Abstract
Background
Systemic renin-angiotensin system (RAS) promotes plasmatic production of angiotensin (Ang) II, which acts through interaction with specific receptors. There is growing evidence that local systems in various tissues and organs are capable of generating angiotensins independently of circulating RAS. The aims of this work were to: 1) study the expression and localization of RAS components in rat gingival tissue and 2) evaluate the in vitro production of Ang II and other peptides catalyzed by rat gingival tissue homogenates incubated with different Ang II precursors.
Methods
Reverse transcription-polymerase chain reaction (RT-PCR) assessed mRNA expression. Immunohistochemical (IHC) analysis aimed to detect and localize renin. Standardized fluorimetric method with tripeptide Hippuryl-Histidyl-Leucine (Hip-His-Leu) was used to measure tissue ACE activity, while high performance liquid chromatography (HLPC) showed products formed after incubation of tissue homogenates with Ang I or tetradecapeptide renin substrate (TDP).
Results
mRNA for renin, angiotensinogen, ACE and Ang II receptors (AT1a, AT1b and AT2) was detected in gingival tissue; cultured gingival fibroblasts expressed renin, angiotensinogen and AT1a receptor. Renin was present in the vascular endothelium and intensely expressed in the epithelial basal layer of periodontally affected gingival tissue. ACE activity was detected (4.95±0.89 nmol His-Leu/g.min). When Ang I was used as substrate, Ang 1-9 (0.576±0.128 nmol/mg.min), Ang II (0.066±0.008 nmol/mg.min) and Ang 1-7 (0.111±0.017 nmol/mg.min) were formed, whereas these same peptides (0.139±0.031; 0.206±0.046 and 0.039±0.007 nmol/mg.min, respectively) and Ang I (0.973±0.139 nmol/mg.min) were formed when TDP was the substrate.
Conclusion
Results presented here clearly show existence of a local RAS in rat gingival tissue, which is capable of generating Ang II and other vasoactive peptides in vitro.
Keywords: renin-angiotensin system, gingival fibroblasts, gingival tissue, angiotensin, Connective tissue biology, Experimental design, Fibroblast(s), Gene expression, Histology, Molecular biology, Oral medicine, Vascular biology
Circulating renin-angiotensin system (RAS) is an endocrine system that promotes the formation of angiotensin (Ang) II, which exerts its effects through the interaction with specific AT1 and AT2 receptors. Ang II is generated by renin action, an enzyme produced in the kidneys, on plasmatic angiotensinogen, produced in the liver, thus forming the biologically inactive decapeptide Ang I (Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10), which is cleaved in its Phe8-His9 bond by angiotensin I-converting enzyme (ACE), present in abundance on pulmonary endothelium, to finally generate the active octapeptide Ang II (Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8).1-3
This classic concept of the circulating RAS has been modified since evidence strongly suggests the participation of different tissues and organs in the formation of Ang II. There is substantial evidence for the presence of essential components of RAS in a variety of tissues, including brain, heart, kidney, gonads, ovary, bone, skeletal muscle, adipose tissue, pancreas, carotid artery, umbilical cord and dental pulp, thus suggesting the existence of a local RAS capable of generating angiotensins independently of the circulating RAS.2-8 Besides Ang II, other vasoactive peptides like Ang 1-9 and Ang 1-7 may also be formed by the enzymatic actions of carboxypeptidases such as angiotensin-converting enzyme-2 (ACE2)3,9-11 and neutral endopeptidases.11,12
Recent data support the hypothesis that RAS is key mediator of inflammation. Besides being a potent vasoactive peptide, Ang II increases adhesion molecules, cytokines and chemokines and exerts a proinflammatory effect on leucocytes, endothelial cells and vascular smooth muscle cells. Acting via the type 1 receptor, Ang II initiates an inflammatory cascade of reduced nicotinamide-adenine dinucleotide phosphate oxidase, reactive oxygen species (ROS) and nuclear factor-kappaB, which mediates transcription and gene expression and increases adhesion molecules and chemokines. AngII also participates in tissue repair and remodeling, through the regulation of cell growth and matrix synthesis.13-15 It has been demonstrated that Ang II stimulates proliferation of guinea-pig and rabbit gingival fibroblasts,16,17 and in human gingival fibroblasts it induces prostaglandin E2 release, which is involved in the pathogenesis of periodontal disease.18
A few reports have provided evidence on the existence of some RAS components in oral tissues, especially AT1 and AT2 receptors, in gingival tissue and cultured gingival fibroblasts of different species.8,16-19 To date no report has unequivocally documented the existence of other important RAS components, such as renin and angiotensinogen, in the gingival tissue. Therefore, the aims of this work were to: 1) study the expression and localization of RAS components in rat gingival tissue and 2) evaluate the in vitro production of Ang II and other peptides catalyzed by rat gingival tissue homogenates incubated with different precursors of Ang II.
Material and Methods
Ethical aspects
The protocol for this study was approved by the Ethics Committee on Animal Research of the Bauru School of Dentistry, University of São Paulo (protocol #20/2004). A total of 25 male Wistar rats weighing from 300 to 400 g were used. These animals were housed in temperature-controlled rooms and received water and food ad libitum. In order to obtain all the gingival samples from the first molar perimeter employed in this study, rats were euthanized with an excessive dose of thiopental§§ (60 mg/kg, intraperitoneally).
Culture of gingival fibroblasts
Gingival fibroblasts were cultured and used for total RNA extraction. These cells were cultured using an explant technique. Samples of the gingival tissue from 3 animals were pooled and minced with a blade into small fragments and grown in proper medium*** supplemented with 15% fetal bovine serum††† and antibiotics‡‡‡ (100 μg/mL penicillin, 100 μg/mL streptomycin and 0.5 mg/mL amphotericin B). Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Confluent cells were detached with 0.25% trypsin and 0.05% EDTA for 5 min. Fibroblasts were used between fourth and eighth passages for total RNA extraction.
Induction of Periodontal Disease
The experimental periodontitis model in rats is described elsewhere.22 Five rats were anesthetized by intraperitoneal injection of thiopental (60 mg/kg), and a silk thread ligature was placed around the lower right first molar in a submarginal position to induce experimental periodontitis. The rats were sacrificed after 30 days, and gingival biopsies were harvested and processed for immunohistochemical analysis.
Total RNA extraction
For the RT-PCR studies, frozen gingival samples (n = 5 animals) embedded in reagent§§§ (0.1 g tissue/1 mL reagent) were homogenized with a tissue homogenizer**** and total RNA was isolated by guanidinium isothiocyanatephenol-chloroform extraction, as we described previously.20-23 Briefly, after homogenization, chloroform extraction, isopropanol precipitation, and 70% (volume/volume) ethanol washing of precipitated RNA were performed. Total RNA from fibroblasts was isolated following the manufacturer's instruction†††† as we previously described.21 Total RNA was resuspended in diethyl pyrocarbonate-treated water and treated with DNase‡‡‡‡, according to the manufacturer's instructions, for 15 min at room temperature to remove any potential genomic DNA contamination. After RNA concentration measurement with a spectrophotometer at 260 nm, and RNA quality assessment it was stored at −80°C until use.
Detection of RAS components mRNA using reverse transcription – polymerase chain reaction (RT-PCR)
First-strand cDNA synthesis was performed using total DNase-treated RNA from gingival biopsies or cultured gingival fibroblasts as we published elsewhere.21-23 Random hexadeoxynucleotides (0.2 μg), RT buffer [45 mM Tris–HCl (pH 8.3), 68 mM KCl, and 9 mM MgCl2], 0.08 mg/mL bovine serum albumin, 15 mM DTT, 1.8 mM dNTPs, and 150 units of reverse transcriptase were utilized, as provided by the manufacturer§§§§. cDNA was synthesized during a 60-min incubation at 37°C, and the reaction was terminated by heating at 90°C for 5 min. RT products (3 μL) served as the template for PCR amplification, using specific primers***** (Table 1). All PCR reactions were performed in a total volume of 50 μL, which was composed of 20 pmol (0.4 μM) of each primer, PCR buffer [20 mM Tris–HCl (pH 8.4), 50 mM KCl], 0.2 mM dNTPs, and 2.5 units of recombinant Taq DNA polymerase†††††. Temperature cycling consisted of an initial denaturation step for 2 min at 94°C, followed by 40 cycles of amplification. In pilot experiments this number of cycles did not reach the saturation of the PCR reaction for all targets. For angiotensinogen (2.0 mM MgCl2),24 renin (2.0 mM MgCl2)24 and the constitutively expressed gene β-actin (1.5 mM MgCl2),20 each round consisted of denaturation for 45 s at 94°C, annealing for 30 s at 58°C, and extension for 1 min at 72°C. For AT1a, AT1b and AT2 receptors (1.5 mM MgCl2),25 each round consisted of denaturation for 60 s at 94°C, annealing for 60 s at 60°C, and extension for 1 min at 72°C. For ACE (1.5 mM MgCl2),26 each round consisted of denaturation for 60 s at 94°C, annealing for 60 s at 61°C, and extension for 2 min at 72°C. Samples were incubated for an additional 10 min-period at 72°C for terminal elongation after the completion of the final cycle. For each set of primers, RT-PCR was performed on sterile water to check for contamination and, for each sample, PCR was conducted on RNA (no-RT) to check for genomic DNA contamination. A 9 μL-aliquot of each sample was electrophoretically size fractionated on a 2% agarose gel containing ethidium bromide (0.5 μg/mL). DNA was visualized under ultraviolet light to detect the presence of PCR amplification products at the anticipated sizes. The size of the PCR products was determined by comparison with the 100-bp ladder‡‡‡‡‡. The agarose gel, containing amplified products, was scanned and analyzed by a computer program§§§§§ to obtain numeric values that permitted a semiquantitative comparison between each target and the constitutive control β-actin.
Table 1.
Sequences of the primers used in the polymerase chain reaction (PCR) for amplification of the different targets and predicted size of the PCR products
| Target | Predicted size |
Sense (5′-3′) | Anti-sense (5′-3′) |
|---|---|---|---|
| β-actin* | 351 bp | AACCGCGAGAAGATGACCCAGATCATGTTT | AGCAGCCGTGGCCATCTCTTGCTCGAAGTC |
| AGTN** | 309 bp | TTCAGGCCAAGACCTCCC | CCAGCCGGGAGGTGCAGT |
| Renin** | 552 bp | ATGCCTCTCTGGGCACTCTT | GTCAAACTTGGCCAGCATGA |
| AT1a*** | 306 bp | CGTCATCCATGACTGTAAAATTC | GGGCATTACATTGCCAGTGTG |
| AT1b*** | 344 bp | CATTATCCGTGACTGTGAAATTG | GCTGCTTAGCCCAAATGGTCC |
| AT2*** | 445 bp | GGAGCGAGCACAGAATTGAAAGC | TGCCCAGAGAGGAAGGGTTGCC |
| ACE**** | 407 bp | CAGCTTCATCATCCAGTTCC | CTAGGAAGAGCAGCACCCAC |
Immunohistochemical analysis to detect and localize renin
Immunohistochemical analysis was performed as previously described, with minor modifications.22,24 Gingival biopsies (n = 2 animals without periodontal disease induction and n = 5 animals in which periodontal disease was induced) were harvested and fixed on 10% formaldehyde solution for 48 hours. Samples were routinely processed for paraffin embedding in blocks of approximately 8×10×6 mm. Sections 5-μm thick were obtained and adhered on slides for immunohistochemical analysis. Tissue sections were deparaffinized and the activity of endogenous peroxidase was blocked with 0.3% hydrogen peroxide solution in methanol (0.01 M) for 30 min. Non-specific binding was blocked by incubating the sections for 30 min with blocking solution******. Sections were incubated with primary goat polyclonal anti-renin antibody†††††† diluted 1:200 in Tris buffered saline (TBS, 0.03 M Tris-HCl containing 0.15 M NaCl, pH 8.1) containing 1% horse serum‡‡‡‡‡‡ for 60 min at 4°C. The sections were then rinsed with TBS and incubated with biotinylated horse anti-goat secondary antibody§§§§§§ diluted 1:100 in TBS-1% horse serum for 30 min at room temperature. Sections were rinsed in TBS containing 0.1% Triton X-100*******, and incubated with the ABC reagent for 30 min at room temperature according to the manufacturer's instructions†††††††. Peroxidase activity was visualized with 3,3'-diaminobenzidine tetrahydrochloride‡‡‡‡‡‡‡ solution containing 1 mL/L hydrogen peroxide solution for 3 min under light protection. Reaction was halted by rinses with distilled water, and slides were then counterstained with hematoxylin§§§§§§§ for 2 min at room temperature according to the manufacturer's instructions. The primary antibody was not used in negative controls. Positive controls were performed on paraffin-embedded longitudinal sections of rat renal cortex.
Measurement of ACE activity
We followed the description of previous works by our group and others in order to measure ACE activity using Hip-His-Leu as substrate.27-31 Gingival biopsies (n = 5 animals) were thawed, weighed and homogenized******** in 0.05 M sodium borate buffer, pH 7.4 (adjusted with 0.5 M NaOH), containing 0.32 M sucrose. After centrifugation, supernatants (20 μL) were incubated at 37°C with 200 μL of a 5 mM Hip-Lis-Leu solution in TBS. After 60 min, the enzyme reaction was stopped by the addition of 1.0 mL of 0.5 M NaOH. The dipeptide His-Leu was detected by the addition of 0.1 mL of 1% o-phthaldialdehyde (weight/volume, in ethanol), followed 4 min later by the addition of 0.2 mL of 6 M HCl. The dipeptide His-Leu was measured fluorimetrically (365 nm for excitation and 495 nm for emission††††††††). Standard curves for His-Leu (0–20 nmol) were prepared under the same conditions. All measurements were made in duplicate. ACE activity was expressed in nmoles of His-Leu per minute per gram of gingival tissue.
Measurement of enzymatic activities through high performance liquid chromatography (HPLC)
Rat gingival biopsies (n = 5 animals) were thawed, weighed and homogenized in TBS. After centrifugation, supernatants (10 µL) were incubated separately with either Ang I (30 nmol), renin substrate tetradecapeptide (TDP, 25 nmol) or Ang II (30 nmol) in TBS, in a final volume of 150 µL, for 20 min at 37°C. Incubations were terminated by the addition of 40 µL of 5% trifluoroacetic acid (TFA) in 4% glycerol for each reaction tube. The cleavage of Ang I, TDP and Ang II was assessed by reversed-phase HPLC analysis on equipment‡‡‡‡‡‡‡‡ fitted with a 4 × 250 mm Shim-pak ODS column; peptides were eluted with a linear gradient of acetonitrile concentration (12-32%, 30 min) in 0.1 % TFA, at a flow rate of 1.0 mL/min, and were monitored by absorbance at 215 nm, as we previously described.32 Molar concentrations of Ang II or other products formed were calculated based on the comparison with known concentrations of cognate synthetic substrates. Enzymatic activity was expressed in nmoles of product per minute per milligram of gingival tissue.
RESULTS
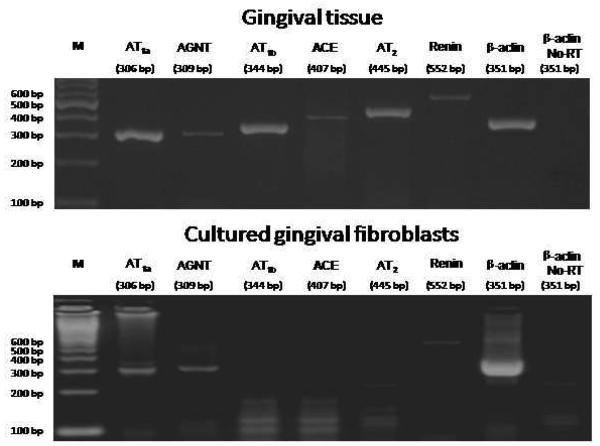
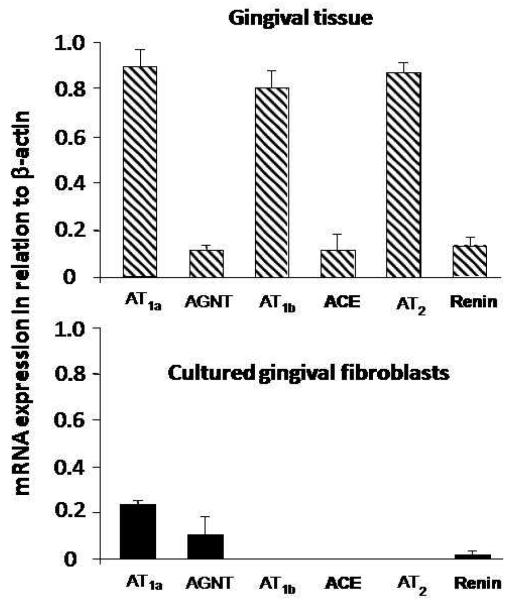
Fig. 1 illustrates mRNA detection in one sample of gingival tissue (upper panel) and cultured gingival fibroblasts (lower panel). While the rat gingival tissue expressed all the RAS components studied, gingival fibroblasts only expressed angiotensinogen, renin and AT1a receptor. Fig. 2 shows a distinct mRNA expression for the different targets in relation to β-actin in the whole gingival tissue as compared with gingival fibroblasts.
Fig. 1.
Ethidium bromide-stained agarose gel of reverse transcription-polymerase chain reaction (RT-PCR) products from rat gingival tissue (upper panel) and cultured rat gingival fibroblasts (lower panel). The cDNAs were amplified by PCR with gene-specific primers for AT1a receptor (306 bp), angiotensinogen (AGTN, 309 bp), AT1b receptor (344 bp), angiotensin I-converting enzyme (ACE, 407 bp), AT2 receptor (445 bp), renin (552 bp) and β-actin (351 bp), from 5 μg of total RNA of a rat gingival tissue sample and 0.8 μg of total RNA from cultured rat gingival fibroblasts. M = 100 bp marker.
Fig. 2.
Expression of mRNA by reverse transcription-polymerase chain reaction (RT-PCR), for different components of the renin-angiotensin system, from 5 μg of total RNA of rat gingival tissue samples (upper panel) and 0.8 μg of total RNA from cultured rat gingival fibroblasts (lower panel), in relation to β-actin (n=5). AGTN = angiotensinogen; ACE = angiotensin I-converting enzyme. Data are expressed as means±standard error of means.
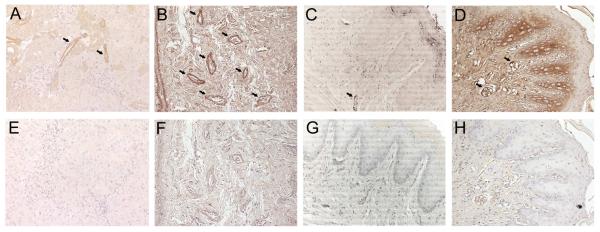
Fig. 3 unequivocally illustrates the presence of renin predominantly in vascular endothelium (arrows). The negative control, derived from a serial cut, did not show any signal of positive staining. Antibody specificity and sensitivity were tested in immunohistochemical analysis of sections known to contain the specific antigen. Examination of renal cortex stained with renin antibody demonstrated specific staining of renin in juxtaglomerular cells as expected (Fig. 4A).
Fig. 3.
Immunohistochemical detection of renin in the rat gingival tissue (original magnification ×20). A: positive control in renal cortex (arrows show juxtaglomerular cells). B and C: arrows show the staining on the vascular endothelium of healthy gingival tissue. D: intense brown staining on the epithelial basal layer of gingival tissue affected by induced periodontal disease; arrows indicate the staining on the vascular endothelium. E, F, G and H: negative controls (without the incubation of the primary antibody) of A, B, C and D, respectively.
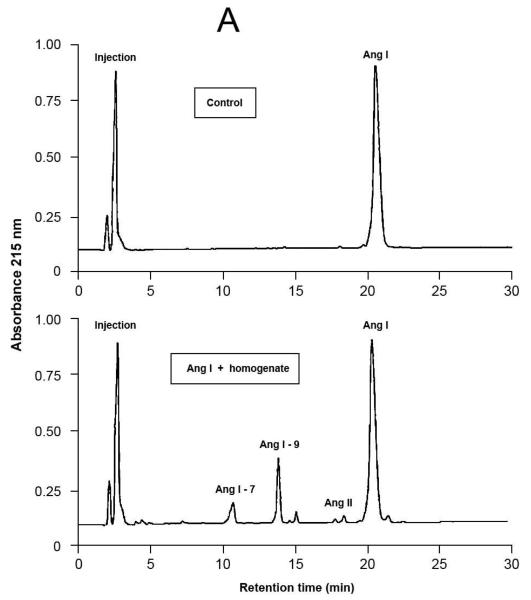
Fig.4.
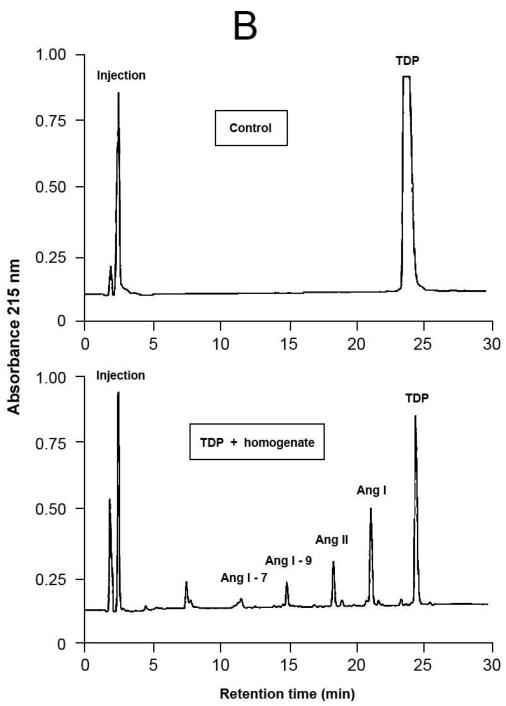
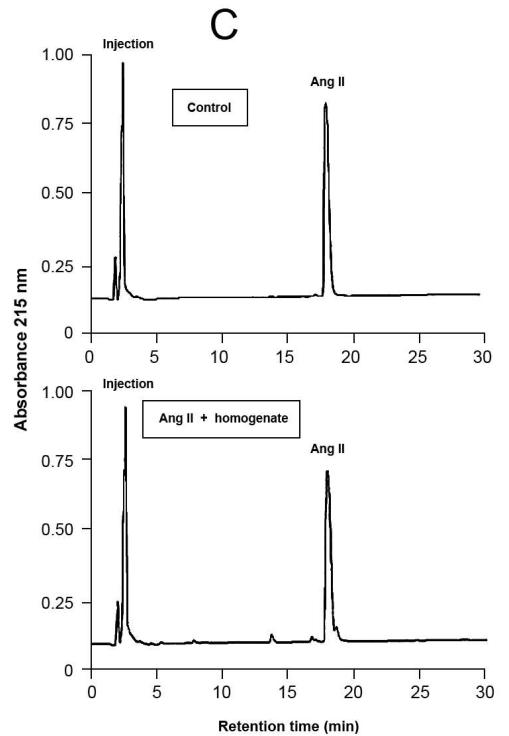
Representative results of proteolytic cleavage of angiotensin (Ang) I [A], renin substrate tetradecapeptide (TDP) [B] and Ang II [C] catalyzed by rat gingival tissue homogenate. Homogenate sample was incubated with either Ang I (30 nmol), TDP (25 nmol) or Ang II (30 nmol), in a final volume of 150 μL, in Tris buffered saline (0.03 M Tris-HCl containing 0.15 M NaCl, pH = 8.1) at 37°C for 20 min. Enzymatic reaction was stopped with the addition of 40 μL of 5% trifluoroacetic acid in 4% glycerol. Upper panels show the substrates without the incubation with the homogenate (control), while the lower panels show the hydrolysis products of the substrates by enzymatic activities present in the homogenate. Chromatograms were generated by reversed-phase HPLC analysis on a C-18 column (4 × 250 mm) developed at a flow rate of 1.0 mL/min with a linear gradient of acetonitrile (12–32%, 30 min) in 0.1% TFA. Peptides were detected by absorbance at 215 nm and quantified by comparison with known concentrations of cognate synthetic substrates.
The enzymatic assay with the tripeptide Hip-His-Leu tested the in vitro functionality of the gingival RAS detected by RT-PCR and immunohistochemistry. Results shown in Table 2 are complementary to those from Fig. 1 since they demonstrate that rat gingival tissue not only express mRNA for ACE, but also produces the active enzyme, which is present in the gingival homogenate.
Table 2.
Values of angiotensin converting enzyme (ACE) activity in different samples of rat gingival tissue (nmol His-Leu/g.min)
| Sample | ACE activity (nmol His-Leu/g.min) |
|---|---|
| 1 | 5.71 |
| 2 | 2.20 |
| 3 | 5.11 |
| 4 | 4.16 |
| 5 | 7.59 |
| Mean±SEM | 4.95±0.89 |
Note: For experimental conditions, see Material and Methods. SEM = standard deviation of mean.
HPLC was used to detect enzymatic activities capable of generating Ang II and other peptides from the Ang II precursors, Ang I (Table 3 and Fig.4A) and TDP (Table 3 and Fig.4B). Besides, HPLC was also used to investigate the existence of enzymatic activities that could degrade Ang II. The hydrolysis products revealed the formation of Ang 1-9, Ang II and Ang 1-7 with both precursors. Additionally, Ang I was formed when TDP was used as the substrate, and a very low Ang II-degrading activity could be detected, under the conditions employed (Table 3 and Fig. 4C).
Table 3.
Hydrolysis products formed by the action of rat gingival tissue homogenates towards the substrates angiotensin (Ang) I (30 nmol), renin substrate tetradecapeptide (TDP, 25 nmol) and Ang II (30 nmol) for 20 min at 37°C in Tris buffered saline (0.03 M Tris-HCl containing 0.15 M NaCl, pH = 8.1)
| Substrate | Product formed (nmol/mg.min) | |||
|---|---|---|---|---|
| Ang I | Ang 1-9 | Ang II | Ang 1-7 | |
| Ang I | - | 0.576±0.128 | 0.066±0.008 | 0.111±0.017 |
| TDP | 0.973±0.139 | 0.139±0.031 | 0.206±0.046 | 0.039±0.007 |
| Ang II | - | - | - | 0 |
Note: For experimental conditions see Material and Methods. - indicates that the product cannot be formed. Data are expressed as means±standard error of means.
DISCUSSION
The present work first documents the existence of a local RAS in the rat gingival tissue. The few reports in the literature on this subject had only showed the presence of some RAS components, mainly Ang II receptors, in the rat gingival tissue and cultured gingival fibroblasts from different species.16-19 To date, the presence of other important RAS components, such as renin and angiotensinogen, had never been shown in the gingival tissue. Therefore, our work represents an important contribution to the study of local RAS in specific tissues independently of the circulating RAS.
The isolated analysis of the positive results in immunohistochemical experiments that detected and localized renin in endothelial cells (Fig. 3B) could not definitely bring conclusions as to the origin of this enzyme since the positive staining could have occurred due to circulating renin uptake by endothelial cells.33 On the other hand, the results obtained by means of RT-PCR (Figs. 1 and 2) showed that rat gingival tissue and cultured gingival fibroblasts are capable of expressing mRNA for this enzyme. Even though the possibility of renin uptake by gingival vascular endothelium may not be excluded, the combined analysis of both immunohistochemical and RT-PCR results strongly suggests that renin is locally produced in the rat gingival tissue, for example in gingival fibroblasts (mRNA expression, Figs. 1 and 2) and endothelial cells (immunostaining, Fig. 3B). Additionally, the intense renin staining in the epithelial basal layer of periodontally diseased samples, as opposed to the absence of staining in the same region of healthy samples, provides further evidence of a local renin production.
RT-PCR experiments also demonstrated the ability of the rat gingival tissue to express ACE mRNA. In order to check whether this enzyme is locally produced, a specific method to measure ACE activity with the tripeptide Hip-His-Leu was used to detect the release of the dipeptide His-Leu by an enzymatic activity present in rat gingival tissue homogenates. The results clearly showed the presence of ACE activity in all the samples tested (Table 2). As far as the cellular source of ACE, the data obtained in this work suggest that gingival fibroblasts do not produce this enzyme since ACE mRNA was not expressed by these cells. Although we did not perform confirmatory experiments, reports in the literature document that endothelial cells represent a potential source of ACE.34,35
RT-PCR experiments showed difference in the level of mRNA expression for the target proteins studied in this work. Gingival tissue expresses angiotensinogen, renin, ACE and the receptors AT1a, AT1b and AT2 with different intensities in relation to β-actin. The whole tissue shows high expression of AT1a, AT1b and AT2 mRNA and low expression of angiotensinogen, renin and ACE, whereas cultured gingival fibroblasts only express angiotensinogen, renin and AT1a receptor. The lower expression of renin and AT1a receptor mRNA by cultured fibroblasts in comparison with the whole gingival tissue suggests that other cellular types of this tissue express mRNA for such proteins. For example, epithelial cells express Ang II receptors,36,37 while mast cells represent an abundant source of renin.38,39 Interestingly, in the presence of periodontal disease, our results showed that besides endothelial cells, other gingival cell types, such as epithelial cells, produce great amounts of renin, as opposed to an undetectable renin production in the epithelium of healthy gingiva (Fig.3C and D).
Our findings corroborate those by other authors concerning the expression of AT1 receptors by cultured gingival fibroblasts from different species such as guinea pig, human, ferret and rabbit.16-19 On the other hand, our data contrast with those by Ohuchi et al.,17 who documented the production of AT2 receptor through Western blot technique in cultured rabbit gingival fibroblasts. The results presented here also contrast with those by Ohuchi et al.,16 since these authors demonstrated that treatment with captopril, an ACE inhibitor, decreased the proliferation of cultured ferret gingival fibroblasts stimulated with Ang II, that is, indirectly the authors demonstrated ACE presence in these cells. Under the conditions employed in the present research, ACE mRNA expression was not detected in fibroblasts. One factor to be taken into account for the absence of mRNA expression of some RAS components in cultured gingival fibroblasts is the sensitivity of semi-quantitative RT-PCR. Another factor that may be considered is the possibility that gingival tissue or cultured gingival fibroblasts increase their expression of RAS components when exposed to a challenge such as an inflammatory event. There are reports of augmented expression of RAS components in the presence of inflammation that support this assumption.8,40,41 and also our results of intense renin staining in periodontally diseased gingival tissue (Fig. 3D). It is worth mentioning that Ang II itself has proinflammatory actions,42 therefore one could suggest that Ohuchi et al.16 induced an inflammatory process on cultured gingival fibroblasts with exogenous Ang II. It is important to consider that both the gingival tissue and the cultured gingival fibroblasts studied in this work presented a healthy condition compatible with homeostasis (except for the renin immunostaining in periodontally diseased gingiva). Future works should explore more deeply the effect of an inflammatory process (periodontal disease, for example) or proinflammatory substances on the expression of gingival RAS components since we detected an intense renin expression in gingival tissue affected by induced periodontal disease (Fig.3D).
When Ang I was used as substrate, the formation of three main products was observed: Ang1-9, in higher amount, followed by Ang 1-7 and Ang II, in a lower amount. On the other hand, when TDP was used as substrate, Ang I generation was mainly observed, followed by Ang II, Ang 1-9 and Ang 1-7 in descending order of product formation. These results suggest that maybe Ang II was formed due to sequential cleavage of Ang I by carboxypeptidase(s). Currently, much emphasis has been given to ACE2, a carboxypeptidase capable of sequentially hydrolyzing Ang I, leading to the formation of Ang 1-9, Ang II and Ang 1-7; although it is important to stress that this enzyme has a superior catalytic activity towards Ang II than Ang I.9,10,43 ACE may also be a potential candidate to Ang II and Ang 1-7 formation in the rat gingival tissue since the literature documents that this enzyme forms Ang II from Ang I, and Ang 1-7 from Ang 1-9.9,10,43 The generation of Ang I from TDP reveals the existence of renin or renin-like activity in the rat gingival tissue homogenate, and it is worth emphasizing that in this work renin was detected in rat gingival tissue (through RT-PCR and immunohistochemistry) and in cultured rat gingival fibroblasts (through RT-PCR).
It calls attention the fact that the amount of Ang II formed from TDP is much higher than that when Ang I is offered as the substrate to the rat gingival tissue homogenates. A possible explanation to this result may be drawn from the results reported by other authors, who showed ACE inhibition by micromolar concentrations of Ang 1-9.44,45 The numbers presented in Table 3 and illustrated in Fig. 4 allow one to conclude that, under the conditions employed in the present study, Ang 1-9 was formed in the concentration of approximately 20 μM (3 nmol of Ang 1-9 formed in 150 μL of reaction final volume). Consequently, the amount of Ang 1-9 formed in this work, when Ang I is used as the precursor substrate, was high enough to inhibit ACE, and therefore could explain the low amount of Ang II formed.
Additionally, the present results showing higher Ang II formation from TDP in comparison with Ang I strongly suggest that endopeptidases present in the rat gingival tissue homogenates directly cleaved the Phe8-His9 bond of TDP. As a potential candidate to this hydrolysis, one could include rat elastase-2,46,47 whose such an enzymatic activity towards TDP is similar to that of rat tonin48 and superior to that of human rat chymase.49 Besides ACE, other enzymes such as rat elastase-2 and rat chymase I could also have contributed to the formation of Ang II through the cleavage of the Phe8-His9 bond of Ang I.46,47 Although, it is important to take into account that rat chymase I mainly presents an Ang II-degrading activity,50 while rat elastase-2 forms Ang II and does not hydrolyze this octapeptide.46,47 Therefore, the findings presented here showing that Ang II is poorly hydrolyzed by rat gingival tissue homogenate (Table 3 and Fig. 4) are certainly instrumental to elucidate the enzymatic pathways to form and degrade peptides generated from the precursors Ang I and TDP. In order to achieve this goal, other approaches must be outlined in future experiments, such as the use of selective enzymatic inhibitors, specific enzymatic substrates or even molecular experiments that could provide evidence as to the presence of other enzymes, besides those studied in this work, that are also important for the formation and degradation of vasoactive peptides in the rat gingival tissue. One may suggest that this local RAS can contribute to the control of blood flow in the gingival tissue since vasoconstrictor (e.g., Ang II) and vasodilator (e.g., Ang 1-7) peptides can be produced locally.
Many studies on the vascular response to periodontal pathogens show that maintenance of vascular inflammation may be enhanced by the presence of periodontophatic bacteria.51-57 Based on our results of renin increase staining in periodontally diseased gingival tissues, one could speculate that these bacteria increase the expression of the local gingival RAS, thus leading to an increased local production of Ang II, which would exert its proinflammatory actions. Further understanding of the role of the RAS in the inflammatory process may provide important opportunities for clinical research and treatment of inflammatory diseases such as periodontitis.
In conclusion, this work is the first to present a clear existence of a local RAS in rat gingival tissue, which is capable of generating Ang II and other vasoactive peptides in vitro. Further studies are necessary to elucidate the role of this local gingival RAS.
ACKNOWLEDGEMENTS
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), São Paulo/SP, Brazil, grant #2004/13479-3 (C.F.S.). The authors are grateful to Vera Lúcia Rufino Rosa, from the Department of Biological Sciences of the Bauru School of Dentistry, for her excellent secretarial assistance. The authors report no conflicts of interest related to this study.
Footnotes
Thiopentax, Cristália®, Campinas, SP, Brazil
Dulbecco's modified Eagle's medium, Invitrogen Life Technologies, Carlsbad, CA.
Cultilab, Campinas, SP, Brazil.
Invitrogen.
TRIzol, Invitrogen.
Scientific Specialties Incorporated, Lodi, CA.
RNeasy Mini Kit, Qiagen, Valencia, CA.
Promega, Madison, WI.
First-strand cDNA synthesis kit, Amersham Pharmacia, Piscataway, NJ.
Dialab Diagnósticos, Belo Horizonte, MG, Brazil.
Promega.
Low DNA mass ladder, Invitrogen.
Gel-Pro Analyzer, Media Cybernetics, Inc., Silver Spring, MD.
S0809, Dako, Carpinteria, CA.
sc 27318, Santa Cruz Biotechnology, Inc., Santa Cruz, CA.
S-200, Vector Laboratories, Inc, Burlingame, CA.
BA-9500, Vector Laboratories, Inc.
Bio-Rad, Hercules, CA.
PK-6200, Vector Laboratories.
DAB, SK-4100, Vector Laboratories.
H-3401, Vector Laboratories.
Potter S homogenizer, B. Braun, Biotech Intern, Bethlehem, PA.
Shimadzu RF-535, Japan.
Shimadzu SCL-6B, Japan.
REFERENCES
- 1.Peach MJ. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977;57:313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- 2.Leung PS. The peptide hormone angiotensin II: its new functions in tissues and organs. Curr Protein Pept Sci. 2004;5:267–273. doi: 10.2174/1389203043379693. [DOI] [PubMed] [Google Scholar]
- 3.Paul M, Mehr AP, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 4.Campbell DJ. Circulating and tissue angiotensin systems. J Clin Invest. 1987;79:1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips MI, Speakman EA, Kimura B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regul Pept. 1993;43:1–20. doi: 10.1016/0167-0115(93)90403-u. [DOI] [PubMed] [Google Scholar]
- 6.Obermüller N, Gentili M, Gauer S, et al. Immunohistochemical and mRNA localization of the angiotensin II receptor subtype 2 (AT2) in follicular granulosa cells of the rat ovary. J Histochem Cytochem. 2004;52:545–548. doi: 10.1177/002215540405200413. [DOI] [PubMed] [Google Scholar]
- 7.Goker H, Haznedaroglu IC, Beyazit Y, et al. Local umbilical cord blood renin-angiotensin system. Ann Hematol. 2005;84:277–281. doi: 10.1007/s00277-004-0989-x. [DOI] [PubMed] [Google Scholar]
- 8.Souza PPC, Fukada SY, Cunha FQ, Costa CAS, Costa-Neto CM. Regulation of angiotensin II receptors levels during rat induced pulpitis. Regul Pept. 2007;140:27–31. doi: 10.1016/j.regpep.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Raizada MK, Ferreira AJ. ACE2: a new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol. 2007;50:112–119. doi: 10.1097/FJC.0b013e3180986219. [DOI] [PubMed] [Google Scholar]
- 10.Kramkowski K, Mogielnicki A, Buczko W. The physiological significance of the alternative pathways of angiotensin II production. J Physiol Pharmacol. 2006;57:529–539. [PubMed] [Google Scholar]
- 11.Turner AJ, Nalivaeva NN. Proteinase dysbalance in pathology: the neprilysin (NEP) and angiotensin-converting enzyme (ACE) families. Cell Mol Biol (Noisy-le-grand) 2006;52:40–48. [PubMed] [Google Scholar]
- 12.Aulakh GK, Sodhi RK, Singh M. An update on non-peptide angiotensin receptor antagonists and related RAAS modulators. Life Sci. 2007;81:615–639. doi: 10.1016/j.lfs.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Ruiz-Ortega M, Lorenzo O, et al. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 14.Dandona P, Dhindsa S, Ghanim H, et al. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007;21:20–27. doi: 10.1038/sj.jhh.1002101. [DOI] [PubMed] [Google Scholar]
- 15.Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29:367–374. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Ohuchi N, Koike K, Sano M, et al. Proliferative effects of angiotensin II and endothelin-1 on guinea pig gingival fibroblast cells in culture. Comp Biochem Physiol C Toxicol Pharmacol. 2002;132:451–460. doi: 10.1016/s1532-0456(02)00098-4. [DOI] [PubMed] [Google Scholar]
- 17.Ohuchi N, Hayashi K, Koike K, et al. Pharmacological properties of angiotensin II receptors in cultured rabbit gingival fibroblasts. Comp Biochem Physiol C Toxicol Pharmacol. 2004;137:281–289. doi: 10.1016/j.cca.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Berggreen E, Heyeraas KJ. Role of K+ATP channels, endothelin A receptors, and effect of angiotensin II on blood flow in oral tissues. J Dent Res. 2003;82:33–37. doi: 10.1177/154405910308200108. [DOI] [PubMed] [Google Scholar]
- 19.Segawa M, Nakao S, Ogata Y, Sugiya H, Furuyama S. Angiotensin II induces prostaglandin E(2) release in human gingival fibroblasts. Life Sci. 2003;72:795–803. doi: 10.1016/s0024-3205(02)02340-8. [DOI] [PubMed] [Google Scholar]
- 20.Santos CF, Oliveira EB, Salgado MCO, Greene AS. Molecular cloning and sequencing of the cDNA for rat mesenteric arterial bed elastase-2, an angiotensin II-forming enzyme. J Cardiovasc Pharmacol. 2002;39:628–635. doi: 10.1097/00005344-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Santos CF, Caprio MA, Oliveira EB, et al. Functional role, cellular source and tissue distribution of rat elastase-2, an angiotensin II-forming enzyme. Am J Physiol Heart Circ Physiol. 2003;285:H775–H783. doi: 10.1152/ajpheart.00818.2002. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira TM, Sakai VT, Machado MAAM, et al. COX-2 inhibition decreases VEGF expression and the alveolar bone loss during the progression of experimental periodontitis in rats. J Periodontol. 2008;79:1062–1069. doi: 10.1902/jop.2008.070411. [DOI] [PubMed] [Google Scholar]
- 23.Rodini CO, Batista AC, Dionísio TJ, Santos CF, Cunha FQ, Lara VS. Morphologic evaluation and expression of matrix metalloproteinases-2 and 9 and nitric oxide during experimental periodontal disease in rat. J Mol Histol. 2008;39:275–282. doi: 10.1007/s10735-008-9163-4. [DOI] [PubMed] [Google Scholar]
- 24.Agoudemos MM, Greene AS. Localization of the renin-angiotensin system components to the skeletal muscle microcirculation. Microcirculation. 2005;12:627–636. doi: 10.1080/10739680500301664. [DOI] [PubMed] [Google Scholar]
- 25.Linderman JR, Greene AS. Distribution of angiotensin II receptor expression in the microcirculation of striated muscle. Microcirculation. 2001;8:275–281. doi: 10.1038/sj/mn/7800097. [DOI] [PubMed] [Google Scholar]
- 26.Lam SY, Leung OS. Chronic hypoxia activates a local angiotensin-generating system in rat carotid body. Mol Cell Endocrinol. 2003;203:147–153. doi: 10.1016/s0303-7207(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 27.Yang HYT, Neff NH. Distribution and properties of angiotensin converting enzyme of rat brain. J Neurochem. 1972;19:2443–2450. doi: 10.1111/j.1471-4159.1972.tb01298.x. [DOI] [PubMed] [Google Scholar]
- 28.Leite R, Salgado MCO. Increased vascular formation of angiotensin II in one-kidney, one clip hypertension. Hypertension. 1992;19:575–581. doi: 10.1161/01.hyp.19.6.575. [DOI] [PubMed] [Google Scholar]
- 29.Cunha V, Salgado HC, Salgado MCO. Enalapril prevents aortic hyperreactivity and remodelling in one-kidney, one-clip hypertensive rats without reducing arterial pressure. Clin Exp Pharmacol Physiol. 2000;27:474–479. doi: 10.1046/j.1440-1681.2000.03283.x. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira EM, Santos RA, Krieger JE. Standardization of a fluorimetric assay for the determination of tissue angiotensin-converting enzyme activity in rats. Braz J Med Biol Res. 2000;33:755–764. doi: 10.1590/s0100-879x2000000700005. [DOI] [PubMed] [Google Scholar]
- 31.Sivieri DO, Jr, Bispo-da-Silva LB, Oliveira EB, Resende AC, Salgado MCO. Potentiation of bradykinin effect by angiotensin-converting enzyme inhibition does not correlate with angiotensin-converting enzyme activity in the rat mesenteric arteries. Hypertension. 2007;50:110–115. doi: 10.1161/HYPERTENSIONAHA.106.085761. [DOI] [PubMed] [Google Scholar]
- 32.Santos CF, Paula CA, Salgado MCO, Oliveira EB. Kinetic characterization and inhibition of the rat MAB elastase-2, an angiotensin I-converting serine protease. Can J Physiol Pharmacol. 2002;80:42–47. doi: 10.1139/y02-004. [DOI] [PubMed] [Google Scholar]
- 33.Swales JD, Abramovici A, Beck F, Bing RF, Loudon M, Thurston H. Arterial wall renin. J Hypertens Suppl. 1983;1:17–22. [PubMed] [Google Scholar]
- 34.Igić R, Behnia R. Properties and distribution of angiotensin I converting enzyme. Curr Pharm Des. 2003;9:697–706. doi: 10.2174/1381612033455459. [DOI] [PubMed] [Google Scholar]
- 35.Gryglewski RJ, Uracz W, Swies J, et al. Comparison of endothelial pleiotropic actions of angiotensin converting enzyme inhibitors and statins. Ann N Y Acad Sci. 2001;947:229–245. doi: 10.1111/j.1749-6632.2001.tb03945.x. [DOI] [PubMed] [Google Scholar]
- 36.Königshoff M, Wilhelm A, Jahn A, et al. The angiotensin II receptor 2 is expressed and mediates angiotensin II signaling in lung fibrosis. Am J Respir Cell Mol Biol. 2007;37:640–650. doi: 10.1165/rcmb.2006-0379TR. [DOI] [PubMed] [Google Scholar]
- 37.Yahata Y, Shirakata Y, Tokumaru S, et al. A novel function of angiotensin II in skin wound healing. Induction of fibroblast and keratinocyte migration by angiotensin II via heparin-binding epidermal growth factor (EGF)-like growth factor-mediated EGF receptor transactivation. J Biol Chem. 2006;281:13209–13216. doi: 10.1074/jbc.M509771200. [DOI] [PubMed] [Google Scholar]
- 38.Silver RB, Reid AC, Mackins CJ, et al. Mast cells: a unique source of renin. Proc Natl Acad Sci USA. 2004;101:13607–13612. doi: 10.1073/pnas.0403208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veerappan A, Reid AC, Estephan R, et al. Mast cell renin and a local renin-angiotensin system in the airway: Role in bronchoconstriction. Proc Natl Acad Sci USA. 2008;105:1315–1320. doi: 10.1073/pnas.0709739105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dandapat A, Hu CP, Chen J, et al. Over-expression of angiotensin II type 2 receptor (agtr2) decreases collagen accumulation in atherosclerotic plaque. Biochem Biophys Res Commun. 2008;366:871–877. doi: 10.1016/j.bbrc.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 41.Steckelings UM, Henz BM, Wiehstutz S, Unger T, Artuc M. Differential expression of angiotensin receptors in human cutaneous wound healing. Br J Dermatol. 2005;153:887–893. doi: 10.1111/j.1365-2133.2005.06806.x. [DOI] [PubMed] [Google Scholar]
- 42.Douillette A, Bibeau-Poirier A, Gravel SP, et al. The proinflammatory actions of angiotensin II are dependent on p65 phosphorylation by the IkappaB kinase complex. J Biol Chem. 2006;281:13275–13284. doi: 10.1074/jbc.M512815200. [DOI] [PubMed] [Google Scholar]
- 43.Wright JW, Yamamoto BJ, Harding JW. Angiotensin receptor subtype mediated physiologies and behaviors: New discoveries and clinical targets. Prog Neurobiol. 2008;84:157–181. doi: 10.1016/j.pneurobio.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyder RA, Wintroub BU. Inhibition of angiotensin-converting enzyme by des-Leu10-angiotensin I: a potential mechanism of endogenous angiotensin-converting enzyme regulation. Biochim Biophys Acta. 1986;871:1–5. doi: 10.1016/0167-4838(86)90125-1. [DOI] [PubMed] [Google Scholar]
- 45.Marcic B, Deddish PA, Jackman HL, Erdös EG. Enhancement of bradykinin and resensitization of its B2 receptor. Hypertension. 1999;33:835–843. doi: 10.1161/01.hyp.33.3.835. [DOI] [PubMed] [Google Scholar]
- 46.Paula CA, Sousa MV, Salgado MCO, Oliveira EB. Purification and substrate specificity of an angiotensin converting elastase-2 from the rat mesenteric arterial bed perfusate. Biochim Biophys Acta. 1998;1388:227–238. doi: 10.1016/s0167-4838(98)00186-1. [DOI] [PubMed] [Google Scholar]
- 47.Santos CF, Greene AS, Salgado MCO, Oliveira EB. Conversion of renin substrate tetradecapeptide to angiotensin II by rat MAB elastase-2. Can J Physiol Pharmacol. 2004;82:1000–1005. doi: 10.1139/y04-102. [DOI] [PubMed] [Google Scholar]
- 48.Gutkowska J, Corvol P, Figueiredo AF, Inagami T, Bouhnik J, Genest J. Kinetic studies of rat renin and tonin on purified rat angiotensinogen. Can J Biochem Cell Biol. 1984;62:137–142. doi: 10.1139/o84-020. [DOI] [PubMed] [Google Scholar]
- 49.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348–22357. [PubMed] [Google Scholar]
- 50.Le Trong H, Neurath H, Woodbury RG. Substrate specificity of the chymotrypsin-like protease in secretory granules isolated from rat mast cells. Proc Natl Acad Sci. 1987;84:364–367. doi: 10.1073/pnas.84.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deshpande RG, Khan MB, Genco CA. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect Immun. 1998;66:5337–5343. doi: 10.1128/iai.66.11.5337-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi EK, Park SA, Oh WM, et al. Mechanisms of Porphyromonas gingivalis-induced monocyte chemoattractant protein-1 expression in endothelial cells. FEMS Immunol Med Microbiol. 2005;44:51–58. doi: 10.1016/j.femsim.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Chun YH, Chun KR, Olguin D, et al. Biological foundation for periodontitis as a potential risk factor for atherosclerosis. J Periodontal Res. 2005;40:87–95. doi: 10.1111/j.1600-0765.2004.00771.x. [DOI] [PubMed] [Google Scholar]
- 54.Ford PJ, Gemmell E, Chan A, Carter CL, Walker PJ, Bird PS, West MJ, Cullinan MP, Seymour GJ. Inflammation, heat shock proteins and periodontal pathogens in atherosclerosis: an immunohistologic study. Oral Microbiol Immunol. 2006;21:206–211. doi: 10.1111/j.1399-302X.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- 55.Koo TH, Jun HO, Bae SK, et al. Porphyromonas gingivalis, periodontal pathogen, lipopolysaccharide induces angiogenesis via extracellular signal-regulated kinase 1/2 activation in human vascular endothelial cells. Arch Pharm Res. 2007;30:34–42. doi: 10.1007/BF02977776. [DOI] [PubMed] [Google Scholar]
- 56.Roth GA, Moser B, Roth-Walter F, et al. Infection with a periodontal pathogen increases mononuclear cell adhesion to human aortic endothelial cells. Atherosclerosis. 2007;190:271–281. doi: 10.1016/j.atherosclerosis.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 57.Colhoun HM, Slaney JM, Rubens MB, et al. Antibodies to periodontal pathogens and coronary artery calcification in type 1 diabetic and nondiabetic subjects. J Periodontal Res. 2008;43:103–110. doi: 10.1111/j.1600-0765.2007.01001.x. [DOI] [PubMed] [Google Scholar]