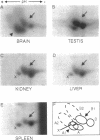
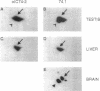
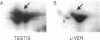Abstract
Cytoplasmic dynein is the microtubule minus-end-directed motor for the retrograde axonal transport of membranous organelles. Because of its similarity to the intermediate chains of flagellar dynein, the 74-kDa intermediate chain (IC74) subunit of dynein is thought to be involved in binding dynein to its membranous organelle cargo. Previously, we identified six isoforms of the IC74 cytoplasmic dynein subunit in the brain. We further demonstrated that cultured glia and neurons expressed different dynein IC74 isoforms and phospho-isoforms. Two isoforms were observed when dynein from glia was analyzed. When dynein from cultured neurons was analyzed, six IC74 isoforms were observed, although the relative amounts of the dynein isoforms from cultured neurons differed from those found in dynein from brain. To better understand the role of the neuronal IC74 isoforms and identify neuron-specific IC74 dynein subunits, the expression of the IC74 protein isoforms and mRNAs of various tissues were compared. As a result of this comparison, the identity of each of the isoform spots observed on two-dimensional gels was correlated with the products of each of the IC74 mRNAs. We also found that between the fifteenth day of gestation (E15) and the fifth day after birth (P5), the relative expression of the IC74 protein isoforms changes, demonstrating that the expression of IC74 isoforms is developmentally regulated in brain. During this time period, there is relatively little change in the abundance of the various IC74 mRNAs. The E15 to P5 time period is one of rapid process extension and initial pattern formation in the rat brain. This result indicates that the changes in neuronal IC74 isoforms coincide with neuronal differentiation, in particular the extension of processes. This suggests a role for the neuronal IC74 isoforms in the establishment or regulation of retrograde axonal transport.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aniento F., Emans N., Griffiths G., Gruenberg J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J Cell Biol. 1993 Dec;123(6 Pt 1):1373–1387. doi: 10.1083/jcb.123.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M., Parton R., Kuznetsov S. A., Schroer T. A., Gruenberg J. Microtubule- and motor-dependent fusion in vitro between apical and basolateral endocytic vesicles from MDCK cells. Cell. 1990 Aug 24;62(4):719–731. doi: 10.1016/0092-8674(90)90117-w. [DOI] [PubMed] [Google Scholar]
- Brady S. T. Molecular motors in the nervous system. Neuron. 1991 Oct;7(4):521–533. doi: 10.1016/0896-6273(91)90365-7. [DOI] [PubMed] [Google Scholar]
- Collins C. A., Vallee R. B. Preparation of microtubules from rat liver and testis: cytoplasmic dynein is a major microtubule associated protein. Cell Motil Cytoskeleton. 1989;14(4):491–500. doi: 10.1002/cm.970140407. [DOI] [PubMed] [Google Scholar]
- Corthésy-Theulaz I., Pauloin A., Pfeffer S. R. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol. 1992 Sep;118(6):1333–1345. doi: 10.1083/jcb.118.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman J. F., 3rd, Pfister K. K. Differential phosphorylation in vivo of cytoplasmic dynein associated with anterogradely moving organelles. J Cell Biol. 1994 Dec;127(6 Pt 1):1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath K. R., Trimbur G. M., Burgess D. R. Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells. J Cell Biol. 1994 Aug;126(3):661–675. doi: 10.1083/jcb.126.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. R., Cleveland D. W., Schroer T. A. Characterization of DLC-A and DLC-B, two families of cytoplasmic dynein light chain subunits. Mol Biol Cell. 1994 Jun;5(6):645–654. doi: 10.1091/mbc.5.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur E. L., Vallee R. B. DYNEINS: molecular structure and cellular function. Annu Rev Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- King S. M., Patel-King R. S., Wilkerson C. G., Witman G. B. The 78,000-M(r) intermediate chain of Chlamydomonas outer arm dynein is a microtubule-binding protein. J Cell Biol. 1995 Oct;131(2):399–409. doi: 10.1083/jcb.131.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. M., Wilkerson C. G., Witman G. B. The Mr 78,000 intermediate chain of Chlamydomonas outer arm dynein interacts with alpha-tubulin in situ. J Biol Chem. 1991 May 5;266(13):8401–8407. [PubMed] [Google Scholar]
- King S. M., Witman G. B. Localization of an intermediate chain of outer arm dynein by immunoelectron microscopy. J Biol Chem. 1990 Nov 15;265(32):19807–19811. [PubMed] [Google Scholar]
- Lafont F., Burkhardt J. K., Simons K. Involvement of microtubule motors in basolateral and apical transport in kidney cells. Nature. 1994 Dec 22;372(6508):801–803. doi: 10.1038/372801a0. [DOI] [PubMed] [Google Scholar]
- Mikami A., Paschal B. M., Mazumdar M., Vallee R. B. Molecular cloning of the retrograde transport motor cytoplasmic dynein (MAP 1C). Neuron. 1993 May;10(5):787–796. doi: 10.1016/0896-6273(93)90195-w. [DOI] [PubMed] [Google Scholar]
- Mitchell D. R., Kang Y. Identification of oda6 as a Chlamydomonas dynein mutant by rescue with the wild-type gene. J Cell Biol. 1991 May;113(4):835–842. doi: 10.1083/jcb.113.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. R., Kang Y. Reversion analysis of dynein intermediate chain function. J Cell Sci. 1993 Aug;105(Pt 4):1069–1078. doi: 10.1242/jcs.105.4.1069. [DOI] [PubMed] [Google Scholar]
- Neely M. D., Erickson H. P., Boekelheide K. HMW-2, the Sertoli cell cytoplasmic dynein from rat testis, is a dimer composed of nearly identical subunits. J Biol Chem. 1990 May 25;265(15):8691–8698. [PubMed] [Google Scholar]
- Ogawa K., Kamiya R., Wilkerson C. G., Witman G. B. Interspecies conservation of outer arm dynein intermediate chain sequences defines two intermediate chain subclasses. Mol Biol Cell. 1995 Jun;6(6):685–696. doi: 10.1091/mbc.6.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Mikami A., Pfister K. K., Vallee R. B. Homology of the 74-kD cytoplasmic dynein subunit with a flagellar dynein polypeptide suggests an intracellular targeting function. J Cell Biol. 1992 Sep;118(5):1133–1143. doi: 10.1083/jcb.118.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Shpetner H. S., Vallee R. B. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J Cell Biol. 1987 Sep;105(3):1273–1282. doi: 10.1083/jcb.105.3.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K. K., Salata M. W., Dillman J. F., 3rd, Vaughan K. T., Vallee R. B., Torre E., Lye R. J. Differential expression and phosphorylation of the 74-kDa intermediate chains of cytoplasmic dynein in cultured neurons and glia. J Biol Chem. 1996 Jan 19;271(3):1687–1694. doi: 10.1074/jbc.271.3.1687. [DOI] [PubMed] [Google Scholar]
- Schroer T. A., Sheetz M. P. Functions of microtubule-based motors. Annu Rev Physiol. 1991;53:629–652. doi: 10.1146/annurev.ph.53.030191.003213. [DOI] [PubMed] [Google Scholar]
- Toyoshima I., Yu H., Steuer E. R., Sheetz M. P. Kinectin, a major kinesin-binding protein on ER. J Cell Biol. 1992 Sep;118(5):1121–1131. doi: 10.1083/jcb.118.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. P. The roles of microtubule-associated proteins in brain morphogenesis: a review. Brain Res Brain Res Rev. 1990 May-Aug;15(2):101–120. doi: 10.1016/0165-0173(90)90013-e. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., Bloom G. S. Mechanisms of fast and slow axonal transport. Annu Rev Neurosci. 1991;14:59–92. doi: 10.1146/annurev.ne.14.030191.000423. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., Shpetner H. S., Paschal B. M. The role of dynein in retrograde axonal transport. Trends Neurosci. 1989 Feb;12(2):66–70. doi: 10.1016/0166-2236(89)90138-0. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., Wall J. S., Paschal B. M., Shpetner H. S. Microtubule-associated protein 1C from brain is a two-headed cytosolic dynein. Nature. 1988 Apr 7;332(6164):561–563. doi: 10.1038/332561a0. [DOI] [PubMed] [Google Scholar]
- Vaughan K. T., Vallee R. B. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995 Dec;131(6 Pt 1):1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson C. G., King S. M., Koutoulis A., Pazour G. J., Witman G. B. The 78,000 M(r) intermediate chain of Chlamydomonas outer arm dynein isa WD-repeat protein required for arm assembly. J Cell Biol. 1995 Apr;129(1):169–178. doi: 10.1083/jcb.129.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]