for rodents: Grade 1 (non-cancerous), Grade 2 (low-grade PIN), Grade 3 (high-grade PIN), Grade 4 (well-differentiated lesion), Grade 5 (moderately differentiated lesion), or Grade 6 (poorly differentiated lesion) (25,30). In addition to evaluating the prostate as a whole, the VP, DLP, dorsal (DP), lateral (LP) and anterior (LP) lobes for each sample were assigned histopathological scores. From those organs of frequent micrometastases, we had a minimum of one 5 μm section cut, stained and evaluated for pathology. Metastatic lesions were defined as high grade tumor with masses of cells with dark large nuclei and high nuclear cytoplasmic ratio, frequent mitotic figures, apoptotic cells and focal necrotic areas.
Confirming SV-40 Tag
Immunohistochemistry (IHC) was employed to measure SV-40 Tag expression in the VP and DLP of 12 week old control- and polyphenol treated-transgenic rats. SV-40 Tag expression was semi-quantitated and localization was evaluated as described previously (10). SV-40 Tag was measured to determine if the effects of polyphenol treatment was due to a direct biological effect on the prostate or an indirect effect caused by an alteration in transgene expression. Non-transgenic C57/BL6 mouse prostate and rat mammary gland were used as negative controls.
Polyphenol Concentrations in Blood Serum and Prostate
For the determination of total genistein and resveratrol concentrations in blood serum, 12 week-old rats (treated ad libitum since parturition) were used. Since rodents are nocturnal animals and eat predominately at night, blood samples were collected in the morning between 9:00–10:00 AM. Immediately following blood collection, blood was centrifuged at 1200 × g for 15 minute, and serum stored at −80°C until assayed. Genistein and resveratrol stock solutions were prepared in 80% methanol in water at a concentration of 1.0 mg/ml. Rodent serum samples (0.2 ml) were diluted into ammonium acetate buffer, pH 5, and reacted with Helix pomatia β-glucuronidase-sulfatase at 37°C for 4 hours and the aglycones were extracted twice with 2 ml ethyl ether (for extraction of genistein) or ethyl acetate (for extraction of resveratrol) by vortexing for 1 minute in 5 ml glass test tubes. The ethyl ether (genistein) and ethyl acetate (resveratrol) phases were separated by centrifugation for 10 minutes at 3000g. The extracts were evaporated to dryness under nitrogen and then reconstituted in 100 μl of 80% aqueous methanol. Aliquots (20 μl) of the reconstituted extract were injected onto a 2.0 × 100 mm Luna 3 μm Phenyl-hexyl reverse-phase column. The mobile phases were (A) 10 mM NH4OAc and (B) acetonitrile-10 mM NH4OAc. The column was pre-equilibrated in 20% B: 80% A. After injection, the concentration of B in the mobile phase was increased at 5%/minute to 100% B over a 0 to 5 minute period. Then, the concentration of B was decreased to 0% at 50%/minute for 1 minute and then held isocratically for another 4 minutes. The total cycle time was 10 minutes per sample. The mobile phase flow rate was 0.2 ml/minute. The column eluate was passed into the chemical ionization interface of a MDS-Sciex 4000 Q TRAP® LC/MS/MS System (Applied Biosystems, Foster City, CA). The interface was operated in the negative mode with a source temperature of 400°C and a nebulizing current of −3 amps. Genistein and resveratrol were detected by selecting their [M-H]− molecular ions in the first quadrupole, causing collisional dissociation with nitrogen gas and selecting a specific daughter ion fragment in the third quadrupole. Selected fragment ions for genistein and resveratrol were used for specific and quantitative measurements using multiple reaction ion monitoring-mass spectrometry (MRM-MS). The cycle time for the complete group was 0.93 second. All sera were independently analyzed in duplicate. Individual ion chromatograms representing each compound were analyzed by Analyst 1.4.1 and the area under the peak eluting in the correct time window was determined. This was normalized to the chrysin internal standard peak and the relative peak area was plotted against concentration of the standard. After fitting the response curve to a polynomial function, the relative areas for the unknowns were calculated by interpolation. The lower limit of quantification (LOQ) using these methods was at a signal-to-noise ratio of 10:1 (5 nM). Interassay coefficients of variation were <30% at LOQ. Standard working solutions (0.05–25 μM) containing internal standards (100 nM each) of chrysin (recovery marker for aglycones) and phenolphthalein glucuronide and 4-methylumbelliferone sulfate (as markers of effective hydrolysis) were prepared by appropriate dilution with 80% methanol in water.
Cell Proliferation
Prostate tissues were harvested and processed for detecting Ki-67, a marker of cell proliferation, as previously described (31). Slides were viewed using a Nikon Labophot-2 microscope (Nikon Corporation, Tokyo, Japan) and digitally recorded using a Nikon 8.0 Mega Pixels CoolPix 8700 Digital Camera (Nikon). For Ki67 quantitation, epithelial cells were counted using Image J software (Image J, NIH). The VP and DLP were analyzed separately (a minimum of 1,000 cells counted per lobe per slide. The epithelial cells staining positive (brown) for Ki67 were counted as well as the non-proliferative epithelial cells (stained blue). The proliferative index was defined as the number of positively stained epithelial cells divided by the total number of epithelial cells counted X 100. Twenty-eight week old TRAMP prostate tumor with and without Ki67 primary antibody was used as positive and negative controls, respectively.
Apoptosis
The ApopTag® Plus Peroxidase In Situ Apoptosis Detection Kit (Chemicon International, Temecula, CA) was used to measure apoptosis following the manufacturer’s instructions. The apoptotic index was defined as the number of epithelial cells stained positive (brown) for apoptosis divided by the total number of epithelial cells counted × 100. Visualization was performed using a Nikon light microscope, Nikon digital camera, and analyzed using Image J software (NIH).
Immunoblot Analyses
When possible, protein expression levels of sex steroid and growth factor receptors and their ligands were measured by western blot analysis as described previously (5,10,25,31). Antibodies for SRC-2 (BD610985) and SRC-3 (BD78820) were purchased from BD Biosciences, San Jose, CA. Antibodies for SRC-1 (SC8995), AR (SC-816), insulin-like growth factor-1 receptor alpha (IGF-1R-α) (SC-712), and β-actin (SC-47778) were from Santa Cruz Biotechnology (Santa Cruz, CA). Positive protein controls purchased from the suppliers of the corresponding antibodies and the use of Kaleidoscope Precision Plus Protein and Pre-stained SDS-PAGE Broad Range standards (BioRad Hercules, CA) were employed to identify the protein of interest.
Sex Steroid Hormone Concentrations in Blood Serum
Serum total testosterone, dihydrotestosterone (DHT), and estradiol concentrations were measured in the blood serum using coated well enzyme- and radio-immunoassays (Diagnostic Systems Laboratories, Inc., Webster, TX) as described by the manufacturer. The following kits were used DSL 10-4000 (total testosterone), DSL 9600 (DHT), and DSL 10-4300 (estradiol). All samples were run in duplicate with eight samples per group by Dr. Richard Parker (OB/GYN Department, UAB). Standards provided by the manufacturer were utilized: 0.1–25 ng/mm for total testosterone, 25–2500 pg/mL for DHT, and 20–6000 pg/mL for estradiol. Sensitivity for total testosterone, DHT, and estradiol were 0.04 ng/ml, 4 pg/ml, and 7 pg/ml, respectively.
IGF-1 Concentrations in Prostate and Blood Serum
IGF-1 protein levels in the prostate were quantified by enzyme linked immunosorbent assay (ELISA) as described by Crowther et al. (32) with modifications (33). Prior to analysis, kinetic curves were set up to establish zero order kinetics. Samples were run in triplicate and the absorbance at 450 nm was read in an OPTI max Microplate reader (Molecular Devices, Sunnyvale, CA). Rat liver with, and without, IGF-1 primary antibody was used as a positive and negative control, respectively.
For IGF-1 in the blood serum, we used a coated well ELISA kit as described by Diagnostic Systems Laboratories, Inc. All samples were run in duplicate with eight samples per group. Concentration (ng/ml) of samples and controls were determined by plotting the mean absorbance readings of the controls and unknowns against the mean absorbance readings of all standards in a four-parameter curve fit (25).
Statistics
Power analysis for animals/group per experiment has been previously determined to be 30/group and eight/group for chemoprevention and biochemical studies, respectively (4,5,9,31,33). Fisher’s exact test was used to evaluate histopathological grade frequencies among treatment groups. One way ANOVA analysis followed by the Tukey test was used to evaluate multiple groups (> 2). P < 0.05 was considered to be significant. GraphPad™ Prism 5.0 and GraphPad™ InStat 3.0 (San Diego, CA) were used to assist in statistical analysis.
RESULTS
Genistein and Resveratrol alone and in combination
Resveratrol (250 mg/kg diet), genistein (250 mg/kg diet), the low dose combination (83 mg genistein + 83 mg resveratrol/kg diet), and the high dose combination (250 mg genistein + 250 mg resveratrol/kg diet) were well-tolerated and did not show evidence of overt toxicity in SV-40 Tag rats. There was no significant difference in food or water intake in polyphenol treated animals when compared to controls (data not shown). In 12 week old polyphenol treated-SV-40 Tag rats, there was no significant difference in the body, testes, VP, seminal vesicle, and abdominal-groin fat pad weights (Table I). However, genistein as a single agent (250 mg genistein/kg diet) and the low dose combination treatment (83 mg genistein + 83 mg resveratrol/kg diet) significantly lowered DLP weights by 15%. Calculations of VP, DLP and testes to body weight ratios showed slight, but not significant decreases from all polyphenol treatments at 12 week (data not shown).
Table I.
Body and tissue weights in 12 week old SV-40 Tag rats fed genistein and resveratrol, alone or in combination
| Treatment | BW (g) | Testes (mg) | Ventral Prostate (mg) | Dorsolateral Prostate (mg) | Seminal Vesicles (mg) |
|---|---|---|---|---|---|
| Control (AIN-76A diet) | 418 ± 7 | 3,220 ± 47 | 356 ± 18 | 364 ± 11 | 1,467 ± 73 |
| 250 mg Genistein/kg diet | 427 ± 11 | 3,237 ± 55 | 343 ± 18 | 318 ± 12* | 1,399 ± 65 |
| 250 mg Resveratrol/kg diet | 414 ± 6 | 3,231 ± 44 | 328 ± 11 | 335 ± 11 | 1,438 ± 65 |
| 83 mg Genistein + 83 mg Resveratrol/kg diet | 426 ± 10 | 3,164 ± 91 | 370 ± 19 | 318 ± 12* | 1,432 ± 81 |
| 250 mg Genistein + 250 mg Resveratrol/kg diet | 429 ± 9 | 3,328 ± 70 | 332 ± 16 | 348 ± 13 | 1,489 ± 128 |
Body, testes, ventral prostate, dorsolateral prostate and seminal vesicles weights represent a minimum of 30 animals per group. Values are presented as the mean ± SEM.
P < 0.05 when compared to control treatment by One-Way ANOVA followed by the Tukey Test.
Chemoprevention
Entire Prostate
Evaluating the entire prostates of 30 week old SV-40 Tag rats fed control diet, we found the incidence of prostate cancer (Grades 4–6) was 98%, of which 29% were poorly differentiated tumors (Grade 6) (Table II). None of the 42 control-treated animals were graded normal (Grade 1), low-grade PIN (Grade 2), or moderately differentiated cancer (Grade 4). In addition, only one animal received a score of high-grade PIN (Grade 3).
Table II.
Histopathological analysis of the entire prostates of 30 week old SV-40 Tag rats fed genistein or resveratrol, alone or in combination
| Grade Level |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | n | 1 | 2 | 3 | 4 | 5 | 6 | Prostate Cancer (4–6) | Cancer-free (1–3) |
| Control (AIN-76A diet) | 42 | 0% | 0% | 2% | 0% | 69% | 29% | 98% | 2% |
| 250 mg Genistein/kg diet | 40 | 5% | 3% | 10% | 15% | 58% | 10%* | 83%* | 18%* |
| 250 mg Resveratrol/kg diet | 39 | 0% | 8% | 13% | 4% | 59% | 15% | 79%* | 21%* |
| 83 mg Genistein + 83 mg Resveratrol/kg diet | 34 | 0% | 3% | 3% | 3% | 68% | 24% | 94% | 6% |
| 250 mg Genistein + 250 mg Resveratrol/kg diet | 35 | 14%* | 6% | 3% | 6% | 63% | 9%* | 77%** | 23%** |
Tissue sections were histopathologically evaluated and given a score of 1 (normal tissue), 2 (low-grade PIN), 3 (high-grade PIN), 4 (well-differentiated tumor), 5 (moderately differentiated tumor), and 6 (poorly differentiated tumor) depending on the presence and progression of lesions. Results are the percentage of mice prostates as a function of the pathological score. The incidence of each individual grade (1–6) as well as the overall prostate cancer (a sum of Grades 4–6) and cancer-free (a sum of Grades 1–3) incidence is included.
P < 0.05 and
P < 0.01 compared to control treatment by Fisher’s Exact Test.
Lifetime administration of genistein in the diet (250 mg/kg diet) as a single agent significantly decreased the incidence of prostate cancer (Grades 4–6) by 15% and suppressed poorly differentiated tumors (Grade 6) by 3-fold in the entire prostate when compared to control group (Table II). SV-40 Tag rats receiving resveratrol in the diet (250 mg/kg diet) had a significantly lower incidence (19%) of prostate cancer (Grades 4–6). Hence, the single genistein and resveratrol treatments resulted in rats being 18% and 21% prostate cancer free, respectively compared to the animals fed AIN-76A diet only being only 2% prostate cancer free.
While the low dose combination treatment (83 mg genistein + 83 mg resveratrol/kg diet) did not significantly alter any of the cancer grades, there was a 3-fold increase in cancer-free grades. On the other hand, the high dose combination treatment (250 mg genistein + 250 mg resveratrol/kg diet) resulted in protection against prostate cancer among all treatment groups, an 11.5-fold decrease in prostate cancer incidence (Grades 4–6) compared to control fed transgenic rats (23% versus 2% cancer free). When evaluating the entire prostate, the high dose combination treatment significantly reduced the overall incidence of prostate cancer (Grades 4–6) by 21% and poorly differentiated tumors by 69% (29% versus. 9%). In addition, there was a significantly higher incidence of animals with normal (Grade 1) scores in the high dose combination group (14%) when compared to controls (0%). While we did not see additive or synergistic response from the combination treatment, there is a hint of a dose response effect, with the cancer free incidence going from 2% to 6% and 23% with increasing concentrations genistein and resveratrol in the diet.
Individual Lobes
At time of necropsy (30 weeks) poorly differentiated tumors (Grade 6) occurred at the highest rate in the VP (20%) followed by the LP (13%), DP (2%), and AP (0%) of SV-40 Tag rats (Table III). Furthermore, the highest incidence of prostate cancer (Grades 4–6) in SV-40 Tag rats fed control diet occurred in the LP (98%) followed by the VP (95%), DP (83%), and AP (13%). Investigating the effects on individual lobes showed that genistein (250 mg/kg diet) significantly reduced the overall incidence of prostate cancer (Grades 4–6) in the DP (39%) and LP (19%), but had no significant effect on the VP and AP. Genistein was the only treatment that significantly suppressed prostate cancer in the DP. In addition, genistein significantly decreased the incidence of poorly differentiated tumors (Grade 6) in the VP by 7-fold. Dietary genistein also significantly increased the incidence of normal (Grade 1) scores in the DP from 0% in controls to 10% in genistein-treated animals.
Table III.
Histopathological analysis of the dorsal, lateral, ventral, and anterior prostate lobes of 30 week old SV-40 Tag rats fed genistein or resveratrol alone, or in combination
| Grade Level |
||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | n | 1 | 2 | 3 | 4 | 5 | 6 | Prostate Cancer 4–6 |
| Dorsal Prostate | ||||||||
| Control (AIN-76A) | 42 | 0% | 0% | 17% | 21% | 60% | 2% | 83% |
| 250 mg Genistein | 39 | 10%* | 5% | 33% | 13% | 36% | 3% | 51%** |
| 250 mg Resveratrol | 39 | 10%* | 0% | 18% | 18% | 46% | 8% | 72% |
| 83 mg Genistein + 83 mg Resveratrol | 35 | 11%* | 3% | 0% | 29% | 51% | 6% | 86% |
| 250 mg Genistein + 250 mg Resveratrol | 37 | 21%** | 0% | 9% | 12% | 56% | 3% | 71% |
| Lateral Prostate | ||||||||
| Control (AIN-76A) | 40 | 0% | 0% | 3% | 5% | 80% | 13% | 98% |
| 250 mg Genistein | 39 | 8% | 0% | 13% | 21% | 54% | 5% | 79%** |
| 250 mg Resveratrol | 37 | 14%* | 0% | 11% | 11% | 54% | 11% | 76%** |
| 83 mg Genistein + 83 mg Resveratrol | 35 | 9% | 3% | 3% | 3% | 71% | 11% | 86% |
| 250 mg Genistein + 250 mg Resveratrol | 35 | 17%* | 9% | 9% | 0% | 60% | 6% | 66%*** |
| Ventral Prostate | ||||||||
| Control (AIN-76A) | 41 | 0% | 0% | 5% | 5% | 71% | 20% | 95% |
| 250 mg Genistein | 39 | 8% | 0% | 5% | 26% | 59% | 3%* | 87% |
| 250 mg Resveratrol | 39 | 13%* | 0% | 5% | 8% | 67% | 8%* | 82% |
| 83 mg Genistein + 83 mg Resveratrol | 35 | 3% | 3% | 3% | 3% | 71% | 17% | 91% |
| 250 mg Genistein + 250 mg Resveratrol | 34 | 15%* | 6% | 3% | 6% | 71% | 0%** | 76%** |
| Anterior Prostate | ||||||||
| Control (AIN-76A) | 40 | 43% | 20% | 25% | 10% | 3% | 0% | 13% |
| 250 mg Genistein | 38 | 58% | 26% | 11% | 5% | 0% | 0% | 5% |
| 250 mg Resveratrol | 34 | 32% | 24% | 35% | 6% | 3% | 0% | 9% |
| 83 mg Genistein + 83 mg Resveratrol | 34 | 68% | 15% | 12% | 0% | 3% | 3% | 6% |
| 250 mg Genistein + 250 mg Resveratrol | 35 | 51% | 17% | 14% | 17% | 0% | 0% | 17% |
Tissue sections were histopathologically evaluated with scores of 1 (normal tissue), 2 (low-grade PIN), 3 (high-grade PIN), 4 (well-differentiated tumor), 5 (moderately differentiated tumor), and 6 (poorly differentiated tumor) depending on the presence and progression of lesions. Results are the percentage of mice as a function of the pathological score. The incidence of each individual grade (1–6) as well as the overall prostate cancer incidence (a sum of Grades 4–6) is included.
P < 0.05,
P < 0.01, and
P < 0.001 compared to control treatment by Fisher’s Exact Test.
Resveratrol (250 mg/kg diet) acted primarily on the LP where it significantly suppressed prostate cancer (Grades 4–6) by 22%. In the VP, poorly differentiated tumors (Grade 6) were significantly decreased from 20% to 8%. Resveratrol significantly increased the incidence of normal (Grade 1) scores from zero in controls to 10%, 14% and 13% in the DP, LP, and VP, respectively.
In comparison, the high dose combination treatment (250 mg genistein + 250 mg resveratrol/kg diet) significantly reduced the overall incidence of prostate cancer (Grades 4–6) in the LP and VP by 33% and 20%, respectively. The high dose combination treatment group was the only group that significantly protected against overall prostate cancer (Grades 4–6) in the VP. Remarkably, there were no animals with poorly differentiated lesions in the VP of high dose combination-treated rats. Poorly differentiated tumors (Grade 6) in the VP were statistically reduced from 20% in control-treated animals to 0% in the high dose combination group. In conjunction, the high dose combination treatment significantly increased the incidence of normal (Grade 1) prostate tissue in DP, LP, and VP, but not in the AP.
In contrast, the incidence of overall and poorly differentiated cancer in the low dose combination (83 mg genistein + 83 mg resveratrol/kg diet) did not statistically differ from control-treated animals in any comparison. However, similar to genistein as a single agent, the low dose genistein and resveratrol combination significantly increased the incidence of normal score (Grade 1) in the DP only.
Metastasis
Although metastasis in the SV-40 Tag rat model has been reported to be absent or extremely low, we evaluated and found some, but no statistical change in latency, number of tumors per animal, tumor weight, or number of lung, liver, kidney, lymph node, or neck metastases between the control-and polyphenol-treated SV-40 Tag rats at 30 weeks of age. Metastasis was observed in 5%, 3%, 5%, 7% and 8% in control-, genistein-, resveratrol-, low dose combination-, and high dose combination-treated SV-40 Tag rats, respectively.
SV-40 Tag Expression in Transgenic Rats
At 12 weeks, SV-40 Tag protein expression was assessed via IHC to determine if the effect of polyphenols on histopathology and biomarkers was due to regulation of the probasin promoter. Then, following necropsy, animals were confirmed to be transgenic by a second round of tail-clipping and PCR for SV-40 Tag. There was no treatment effect on SV-40 Tag expression in the VP and DLP epithelia (data not shown).
Genistein and Resveratrol Concentrations in the Blood Serum
Genistein and resveratrol concentrations were measured in the blood sera of 12 week old SV-40 Tag rats exposed to these polyphenols. Rats fed 250 mg genistein/kg diet or 250 mg resveratrol/kg diet had total genistein and resveratrol concentrations in the blood serum of 2,160 ± 497 pmol/ml and 211 ± 53 pm/ml, respectively (Table IV). In the low dose combination treatment, blood genistein concentrations were 2.4-fold lower than concentrations observed with high dose genistein as a single agent. In high dose combination treatment (250 mg genistein + 250 mg resveratrol/kg diet), total genistein concentrations were 37% higher than concentrations observed at the same dose with the single agent genistein treatment.
Table IV.
Genistein and resveratrol concentrations in blood serum of 12 week old SV-40 Tag rats fed genistein or resveratrol alone, or in combination
| Treatment | n | Genistein (pmol/ml) | Resveratrol (pmol/ml) |
|---|---|---|---|
| Control (AIN-76A diet) | 3 | 5 ± 3 | 37 ± 1 |
| 250 mg Genistein/kg diet | 7 | 2,160 ± 497 | 21 ± 5 |
| 250 mg Resveratrol/kg diet | 8 | 30 ± 11 | 211 ± 53 |
| 83 mg Genistein + 83 mg Resveratrol/kg diet (Low Dose Combination) | 8 | 890 ± 369 | 305 ± 155 |
| 250 mg Genistein + 250 mg Resveratrol/kg diet (High Dose Combination) | 6 | 2,967 ± 787 | 501 ± 166 |
Blood serum underwent liquid-liquid extraction and polyphenols were quantitated by HPLC/MS. Values are presented as Mean ± SEM. There were no significant differences between combination treatment groups and single agent treatments when compared by One-Way ANOVA.
In contrast, blood resveratrol concentrations were 2.4-fold higher from combination high dose genistein and resveratrol treatment than high dose resveratrol treatment alone. Also, combination low dose genistein and resveratrol treatment (one-third dose) resulted in a 45% higher blood resveratrol concentration than those found in the single agent high dose resveratrol treatment alone. However, there was no statistical difference between combination treatment groups and single agent treatment groups.
Cell Proliferation
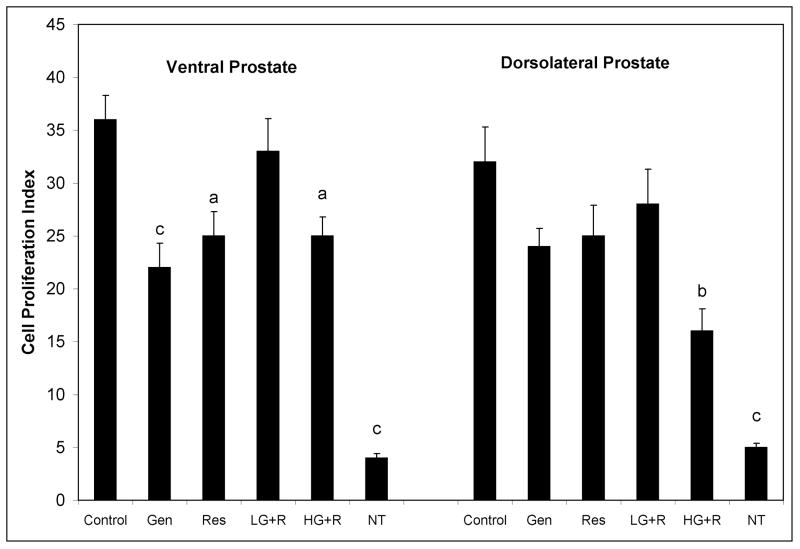
After demonstrating prostate cancer chemoprevention with genistein and resveratrol in the SV-40 Tag rat, we focused on cell turnover (proliferation and apoptosis) to explain how genistein and resveratrol suppress prostate cancer development. For this and other mechanism studies, we focused our attention on the VP and the DLP (26,27). Cell proliferation, as determined by the Ki67 assay, did not differ significantly between the VP and DLP of 12 week old non-transgenic rats (Figure 1). However, when compared to non-transgenic rats, cell proliferation indices in VP and DLP of control treated SV-40 Tag rats were significantly increased by 9-fold and 6.4-fold in the VP and DLP, respectively.
Figure 1.
Cell proliferation as determined by immunohistochemical staining of Ki-67 in ventral and dorsolateral prostate lobes of 12 week old SV-40 Tag rats and non-transgenic rats. SV-40 Tag rats were fed control AIN-76A diet (Cntr), 250 mg genistein/kg diet (Gen), 250 mg resveratrol/kg diet (Res), 83 mg genistein/kg diet + 83 mg resveratrol/kg diet (L-G+R), 250 mg genistein/kg diet + 250 mg resveratrol/kg diet (H-G+R), or non-transgenic rats (NT) were fed control diet starting at birth. Values are presented as total number of Ki-67 staining epithelial cells divided by total number of epithelial cells X 100 ± SEM. Each group contained eight samples. aP < 0.05, bP < 0.01 and cP < 0.001 when compared to control treatment (Cntr) by One-Way ANOVA followed by the Tukey test.
In the VP, genistein was the most effective treatment in regulating cell proliferation, significantly reducing cell proliferation by 39% (Figure 1). However, genistein did not statistically change cell proliferation in the DLP. Resveratrol significantly decreased cell proliferation in the VP by 31%, but had no significant effect on the proliferative index in the DLP. The low dose combination treatment (83 mg genistein + 83 mg resveratrol/kg diet) had no significant change on cell proliferation in the VP and DLP. On the other hand, the high dose combination treatment (250 mg genistein + 250 mg resveratrol/kg diet) resulted in significant reductions in cell proliferation of 31% and 50% in the VP and DLP, respectively.
Apoptosis
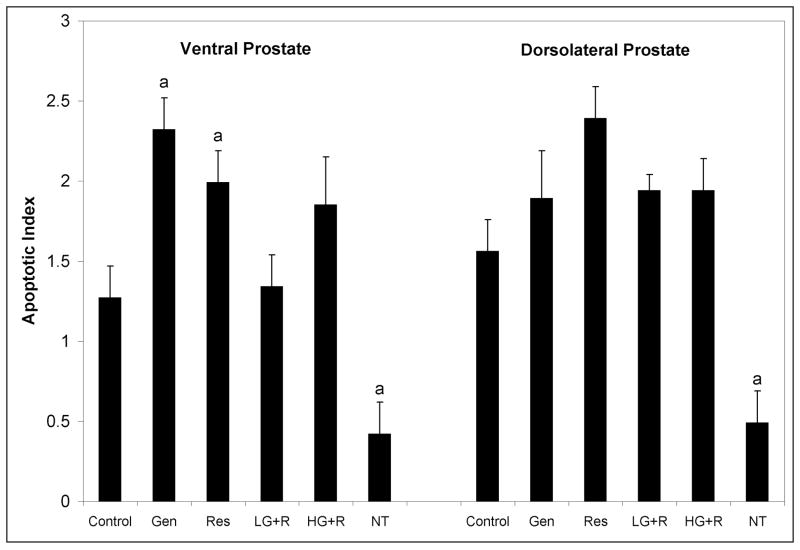
Apoptotic indices in the VP and DLP of control-fed non-transgenic Sprague Dawley rats were similar and low at 0.4% and 0.5%, respectively (Figure 2). Likewise, the apoptotic indices in the VP and DLP of SV-40 Tag rats were similar to each other, 1.3% and 1.6%, respectively. This demonstrates the increased rate of apoptosis in the VP and DLP of the SV-40 Tag rats compared to the non-transgenic rats, by 3-fold in each lobe. In genistein- and resveratrol-treated rats, apoptosis in the VP was significantly increased by 83% and 57% compared to controls. However, genistein and resveratrol combination treatments did not alter apoptosis in the VP. In the DLP, genistein and resveratrol alone or in combination did not significantly change the rate of apoptosis.
Figure 2.
Apoptosis in ventral and dorsolateral prostates of SV-40 Tag and non-transgenic rats. SV-40 Tag rats were fed control AIN-76A diet (Cntr), 250 mg genistein/kg diet (Gen), 250 mg resveratrol/kg diet (Res), 83 mg genistein/kg diet + 83 mg resveratrol/kg diet (L-G+R), 250 mg genistein/kg diet + 250 mg resveratrol/kg diet (H-G+R), or non-transgenic (NT) were rats fed control diet starting at birth. Values are presented as total number of apoptotic epithelial cells divided by total number of epithelial cells X 100 ± SEM. Each group contained eight samples. aP < 0.05 compared to control treatment (Cntr) by One-Way ANOVA followed by the Tukey test.
IGF-1 Signaling Proteins
IGF-1
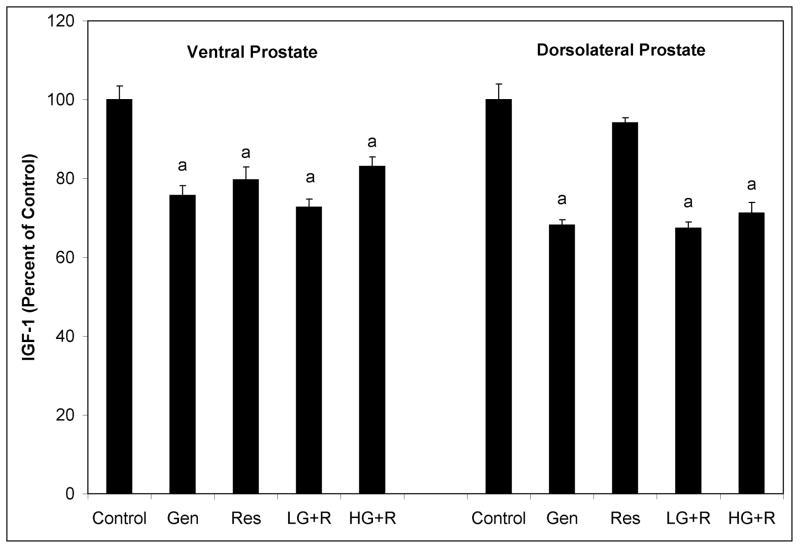
In the VP of 12 week old SV-40 Tag rats, genistein, resveratrol, low dose genistein plus resveratrol combination, and high dose genistein plus resveratrol combination significantly reduced IGF-1 levels by 24%, 20%, 27%, and 17%, respectively (Figure 3). In the DLP, high dose genistein alone, low dose genistein plus resveratrol combination, and high dose genistein plus resveratrol combination, but not high dose resveratrol alone, significantly reduced IGF-1 levels by 32%, 33%, and 29%, respectively. However in the blood serum of 12 week old SV-40 Tag rats these polyphenol treatments did not significantly alter IGF-1 concentrations when compared to controls (data not shown).
Figure 3.
Insulin-like growth factor-1 protein expression in ventral and dorsolateral prostates of 12 week old SV-40 Tag rats. SV-40 Tag rats were fed control AIN-76A diet (Cntr), 250 mg genistein/kg diet (Gen), 250 mg resveratrol/kg diet (Res), 83 mg genistein/kg diet + 83 mg resveratrol/kg diet (L-G+R), or 250 mg genistein/kg diet + 250 mg resveratrol/kg diet (H-G+R), starting at birth. IGF-1 protein expression was determined via ELISA. Each sample was run in triplicate and each group contained eight samples. Values are expressed as mean ± SEM relative to controls. *P < 0.05 when compared to control treatment (Cntr) by One-Way ANOVA followed by the Tukey test.
IGF-1R
Using two different antibodies, we measured both the extracellular α-subunit and the transmembrane β-subunit of IGF-1R that undergoes conformational change leading to autophosphorylation of the tyrosine kinase domain. Genistein and resveratrol treatments did not significantly alter IGF-1Rα and IGF-1Rβ in the VP and DLP when compared to control-treated rats (data not shown).
Sex Steroid Receptor Signaling Proteins
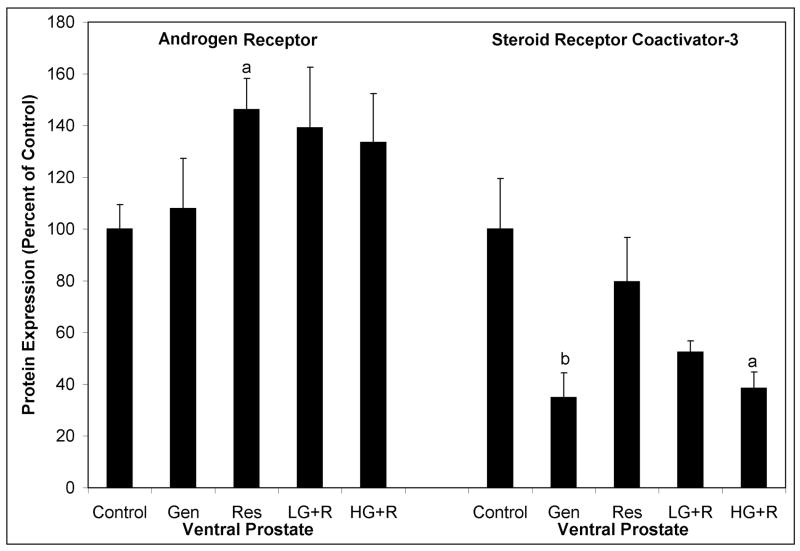
AR protein expression in the VP of 12 week old SV-40 Tag rats was significantly increased 46% by dietary resveratrol (Figure 4). However, resveratrol in combination with genistein (and genistein alone) did not significantly alter AR protein expression in the VP. Genistein and resveratrol alone and in combination did not alter AR protein expression in the DLP (data not shown).
Figure 4.
Androgen receptor and steroid receptor coactivator-3 protein levels as measured by western blot analysis from ventral prostates of 12 week old SV-40 Tag rats. SV-40 Tag rats were fed control AIN-76A diet (Cntr), 250 mg genistein/kg diet (Gen), 250 mg resveratrol/kg diet (Res), 83 mg genistein/kg diet + 83 mg resveratrol/kg diet (L-G+R), 250 mg genistein/kg diet + 250 mg resveratrol/kg diet (H-G+R) starting at birth. Each treatment group contained eight samples. The graph illustrates mean density ± SEM as a percent of the SV-40 Tag control group (Cntr). aP < 0.05 and bP < 0.01 compared to control treatment (Cntr) by One-Way ANOVA followed by the Tukey test.
Since co-activators regulate the activity of sex steroid receptors, we measured SRC proteins in the prostates of 12 week old SV-40 Tag rats. Genistein, alone and in combination (high dose), significantly decreased SRC-3 protein expression in the VP by 65% and 61%, respectively (Figure 4). No changes in SRC-1 or SRC-2 in the VP or of any of the steroid coactivators (SRC-1, SRC-2 and SRC-3) were observed in the DLP.
Sex Steroid Hormone Concentrations
Since alterations in sex steroid hormones could account for sex steroid receptor regulation via compensation or feedback inhibition mechanisms, we quantified sex steroid hormone concentrations in the blood sera of 12 and 30 week old SV-40 Tag rats. Serum testosterone, DHT, and estradiol concentrations were not statistically different between polyphenol- and control-treated rats at both ages (data not shown). However, as expected (34), there was an age effect with testosterone concentrations being significantly less in 30 week old rats compared to those in 12 weeks rats treated with polyphenols (data not shown).
DISCUSSION
Chemoprevention in the SV-40 Tag Rat Model
Using SV-40 Tag rats, we were able to show that genistein alone, resveratrol alone, and genistein plus resveratrol in combination (high dose) suppressed the most severe grade of prostate cancer (poorly differentiated lesions (Grade 6) in these transgenic animals by 2.9-, 1.9- and 3.2-fold, respectively compared to SV-40 Tag rats fed control diet. This resulted in a 9-, 10.5- and 11.5-fold increase in prostate cancer free animals compared to SV-40 Tag rats fed control diet.
It is in the VP where prostate cancer is initiated and where most of the poorly differentiated tumors develop in SV-40 Tag rats (25). Likewise, this is where genistein is the most effective in reducing the percent of poorly differentiated tumors, from 20% to 3% in the VP. The overall inhibition of prostate cancer (Grades 4–6) was most evident in the DP (83% to 51%). In the DP, only 2% of the animals had the most severe lesions, poorly-differentiated tumors (Grade 6). On the other hand, resveratrol alone appears to be eliciting most of its effect on the LP since it was the only lobe in which resveratrol significantly suppressed prostate cancer (98% to 76%). The LP is the lobe with the second highest incidence of poorly developing prostate tumors (13%). The difference in lobe pathology suggests that genistein and resveratrol have some tissue selectivity and could act via different mechanisms.
In our study, the high dose combination (250 mg genistein + 250 mg resveratrol/kg diet) treatment in SV-40 Tag rats had the most pronounced effect among all treatment groups in regards to chemoprevention, although it did not exhibit a synergistic protective effect. Poorly differentiated tumors (Grade 6) and overall prostate cancer (Grades 4–6) in the entire prostate were suppressed by 69% and 21%, respectively. Only three rats had poorly differentiated tumors in the high dose combination treatment compared to 12 in the control-treated group. A closer look into the high dose combination treated-animals revealed that their prostates contained only a small percent area of histopathological Grade 6 tumors. In the three rats that had poorly differentiated tumors in the high dose combination treatment, each had poorly differentiated tumors in only one lobe and the percent area of Grade 6 tumors was only 1%, 5%, and 10%. This demonstrates that even when poorly differentiated tumors are present, progression is slow and they only occupy a small area of the prostate.
In addition, the high dose combination treatment suppressed PIN and prostate cancer in the entire prostate in 14% of the genistein and resveratrol-treated animals compared to 0% in control SV-40 Tag rats. The high dose combination treatment group had five out of 35 animals with prostates that remained normal in pathology. The suppression of pre-malignant lesions and prostate cancer confirms the potential of these dietary agents in chemoprevention (4,5,10,33). Furthermore, the prostates in the majority of these animals displayed atrophic glands and resembled chemical castration. Atrophic glands in SV-40 Tag rats have also been reported using raloxifene, a selective estrogen receptor modulator (SERM) (35).
The high dose genistein and resveratrol combination treatment in SV-40 Tag rats elicited most of its effects on the ventral and lateral prostate lobes. It reduced overall prostate cancer in the lateral prostate and ventral prostate lobes by 32% and 20%, respectively, but did not have an effect in the dorsal prostate lobe where genistein as a single agent was most protective. In the VP, the high dose combination was the only treatment to be completely protective against poorly differentiated tumors (from 20% to 0%) and suppressed overall prostate cancer (Grade 4–6) from 95% to 76%.
The low dose combination treatment (83 mg genistein + 83 mg resveratrol/kg diet) did not demonstrate protection against prostate cancer at 30 weeks of age in this model. This suggests that this concentration was below the effective dose for causing significant histopathological changes in this model. Changes early in life by the low dose combination were most likely overwhelmed by prostate cancer as it later progressed.
Polyphenol Concentrations in the Blood Serum
Serum total genistein concentration in 12 week old SV-40 Tag rats fed 250 mg genistein/kg diet was 2,160 ± 497 pmol/ml, a concentration that is two- to three-fold higher than previously reported in the blood serum of Lobund-Wistar rats (4,36). The differences observed between these studies may be due to differences in strains, metabolism between transgenic and non-transgenic animals, and sera collection times. Interestingly, serum genistein concentrations in Japanese men eating a traditional diet high in soy has been reported to be 276 pmol/ml, with the maximum being a comparative 2,500 pmol/ml (37). Since genistein concentrations in the blood serum may peak at eight hours and gradually decline after 24 hours, we collected blood serum in the morning, but it is impossible to assure that all rats in a cage eat on a continuum.
Resveratrol concentrations in the blood serum were approximately 10-fold lower than blood genistein concentration even with the same polyphenol diet concentration (250 mg per kg AIN-76A diet). This supports the lack of stability and/or poor bioavailability of resveratrol observed in other in vivo studies (38). In addition, steady state concentrations have been reported to last only two to four hours after red wine ingestion and gradually return to baseline (39), emphasizing the importance of carefully selecting the time to collect specimens when determining polyphenol concentrations. According to Goldberg et al., healthy human subjects who ingested an oral dose of 25 mg resveratrol/70 kg BW had peak total resveratrol concentrations of 10–40 pmol/ml (40), whereas Walle et al. observed a 2,000 pmol/ml peak total resveratrol concentration in the blood serum after administering the same dose (38). In our study, SV-40 Tag rats fed 250 mg resveratrol/kg diet had total resveratrol concentrations of 211 ± 53 pmol/ml, a concentration in between the reported human blood serum concentrations.
While circulating genistein and resveratrol concentrations are significantly different in 12 week old rats fed the same concentrations of these polyphenols, the amount of diet that they ingested was the same. For the 250 mg genistein or resveratrol/Kg diets, 12 week old male rat weighing 420 grams ate approximately 20 grams per day. This calculates to 11.9 mg of genistein or resveratrol per Kg body weight per day.
When 250 mg genistein/kg and 250 mg resveratrol/kg AIN-76A were given in combination, genistein and resveratrol concentrations in the blood serum were 37% and 137% higher in the combination treatments than when these polyphenols were administered alone. This may be due to an enhanced absorption or altered metabolism caused by the accompanying polyphenol. It is likely that the effect on resveratrol is real since it is a 137% increase and that may be due to preferential phase II metabolism of genistein via glucuronidation and sulfation, thus allowing unaltered resveratrol to accumulate. Our data supports other studies that have demonstrated the altered bioavailability of resveratrol by another polyphenol, daidzein (41). It is plausible that the pharmacokinetics and pharmacodynamics of resveratrol and genistein are altered when given in combination and this should be investigated in future studies.
Polyphenol Mechanism of Action in the SV-40 Tag Rat
It has been suggested that polyphenols, acting as weak estrogens, could alter steroid hormone levels. Hence, sex steroid levels were measured from the blood serum of 12 and 30 week old SV-40 Tag rats fed genistein and resveratrol alone or in combination, starting at birth. These dietary treatments did not alter circulating concentrations of testosterone, DHT and estradiol in 12 and 30 week old male rats within age groups (data not shown). However, there was an age effect with testosterone concentrations being significantly less in 30 week old rats compared to those in 12 weeks rats treated with polyphenols. Others have also reported this in control animals for testosterone (34).
Cell proliferation and apoptosis are integrally connected to prostate carcinogenesis. When these events are uncontrolled, tumor development and progression may thrive. As expected, we recorded significantly increased cell proliferation in prostates of SV-40 Tag rats compared to the non-transgenic rats. Likewise, the rate of apoptosis was increased in transgenic prostates, presumably due to increase DNA damaged cells. As seen in Table V, cell proliferation to apoptosis ratio was 38% higher in the VP than the DLP of control treated SV-40 Tag rats. This is consistent with earlier and increased susceptibility for carcinogenesis in the VP over the DLP in these transgenic rats (25). Also, important are that polyphenol treatments decreased cell proliferation and increased apoptosis in the prostate. Genistein and resveratrol, alone or in combination at the high dose, resulted in large decreases in cell proliferation to apoptosis ratios in both the VP (66%, 55%, 52%) and DLP (40%, 49%, 60%), respectively. These ratios are consistent with the chemoprevention data, strongly supporting the concept that rate of cell proliferation and apoptosis are good indicators of carcinogenesis and chemoprevention. Future studies should look at biomarker proteins of cell proliferation and apoptosis.
To delve deeper into the mechanism of action of genistein and resveratrol, alone and in combination, we investigated sex steroid receptor, co-regulators and specific growth factor receptors and ligands that are associated with regulation of cell proliferation and apoptosis. Contrary to the prevailing dogma of a down-regulation of AR being associated with a decrease in prostate cancer risk and reported down-regulated AR in rats treated with resveratrol from 3–10 weeks of age (11), we found a 46% increase in AR in the VP of resveratrol-treated SV-40 Tag rats. This supports our previous findings in the TRAMP model where we also observed an increase in AR in the DLP of resveratrol-treated TRAMP mice (10). One explanation for the up-regulation of AR may be a compensation for decreased local androgen concentrations or growth-factor signaling in the prostate. However, we found these polyphenol treatments did not alter testosterone and DHT concentrations in the blood serum. Another postulated mechanism is for AR to function as a tumor suppressor prior to the development of prostate cancer by inhibiting uncontrolled proliferation in normal prostate epithelium (15). Then, upon malignant conversion, AR undergoes a molecular switch to act as an oncogene and stimulate the growth of prostate cancer cells. The up-regulation of AR as tumor suppressor at 12 weeks of age during the transition between high-grade PIN to well differentiated prostate adenocarcinoma supports the decreased cell proliferation observed in the prostate of resveratrol-treated SV-40 Tag rats. In contrast to resveratrol as a single agent, the sex steroid receptor AR was not up-regulated by high dose combination treatment. It may be that when given in combination, genistein may be antagonizing the effects of resveratrol in regards to AR protein expression.
The SRC coactivator proteins play important roles in sex steroid signaling via multiple physiological processes including cell proliferation, survival, and development. One of these, SRC-3, directly binds AR and stimulates its transcriptional activities in an androgen-dependent fashion (16). Moreover, SRC-3 is frequently amplified or over-expressed in breast cancer and cross-talks with the IGF-1 signaling pathway by causing an increase in mammary IGF-1 mRNA and serum IGF-1 protein levels (17). In our study genistein alone, and the genistein and resveratrol combinations, down-regulated SRC-3 protein expression in the VP, further supporting the chemoprevention and decreased cell proliferation noted in those animals. Hence, the decreased expression of SRC-3 could be reducing the transcriptional activity of AR. This may also explain why AR protein expression is increased, to compensate for reduced androgen receptor signaling.
Elevated levels of IGF-1 in the blood serum have been associated with an increased risk of advanced stage prostate cancer in humans (18,42). Additional support in regards to the role of IGF-1 and prostate growth can be found in IGF-1 deficient mice that exhibit decreased prostate size, and increased rat prostate growth with systemic administration of IGF-1 (19,20). In the current study, IGF-1 was down-regulated by all polyphenol treatments in the VP. While the combinational genistein and resveratrol treatments did down regulate IGF-1 concentrations in the VP and DLP, there was, however, no dose response effect. On the other hand, IGF-1 levels in the blood of polyphenol-treated rodents were not altered, strongly suggesting that regulation of IGF-1 at the prostate tissue level occurs and may play a role in the observed chemoprevention by creating an anti-proliferative and pro-apoptotic environment. Accordingly, localized IGF-1 levels at the target tissue would provide a better biomarker for cancer than systemic IGF-1 blood serum concentration that reflects liver IGF-1 production.
CONCLUSIONS
In SV-40 Tag rats, genistein and resveratrol provided in the diet, alone and in combination, significantly reduced the most severe grade of prostate cancer (poorly differentiated lesions) in these transgenic animals by 2.9-, 1.9- and 3.2-fold, respectively compared to control diet. When examining the prostate lobes individually, it was in the VP that genistein was the most effective in suppressing poorly differentiated tumors, a 7 fold decrease. Also, resveratrol was able to suppress poorly differentiated tumors in the VP, by 2.5 fold. Most effective was the high dose genistein plus resveratrol combination that completely suppressed poorly differentiated tumor development in the VP. In accomplishing this, genistein, resveratrol and high dose genistein plus resveratrol treatments resulted in 13%, 18% and 24% cancer free in the VP, respectively. While it is unlikely that polyphenols will wipe out prostate cancer, the data is supportive of genistein and resveratrol being able to suppress prostate cancer development, especially poorly differentiated tumors. In a practical sense, suppressing prostate cancer development in men would be desirable achievement.
Our mechanism of action studies carried out at time of transition from PIN to cancer lesions at 12 weeks (25), demonstrated that polyphenol treatments decreased cell proliferation and IGF-1 protein expression in the prostate. In addition, genistein as a single agent induced apoptosis and decreased SRC-3 in the VP. Resveratrol alone increased AR protein expression in the DLP, supporting the concept of the AR functioning as a tumor suppressor prior to the development of prostate cancer by inhibiting uncontrolled proliferation in normal prostate epithelium (15). The modulation of sex steroid receptor and growth factor signaling in conjunction with a decrease in cell proliferation is consistent with the chemoprevention observed in TRAMP mice (5,10) with genistein and resveratrol.
While it is unlikely that polyphenols alone will wipe out prostate cancer, the data is supportive of genistein and resveratrol being able to suppress prostate cancer development, especially poorly differentiated tumors. In a practical sense, suppressing prostate cancer development by ca. 10–20 years in men would be a hallmark achievement. The dosages administered in our studies are physiologically relevant and biologically achievable, but would require a large consumption of genistein and resveratrol. In the case of genistein, this is achievable via the consumption of tofu or soy supplements. On the other hand, resveratrol is found in low concentrations in red wine and grapes, hence, dietary supplements may be on the only answer at this time.
Acknowledgments
This research was supported by NIH-U54-CA10094. CEH was supported by a National Cancer Institute Cancer Prevention and Control Training Program (NCI Grant CA 47888). We thank Drs. Gail Prins and Steve Swanson of the University of Illinois at Chicago for providing us with SV-40 Tag rats, Dr. Rene Desmond (UAB) for statistical assistance, Dr. Richard Parker (UAB) for steroid hormone analysis, and the UAB Mass Spectrometry Laboratory for assistance with measuring polyphenols in the blood serum. We would also like to acknowledge Dr. Stephen Barnes and UAB’s Mass Spectrometry Facility, for assistance in measuring polyphenol concentrations. The facility is supported by NIH-P30 CA-13148-34.
Footnotes
Disclosures
The authors have no financial or other contractual agreements that might cause conflicts of interest.
References
- 1.Society AC. Cancer Facts and Figures. ACS 2008
- 2.Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA. Environmental and chemical carcinogenesis. Seminars in Cancer Biology. 2004;14(6):473–86. doi: 10.1016/j.semcancer.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Zhou JR, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. The Journal of Nutrition. 1999;129(9):1628–35. doi: 10.1093/jn/129.9.1628. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Eltoum IE, Lamartiniere CA. Dietary genistein suppresses chemically induced prostate cancer in Lobund-Wistar rats. Cancer Letters. 2002;186(1):11–8. doi: 10.1016/s0304-3835(01)00811-4. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Eltoum IE, Lamartiniere CA. Genistein chemoprevention of prostate cancer in TRAMP mice. Journal of Carcinogenesis. 2007;6:3. doi: 10.1186/1477-3163-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science (New York, NY) 1997;275(5297):218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee S, Bueso-Ramos C, Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Research. 2002;62(17):4945–54. [PubMed] [Google Scholar]
- 8.Bhat KP, Lantvit D, Christov K, Mehta RG, Moon RC, Pezzuto JM. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Research. 2001;61(20):7456–63. [PubMed] [Google Scholar]
- 9.Whitsett T, Carpenter M, Lamartiniere CA. Resveratrol, but not EGCG, in the diet suppresses DMBA-induced mammary cancer in rats. Journal of Carcinogenesis. 2006;5:15. doi: 10.1186/1477-3163-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harper CE, Patel BB, Wang J, Arabshahi A, Eltoum IA, Lamartiniere CA. Resveratrol suppresses prostate cancer progression in transgenic mice. Carcinogenesis. 2007;28:1946–1953. doi: 10.1093/carcin/bgm144. [DOI] [PubMed] [Google Scholar]
- 11.Seeni A, Satoru T, Takeshita K, et al. Suppression of prostate cancer growth by resveratrol in the transgenic rat for adenocarcinoma of prostate (TRAP) model. Asian Pacific J Cancer Prevention. 2008;9:7–14. [PubMed] [Google Scholar]
- 12.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nature Medicine. 1999;5(3):280–5. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 13.Culig Z, Hobisch A, Cronauer MV, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Research. 1994;54(20):5474–8. [PubMed] [Google Scholar]
- 14.Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. The Journal of Biological Chemistry. 1996;271(33):19900–7. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 15.Litvinov IV, De Marzo AM, Isaacs JT. Is the Achilles’ heel for prostate cancer therapy a gain of function in androgen receptor signaling? The Journal of Clinical Endocrinology and Metabolism. 2003;88(7):2972–82. doi: 10.1210/jc.2002-022038. [DOI] [PubMed] [Google Scholar]
- 16.Culig Z, Comuzzi B, Steiner H, Bartsch G, Hobisch A. Expression and function of androgen receptor coactivators in prostate cancer. The Journal of Steroid Biochemistry and Molecular Biology. 2004;92(4):265–71. doi: 10.1016/j.jsbmb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Chung AC, Zhou S, Liao L, Tien JC, Greenberg NM, Xu J. Genetic ablation of the amplified-in-breast cancer 1 inhibits spontaneous prostate cancer progression in mice. Cancer Research. 2007;67(12):5965–75. doi: 10.1158/0008-5472.CAN-06-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolk A, Mantzoros CS, Andersson SO, et al. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. Journal of the National Cancer Institute. 1998;90(12):911–5. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 19.Torring N, Vinter-Jensen L, Pedersen SB, Sorensen FB, Flyvbjerg A, Nexo E. Systemic administration of insulin-like growth factor I (IGF-I) causes growth of the rat prostate. The Journal of Urology. 1997;158(1):222–7. doi: 10.1097/00005392-199707000-00075. [DOI] [PubMed] [Google Scholar]
- 20.Ruan W, Powell-Braxton L, Kopchick JJ, Kleinberg DL. Evidence that insulin-like growth factor I and growth hormone are required for prostate gland development. Endocrinology. 1999;140(5):1984–9. doi: 10.1210/endo.140.5.6721. [DOI] [PubMed] [Google Scholar]
- 21.Asamoto M, Hokaiwado N, Cho YM, et al. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Research. 2001;61(12):4693–700. [PubMed] [Google Scholar]
- 22.Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(8):3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wikstrom P, Lindahl C, Bergh A. Characterization of the autochthonous transgenic adenocarcinoma of the mouse prostate (TRAMP) as a model to study effects of castration therapy. The Prostate. 2005;62(2):148–64. doi: 10.1002/pros.20123. [DOI] [PubMed] [Google Scholar]
- 24.Cho YM, Takahashi S, Asamoto M, et al. Age-dependent histopathological findings in the prostate of probasin/SV40 T antigen transgenic rats: lack of influence of carcinogen or testosterone treatment. Cancer Science. 2003;94(2):153–7. doi: 10.1111/j.1349-7006.2003.tb01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harper CE, Patel BJ, Cook LM, et al. Characterization of SV-40 Tag rats as a model to study prostate cancer. BMC Cancer. 2009;9:30. doi: 10.1186/1471-2407-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price D. Comparative aspects of development and structure in the prostate. National Cancer Institute Monograph. 1963;12:1–27. [PubMed] [Google Scholar]
- 27.Shappell SB, Thomas GV, Roberts RL, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Research. 2004;64(6):2270–305. doi: 10.1158/0008-5472.can-03-0946. [DOI] [PubMed] [Google Scholar]
- 28.Gingrich JR, Barrios RJ, Morton RA, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Research. 1996;56(18):4096–102. [PubMed] [Google Scholar]
- 29.Folkvord JM, Viders D, Coleman-Smith A, Clark RA. Optimization of immunohistochemical techniques to detect extracellular matrix proteins in fixed skin specimens. J Histochem Cytochem. 1989;37(1):105–13. doi: 10.1177/37.1.2461979. [DOI] [PubMed] [Google Scholar]
- 30.Wechter WJ, Leipold DD, Murray ED, Jr, et al. E-7869 (R-flurbiprofen) inhibits progression of prostate cancer in the TRAMP mouse. Cancer Research. 2000;60(8):2203–2208. [PubMed] [Google Scholar]
- 31.Harper CE, Patel BB, Wang J, Eltoum IA, Lamartiniere CA. Epigallocatechin-3-Gallate suppresses early stage, but not late stage prostate cancer in TRAMP mice: Mechanisms of action. The Prostate. 2007;67(14):1576–89. doi: 10.1002/pros.20643. [DOI] [PubMed] [Google Scholar]
- 32.Crowther JR. Methods in Molecular Biology. Vol. 149. Clifton; NJ: 2000. The ELISA guidebook; pp. III–IV.pp. p1–413. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Eltoum IE, Lamartiniere CA. Genistein alters growth factor signaling in transgenic prostate model (TRAMP) Mol Cell Endocrinol. 2004;219:171–180. doi: 10.1016/j.mce.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Carruba G. Estrogens and mechanisms of prostate cancer progression. Annals of the New York Academy of Sciences. 2006;1089:201–17. doi: 10.1196/annals.1386.027. [DOI] [PubMed] [Google Scholar]
- 35.Zeng Y, Yokohira M, Saoo K, et al. Inhibition of prostate carcinogenesis in probasin/SV40 T antigen transgenic rats by raloxifene, an antiestrogen with anti-androgen action, but not nimesulide, a selective cyclooxygenase-2 inhibitor. Carcinogenesis. 2005;26(6):1109–16. doi: 10.1093/carcin/bgi056. [DOI] [PubMed] [Google Scholar]
- 36.Dalu A, Haskell JF, Coward L, Lamartiniere CA. Genistein, a component of soy, inhibits the expression of the EGF and ErbB2/Neu receptors in the rat dorsolateral prostate. The Prostate. 1998;37(1):36–43. doi: 10.1002/(sici)1097-0045(19980915)37:1<36::aid-pros6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342(8881):1209–10. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 38.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metabolism and Disposition: the Biological Fate of Chemicals. 2004;32(12):1377–82. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 39.Bertelli AA, Giovannini L, Stradi R, Bertelli A, Tillement JP. Plasma, urine and tissue levels of trans- and cis-resveratrol (3,4′,5-trihydroxystilbene) after short-term or prolonged administration of red wine to rats. International Journal of Tissue Reactions. 1996;18(2–3):67–71. [PubMed] [Google Scholar]
- 40.Goldberg DM, Yan J, Soleas GJ. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clinical Biochemistry. 2003;36(1):79–87. doi: 10.1016/s0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- 41.de Santi C, Pietrabissa A, Mosca F, Pacifici GM. Glucuronidation of resveratrol, a natural product present in grape and wine, in the human liver. Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 2000;30(11):1047–54. doi: 10.1080/00498250010002487. [DOI] [PubMed] [Google Scholar]
- 42.Chan JM, Stampfer MJ, Ma J, et al. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. Journal of the National Cancer Institute. 2002;94(14):1099–106. doi: 10.1093/jnci/94.14.1099. [DOI] [PubMed] [Google Scholar]