Abstract
Disorders of respiratory control are a prominent feature of Rett syndrome (RTT), a severely debilitating condition caused by mutations in the gene encoding methyl-CpG-binding protein 2 (MECP2). RTT patients present with a complex respiratory phenotype that can include periods of hyperventilation, apnea, breath holds terminated by Valsalva maneuvers, forced and deep breathing and apneustic breathing, as well as abnormalities of heart rate control and cardiorespiratory integration. Recent studies of mouse models of RTT have begun to shed light on neurologic deficits that likely contribute to respiratory dysfunction including, in particular, defects in neurochemical signaling resulting from abnormal patterns of neurotransmitter and neuromodulator expression. The authors hypothesize that breathing dysregulation in RTT results from disturbances in mechanisms that modulate the respiratory rhythm, acting either alone or in combination with more subtle disturbances in rhythm and pattern generation. This article reviews the evidence underlying this hypothesis as well as recent efforts to translate our emerging understanding of neurochemical defects in mouse models of RTT into preclinical trials of potential treatments for respiratory dysfunction in this disease.
1. INTRODUCTION
Rett syndrome (RTT) is a complex neurodevelopmental disorder whose underlying pathogenic mechanisms remain poorly understood. RTT affects approximately 1 in 10,000 live female births and is characterized by apparently normal early postnatal development followed by neurological decline around 6 – 18 months of age. The disorder has a highly variable course and affected individuals exhibit a broad array of symptoms that generally includes loss of acquired speech, head growth deceleration, autistic features such as emotional withdrawal and diminished eye contact, motor stereotypies, early hypotonia followed by rigidity, epileptiform seizures, exaggerated responses to stress and severe respiratory and autonomic (cardiac and gastrointestinal) dysfunction (Hagberg et al., 1983; Shahbazian et al., 2002; Vorsanova et al., 2004; Chahrour and Zoghbi 2007, Chahrour, et al., 2008; Ogier and Katz, 2008; Weese-Mayer et al. 2006, 2008). Approximately 25% of RTT patients may die prematurely of cardiorespiratory failure (Kerr et al., 1997).
At least 95% of typical RTT cases result from loss-of-function mutations in the gene encoding methyl-CpG-binding protein 2 (MeCP2; Amir et al., 1999; Shahbazian et al., 2002), one of a number of methyl-binding proteins (Klose and Bird, 2006) that regulate gene expression by repressing transcription at methylated promoters. Over 200 different MECP2 mutations have been found in RTT patients and tend to cluster within two functional domains of the protein; a methyl-binding domain that recognizes methylated CpG dinucleotides with particular flanking sequences (Klose and Bird, 2006), and a transcription repression domain. The MECP2 gene is X-linked, and homozygous mutation in females (or hemizygous mutation in males) is invariably lethal. Thus, affected females are heterozygotes and somatic mosaics for MeCP2, i.e., cells in which the mutated allele occurs on the inactive X are phenotypically normal, whereas cells in which the mutated allele occurs on the active X are mutant. Disease phenotype is therefore affected not only by the specific MECP2 mutation but by the skewing of X chromosome inactivation; individuals in which inactivation is skewed towards the mutant allele are less severely affected, and vice versa. For a more detailed discussion of molecular genetic aspects of RTT the reader is referred to an excellent recent review by Chahrour and Zoghbi (2007).
2. CARDIO-RESPIRATORY PHENOTYPES IN RTT
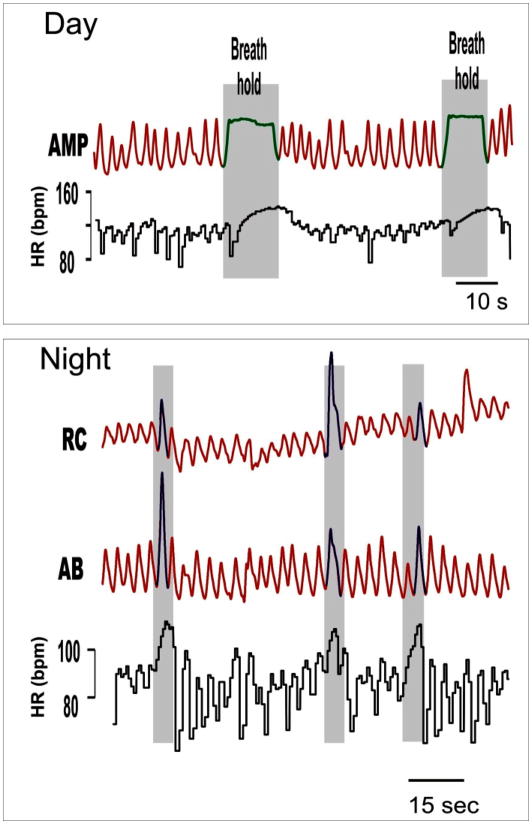
Breathing abnormalities are among the clinical diagnostic criteria for RTT, and include alternating periods of hyperventilation and apneas, breath holds terminated by Valsalva’s maneuvers, forced and deep breathing as well as apneustic breathing (Fig. 1) (cf. Elian and Rudolf, 1991; Julu et al. 2001; Julu and Witt-Engerström, 2005; Marcus et al. 1994, Weese-Mayer et al., 2006, 2008). Perhaps the most striking breathing abnormalities are the frequent breath holds and apneas as they reveal abnormal cardiorespiratory coupling. In particular, each breath hold is associated with an instantaneous subtle decrement in heart rate, followed by an exaggerated increase that is not seen in controls. We hypothesize that the frequent reoccurrence of breath holds with an overshoot in the heart rate response likely results in an alternating exposure to hypoxic and oxidative stress, which may in turn have detrimental long-term consequences on cardiorespiratory homeostasis (Nanduri et al., 2009). Recurring breath holds may have another important consequence for cardiovascular function as they are thought to lead to prolonged QT syndrome (Guideri et al. 1999, 2001, 2004; Sekul et al. 1994). Indeed, decreased heart rate variability and prolonged QTc are characteristic features of RTT patients (Weese-Mayer et al. 2006). A long QT syndrome is significant as it has been linked with sudden death in non-RTT children and could therefore also explain the sudden and unexpected deaths in RTT patients (Weese-Mayer et al. 2006, 2008; Kerr et al. 1997).
Figure 1.
Breaths and breath-holds in a representative child with Rett Syndrome obtained during the day and night using inductance plethysmography waveforms: sum amplitude (AMP), rib cage (RC), abdomen (AB), and heart rate (HR) are shown. Note that respiratory irregularities and characteristic heart rate changes occur during both the day and night. Adapted from Weese-Mayer et al. 2006, 2008.
Another key feature of the RTT breathing phenotype is its state-dependency. Respiratory disturbances in RTT are significantly more severe during wakefulness compared to sleep (Weese-Mayer et al., 2008) and are exacerbated by behavioral arousal. However, it must be emphasized that breathing during sleep is not normal (Weese-Mayer et al. 2008). Indeed, the abnormalities in breathing are reminiscent of those observed during the day, but are less frequent and severe. As during the day, nocturnal breathing is more irregular, and breathing frequency, mean airflow and heart rate are significantly increased in RTT patients compared with controls. Similarly, heart rate variability is decreased during the night. Thus, cardiorespiratory dysregulation in RTT occurs during wakefulness and sleep, but the degree of dysregulation is quantitatively much less dramatic during night time compared to wakefulness. The state-dependence of breathing abnormalities in RTT suggests that problems with modulation of the respiratory rhythm and motor pattern play a critical role in shaping the ventilatory phenotype in these patients. It is therefore tempting to speculate that breathing dysregulation in RTT results from disturbances in mechanisms that modulate the respiratory rhythm, acting either alone or in combination with more subtle disturbances in rhythm and pattern generation. Disturbances in neuromodulatory mechanisms could be responsible in particular for the significant worsening of breathing irregularities during the day, as well as with behavioral arousal and postnatal maturation. These disturbances could primarily reflect dysregulation of aminergic, peptidergic and neurotrophin-mediated inputs to the rhythm generating network that are critical for adaptive responses to sensory feedback and changing behavioral demands as well as postnatal maturation of the respiratory motor pattern. The persistence of breathing irregularities during both day and night could, in addition, reflect disruption of the normal balance between fast excitatory and inhibitory synaptic mechanisms within the rhythm generating network itself. These two possible types of disturbances are discussed in more detail below.
3. RESPIRATORY PHENOTYPES IN MOUSE MODELS OF RTT
The discovery that mutations in MECP2 are responsible for most cases of RTT led to the development of mouse models in which Mecp2 is either deleted, mutated or overexpressed, including 1) Mecp2−/y mice with extended exonic deletion of the Mecp2 gene (Chen et al., 2001; Guy et al., 2001; Pelka et al., 2006), 2) Mecp2308/y mice with truncation of MeCP2 protein at amino acid 308, a human RTT mutation (Shahbazian et al., 2002), 3) Mecp2Flox/y mice expressing a hypomorphic Mecp2 allele (Samaco et al., 2008) and 4) Mecp2Tg1 mice that overexpress MeCP2 protein (Collins et al., 2004). As with RTT patients, homozygous female Mecp2 mutant mice are not viable and heterozygous females are phenotypically heterogeneous due to variable patterns of X-chromosome inactivation. Therefore, most laboratories study hemizygous males (Mecp2−/y), which are completely null for Mecp2 and therefore tend to be more phenotypically homogenous than female heterozygotes (see however Bissonnette and Knopp, 2006, 2008; Bissonnette et al., 2007). In addition, male hemizygotes typically become symptomatic within several weeks after birth, whereas female heterozygotes tend to develop symptoms only after several months.
The different loss-of-function mouse models exhibit varying degrees of RTT-like pathophysiology, including, in most cases, disturbances of breathing that closely resemble respiratory dysfunction in human RTT patients (Fig. 2). All strains of MeCP2 deficient mice studied thus far exhibit erratic breathing, characterized in particular by pronounced apneic spells that appear after an initial period of apparently normal postnatal development, similar to human patients (Viemari et al., 2005; Ogier et al., 2007; for details see below). The developmental course of breathing disorders in RTT is mimicked in male Mecp2 knockout mice (Mecp2tm1.1Bird −/y; Viemari et al. 2005), as the onset of arrhythmic breathing at rest occurs in these animals around postnatal day (P) 30 (Viemari et al. 2005), an age that correlates, at least in some major aspects, with approximately 1 to 1.5 years in humans (Watson et al., 2006). In addition to apneas, Mecp2tm1.1Jae mice (Chen et al., 2001) exhibit significant periods of abnormally fast breathing that result in a 20% increase in mean respiratory frequency (Ogier et al., 2007), as in RTT patients (Weese-Mayer et al., 2008).
Figure 2.
Plethysmographic recordings from P35 WT (Mecp2+/y) and Mecp2 null (Mecp2−/y) mice (Mecp2tm1.1Jae strain). Each horizontal trace = 10s quiet breathing in room air. Adapted from Ogier et al., (2007).
Analysis of the respiratory motor-pattern in Mecp2tm1.1Bird mice (Guy et al., 2001) with an in situ perfused brainstem preparation showed that erratic breathing and apneas are related to spontaneous fluctuation of postinspiratory motor output to laryngeal adductor muscles during the early expiratory phase (Stettner et al. 2007). The disturbed postinspiratory motor activity accounts in particular for apneas with active glottal closure and could therefore underlie the loss of speech and impaired swallowing observed in RTT patients (Stettner et al., 2008). Postinspiratory activity is indeed essential for airflow modulation during speech, while glottal closure is needed to protect the lungs from invasion of noxious substances (Dutschmann et al., 2008) or food during swallowing (Gestreau et al., 2005). Therefore, we speculate that disturbed respiratory related functions in RTT, such as vocalization and swallowing, are also associated to some degree with brainstem dysfunction (Stettner et al., 2007, 2008). Moreover, since neural regulation of the respiratory and cardiovascular systems are closely linked, it is not surprising that RTT patients also suffer from cardiac sympathetic imbalance, peripheral vasomotor disturbances (Guideri et al. 1999; Julu et al. 1997) and exaggerated heart rate responses during breath-holds (Weese-Mayer et al., 2006, 2008). However, abnormal cardiovascular function has not yet been described in mouse models of RTT (cf. Bissonnette et al., 2007).
The phenotypic complexity of RTT is undoubtedly related to the fact that Mecp2 mutations result in the dysregulation of very large numbers of genes, some of which increase in expression whereas others decrease (cf. Chahrour et al., 2008). Despite this complexity, growing evidence from mouse models of RTT points to abnormal synaptic function, particularly related to defects in neurochemical signaling, as a key factor contributing to neuropathophysiology caused by loss of function mutations in Mecp2 (cf Wang et al., 2006; Monteggia and Kavalali, 2009).
4. ABNORMAL NEUROCHEMICAL SIGNALING IN RTT MICE
4.1. Disturbed GABAergic and glutamatergic neurotransmission
Growing evidence suggests that at least some of the neurological endophenotypes in RTT, including breathing abnormalities, are associated with changes in MeCP2-mediated regulation of excitatory glutamatergic or inhibitory GABAergic synaptic transmission (Blue et al., 1999; Samaco et al., 2005; Chao et al., 2007; Stettner et al., 2007). Moreover, significant metabolic anomalies, including altered glutamate metabolism, have been described in Mecp2 null mice (Viola et al., 2007). It is well established that proper function of the respiratory network, i.e. elaboration and adaptation of the rhythmic central drive to behavioral and environmental changes, requires both glutamatergic excitatory and GABAergic inhibitory synapses. Thus, disturbed function of these synapses in the absence of MeCP2 would be expected to have a direct impact on neural control of breathing.
Developmental alterations in the composition of glutamate and GABA receptor subunits are normally associated with postnatal maturation of respiratory cell groups in the medulla and pons (Wong-Riley and Liu, 2005, 2008). As early as postnatal day (P) 7, Mecp2−/y mice exhibit reduced expression of GABA-A2 and GABA-A4 receptor subunits in the rostral ventrolateral medulla oblongata (Medrihan et al., 2008), a region that contains diverse neuronal populations important for cardio-respiratory control, compared to wild type (WT) animals. In addition, GABAergic, but not glycinergic, synaptic transmission is strongly depressed at P7 in Mecp2−/y mice, leading to an imbalance in excitatory and inhibitory neurotransmission. However, whether or not these early changes in GABAergic signaling contribute to respiratory dysfunction in Mecp2 null mice remains unclear, as plethysmographic recordings did not reveal abnormalities in resting ventilation at P7 (Medrihan et al., 2008). Moreover, even in the isolated rhythmic brainstem slice preparation, respiratory motor output appears normal before P14 (Viemari et al., 2005). Further studies are required, however, to determine whether or not GABAergic signaling deficits in Mecp2 null neonates affect respiratory adaptation to behavioral challenges, despite normal resting ventilation in these animals.
Although changes in glutamatergic neurotransmission in brainstem circuits of Mecp2−/y mice have not yet been investigated in detail, pathophysiological changes in the NMDA receptor subunits NR2A and NR2B have been reported in forebrain structures (Asaka et al., 2006; Lee et al., 2008). At the level of the brainstem, exaggerated respiratory responses to glutamate microinjections into the Kölliker-Fuse nucleus in Mecp2−/y mice at P40 provide indirect evidence for altered glutamatergic neurotransmission at an age at which the RTT respiratory phenotype is fully expressed (Stettner et al., 2007).
In addition to neurons, brain glia may be another target at which loss of MeCP2 affects fast excitatory and/or inhibitory transmission. Glial cells normally play a major role in the elimination of glutamate in the extracellular mileu and thereby help to prevent excitotoxic neuronal damage and synaptopathy resulting from glutamate excess (Tilleux and Hermans, 2007; Schousboe et al., 2004; Danbolt, 2001). Recent studies indicate that Mecp2 null astrocytes are abnormal in some respects, particularly regarding their ability to support neurite outgrowth from hippocampal neurons in culture (Ballas et al., 2009). Although defects in glial regulation of brain glutamate metabolism in Mecp2 null mice have not yet been described, these findings raise the possibility that astrocyte dysfunction could contribute to disruption of glutamatergic signaling in RTT.
4.2. Deficits in BDNF expression and synaptic modulation
Brain-Derived Neurotrophic Factor (BDNF) regulates neuronal survival and maturation during early development, and synaptic function and plasticity throughout life, and is required for normal breathing behavior (for review see Katz, 2005). The synaptic modulatory role of BDNF is related to the fact that BDNF is expressed and secreted by many neurons in an activity–dependent manner throughout life (Balkowiec and Katz, 2000, 2002). Indeed, BDNF null mice exhibit abnormal central respiratory output at birth (Balkowiec and Katz, 1998) and die shortly thereafter of apparent respiratory failure (Erickson et al., 1996). Recent findings indicate that the BDNF gene may be a transcriptional target of MeCP2 (Chen et al., 2001; Martinowich et al., 2003) and Mecp2−/y mice exhibit marked deficits in brain content of BDNF mRNA and protein after birth (Chang et al., 2006; Wang et al., 2006; Ogier et al., 2007). Some of the earliest and most dramatic deficits occur in the nodose ganglion (NG) and the nucleus tractus solitarii (nTS) of the dorsal brainstem, structures that play pivotal roles in reflex control of cardiorespiratory output. Specifically, Mecp2tm1.1Jae−/y mice exhibit 40–50% WT levels of BDNF in the NG and brainstem by 5 weeks after birth (Wang et al., 2006). These declines are not due to cell death, because BDNF deficits in Mecp2−/y mice occur after neurons are no longer dependent on BDNF for survival (Wang et al., 2006). Rather, BDNF-expressing neurons have less BDNF available for release (Wang et al., 2006), adversely affecting BDNF-dependent synaptic signaling. Normally, BDNF regulates glutamatergic synaptic function in newborn and adult animals at multiple sites within the respiratory network, including the nTS (Balkowiec et al., 2000), preBötzinger complex (Thoby-Brisson et al., 2003, Bouvier et al., 2008), Kolliker-Fuse nucleus (Kron et al., 2007a, 2007b) and spinal phrenic motoneurons (Baker-Herman et al., 2004). For example, previous studies demonstrated that BDNF can modulate activity of second order neurons in nTS by inhibiting postsynaptic glutamatergic AMPA receptors (Balkowiec et al., 2000). We hypothesize, therefore, that in Mecp2 null mice, one consequence of decreased BDNF expression by NG sensory neurons is a deficit in modulation of fast glutamatergic transmission at primary afferent synapses in nTS. This hypothesis is supported by our recent finding that primary afferent stimulation elicits significantly larger excitatory postsynaptic responses in nTS relay neurons in Mecp2 null mice compared to WT controls (Kline et al., 2008). This functional synaptopathy may in turn explain the finding of Stettner et al. (2007) that Mecp2 null mice exhibit a loss of habituation in the Breuer-Hering reflex, an nTS-mediated response that plays an essential role in regulating the post-inspiratory phase of the respiratory cycle. Similarly, Mecp2−/y mice exhibit exaggerated ventilatory responses to hypoxia at P30 (Voituron et al., 2009), consistent with a deficit in modulation of afferent inputs to nTS.
4.3. Disturbed monoaminergic modulation
There is compelling evidence that monoaminergic neuromodulatory systems show pathophysiological changes in RTT patients and mouse models. In their initial description of RTT, A. Rett and colleagues reported abnormally low values of noradrenaline (NA) and serotonin (5-HT) in the brain of single RTT patients and suggested that a defect in central monoaminergic systems could be an underlying cause of the disease (Riederer et al., 1985, 1986; Brücke et al., 1987). The characteristic symptoms and the polysomnography records of RTT patients are indeed consistent with central defects in NA and 5HT (Nomura et al., 1985). Further, postmortem examination confirmed a 50% reduction of NA and 5HT contents in the substantia nigra in 2/4 patients with typical features of RTT syndrome (Lekman et al., 1989). Zoghbi et al. (1985) reported a significant decrease in NA but not 5HT metabolites in the CSF of six RTT patients. However, other studies of NA and 5HT metabolites in the CSF or urine from larger samples of RTT patients did not confirm significant differences from controls (Lekman et al., 1990; Temudo et al., 2009). Recently, analysis of 5HT binding in postmortem brain samples from eleven girls with RTT revealed an altered 5HT innervation and/or uptake in the dorsal motor nucleus of the vagus, suggesting abnormal 5HT modulation of this major autonomic nucleus (Paterson et al., 2005).
The neurons of the NA and 5-HT arousal systems have been closely studied for their relationship to behavioral state. Neurons in the locus coeruleus and the raphé nuclei, for example, have relatively characteristic, state dependent firing rates; both groups fire fastest during wakefulness and slow down or even stop during NREM and REM sleep, respectively (for an excellent review see Saper et al., 2001). The NA and 5HT neurons are not only involved in the control of the sleep-wake cycle but also have a major role in the neural control of breathing (Hilaire et al., 2004; Richter et al., 2003). NA and 5HT first appear at embryonic stages and are important for perinatal maturation of the respiratory network and its postnatal function (Hilaire, 2006; Bissonnette and Hilaire, 2008; Gargaglioni et al., 2008; see also Viemari and Tryba, 2009, this issue). Genetic alterations of the NA system alter maturation of respiratory control, with severe consequences for postnatal survival. In neonatal rodents, NA released from the brainstem A6, A5, A2 and A1 groups exerts a complex modulation on the activity of the medullary respiratory network. NE neurons from the pontine A5 and A6 groups are synaptically connected to the medullary respiratory network and either reduce (A5) or facilitate (A6) its rhythmic activity (Hilaire et al., 2004), whereas the medullary A1/C1 and A2/C2 neurons either facilitate (A1/C1) or stabilize (A2/C2) the respiratory rhythm (Hilaire, 2006; Zanella et al., 2006). Pharmacological and genetic alterations of the 5HT system also disrupt the formation of central networks (somatosensory, visual and locomotor networks: Cases et al., 1996; Upton et al., 1999; Cazalets et al., 2000, respectively), including the brainstem-cervical respiratory network (Bou-Flores et al., 2000, Burnet et al., 2001; Bras et al, 2008).
In Mecp2 null mice, biogenic amine systems exhibit abnormal postnatal developmental profiles. Ide et al. (2005) demonstrated that whole brain levels of NA and 5HT in Mecp2 null mice are normal at birth and then decline from P14 onwards. Viemari et al (2005) compared the NA and 5HT contents in the forebrain, pons and medulla of WT and Mecp2 null mice and found a significant postnatal decline in NA by P30 that was specific to the medulla. Thereafter, the NA deficit increased, and 5HT content in the medulla was significantly reduced by P60. Interestingly, the reduction of NA contents in the medulla preceded the appearance of overt breathing deficits. Comparison of the number of NA and 5HT neurons in the medulla of WT and Mecp2 null mice revealed an initial reduction of NA neurons in the A2/C2 group of mutants by P30; by P60, the reduction was even more severe and also affected NA neurons in the A1/C1 group without any effect on 5HT cell counts (Viemari et al., 2005). In contrast, comparison of mRNAs encoding 5HT metabolic enzymes, 5-HT receptor subtypes and the 5-HT transporter in the preBötzinger complex of WT and Mecp2 null mice has revealed significant decreases in mutant animals by P40 (Manzke et al., 2008). In addition, it has been recently shown that MeCP2 deficiency in mice results in dysregulation of the gene encoding L-dopa decarboxylase, an enzyme required for monoamine biosynthesis (Urdinguio et al., 2008).
It will be important to understand the potential impact of monoaminergic deficits on progressive respiratory dysfunction in Mecp2 null mice and RTT patients in relationship to the role of these systems in regulation of the sleep-wake cycle. However, the current body of data may not suffice to fully explain how respiratory dysfunction in RTT relates to behavioral arousal. The erratic breathing in RTT occurs predominantly during wakefulness when both NA and 5-HT neurons exhibit their highest firing rates (Saper et al. 2001). Hence, if an aminergic deficit contributes to breathing irregularities, one would expect that breathing stabilizes during the day when monoaminergic drive is enhanced and conversely becomes irregular during the night. However, the opposite is the case. Such considerations might apply to controls with a normal physiological state, but not to RTT patients in whom cardiorespiratory physiology and various aspects of neuromodulation are perturbed. Abnormalities could include, for example, changes in the responsiveness to neuromodulators, in modulatory release mechanisms or in the balance between inhibitory and excitatory aminergic drive. The state-dependency of the breathing abnormalities needs to be considered also in a developmental context. In all mammals a progressive increase in waking time with decreased REM sleep takes place during postnatal maturation (for review see Garcia-Rill et al., 2008). Therefore, the contribution of NA and 5HT systems to the modulation of breathing may be rather minimal at the neonatal stage. As these systems become more active with age and increased waking time, the impact of NA and 5HT deficits on respiratory behavior may become more pronounced. Hence, the appearance of breathing abnormalities later in postnatal development in Mecp2 null mice would be consistent with an increased dependency on monoaminergic drive.
5. EXPERIMENTAL STRATEGIES TO TREAT BREATHING DISORDERS IN Mecp2 NULL MICE
Recent studies in conditional Mecp2 null mice have demonstrated that reactivation of the Mecp2 gene, even in severely symptomatic animals, can rescue neurologic function to a remarkable degree (Guy et al., 2007; see also Giacometti et al., 2007). These findings indicate that deficits caused by loss of MeCP2 function reflect defects in neuronal signaling, rather than neurodegeneration, and, therefore, are potentially reversible. In addition, genetic overexpression of the BDNF gene in Mecp2tm1-1Jae null mice improves somatomotor function and prolongs lifespan (Chang et al., 2006). However, given current technical barriers to effective gene therapy, several laboratories are investigating the possibility of rescuing neurologic function in Mecp2 null mice and, eventually, RTT patients, by pharmacologic means.
A recent study by Ogier et al. (2007) explored the possibility that pharmacologic elevation of endogenous BDNF expression with ampakine drugs could improve respiratory function in Mecp2tm1-1Jae null mice. Ampakines are benzamide derivatives that acutely facilitate the activity of glutamatergic AMPA receptors (Lynch, 2006). In addition, repeated administration of ampakines in rats and mice increases expression of BDNF mRNA and protein in the forebrain for several days (Lauterborn et al., 2003; Rex et al., 2006) and augments BDNF dependent synaptic plasticity (Ingvar et al., 1997; Porrino et al., 2005; Rex et al., 2006; Wezenberg et al., 2007). Indeed, treatment of Mecp2tm1-1Jae−/y mice with the ampakine CX546 for 3 days restores normal respiratory frequency and ventilation (Ogier et al., 2007). These effects on ventilation were accompanied by a significant increase in expression of BDNF protein in vagal afferent neurons in the nodose ganglion. Although further studies are required to elucidate the mechanism of ampakine action in this model, these data are consistent with the hypothesis that decreased BDNF levels contribute to the respiratory phenotype of Mecp2 null mice and that BDNF may be a pharmacological target for improving respiratory function in RTT. It is not yet known whether ampakine treatment improves other aspects of the RTT phenotype.
Other pharmacological approaches are targeting the NA deficiency in Mecp2 null mice. Treatment with desipramine, a clinically approved NA re-uptake inhibitor, yields some improvement in breathing and prolongs the life span of Mecp2 null mice up to P90 (Roux et al., 2007; Zanella et al., 2008). This is consistent with MeCP2 deficiency dysregulating the gene encoding L-dopa decarboxylase, one of the enzymes in the NE biosynthetic pathway (Urdinguio et al., 2008). However, although desipramine treatment delayed the onset of fatal breathing disorders in a subset of Mecp2−/y mice, ultimately, it did not rescue the mouse RTT phenotype.
In RTT girls, pharmacological treatments with bioamine precursors that significantly increased catecholamine metabolites in CSF did not result in clinical improvement (Nielsen et al., 1990). On the other hand, pharmacological treatment of one RTT girl with the 5HT1A receptor agonist buspirone improved her breathing and oxygen saturation (Andaku et al., 2005) and one of three RTT patients treated with the 5HT reuptake blocker fluoxetine showed clinical improvement (Temudo et al., 2009). However, in the absence of large scale, well controlled trials of NA or 5HT active drugs in RTT patients, the potential for such interventions to improve respiratory function in this population remains speculative at this point.
As illustrated in the present review, at least five different neurochemical signaling systems -- glutamate, GABA, BDNF, NA and 5HT – exhibit abnormal expression and/or function in mouse models of RTT and most appear to contribute to breathing abnormalities in these animals. The fact that these are parallel interacting systems suggests that poly-pharmaceutical approaches will be required to effectively treat breathing disorders in RTT. Such approaches may also be synergistic. For example, treatment with a dual NA/5HT re-uptake inhibitor has been shown to significantly elevate BDNF levels in rodent cortex (Mannari et al., 2008). Conversely, elevation of BDNF levels, which are important for development of NA neurons (Akbarian et al., 2002; Copray et al., 1999; Guo et al., 2005) and NA turnover (Jönsson et al., 2008), may help to address depressed monoamine expression in the absence of MeCP2.
6. CONCLUSIONS
Our current understanding of the respiratory phenotype in RTT leads us to the following conclusions:
The RTT breathing phenotype is extremely complex (hyperventilation, breath holds, forced and deep breathing and apneustic breathing) and includes disturbances in cardiorespiratory coupling. This complexity suggests that cardiorespiratory dysfunction in RTT is unlikely to result from a single underlying mechanism.
The RTT breathing phenotype is abnormal during sleep and wakefulness; however, the abnormalities are exaggerated during wakefulness and can be worsened by behavioral arousal. Thus the RTT breathing phenotype may involve two distinct types of disturbances: (a) the strong state-dependence suggests defects in modulation of the respiratory rhythm and motor pattern that may, in addition, be superimposed on (b) more subtle alterations in rhythm and pattern generation.
The RTT breathing phenotype develops relatively late during postnatal development, implying either that processes involved in stabilization of mature respiratory behavior are affected or alternatively that the breathing phenotype is the consequence of a cascade of changes at different levels that result in a cascade of compensatory mechanisms and instabilities as the RTT patient matures. The repeated breath holds could for example lead to a cascade of biochemical changes that would in turn exaggerate the breathing phenotype.
6.1. A Neurochemical Hypothesis
Loss of MeCP2 alters multiple neurochemical signaling mechanisms, and in some cases, these alterations arise from a failure of neurons to maintain their differentiated phenotypes; e.g., BDNF and TH expressions are normal at birth but subsequently decline. The neurochemical systems affected by loss of MeCP2 are important both for acute synaptic signaling and, in some cases, activity-dependent maturation of neuronal circuits (e.g., BDNF). We hypothesize, therefore, that the Mecp2 null respiratory phenotype is the net result of 1) acute synaptopathies (neurons don’t release or respond to their normal complement of transmitters/modulators) and 2) perturbed circuit development and/or loss of plasticity. For example, the loss of BDNF-dependent modulation of glutamatergic transmission may result in an inability of the network to undergo long-term modifications in synaptic strength that underlie emergence of a stable respiratory pattern after birth.
Respiratory network dysfunction in RTT may result in particular from progressive synaptopathies in pathways associated with the processing of, and adaptive responses to, afferent information as well as adaptive responses to modulatory dysfunction. This scenario would reflect the fact that the behavioral repertoire of mammals is naturally increasing in complexity with age. Mecp2 null mice exhibit progressive neurochemical deficits in BDNF, NE and 5-HT systems that play key roles in behavioral adaptation of respiratory motor output. Specific tasks such as exercise, food foraging, vocalization, etc., are associated with multi-modal and multi-synaptic integration of afferent inputs that are required for adaptive responses of the respiratory system to changing behavioral demands. Thus, the effects of synaptic dysfunction coupled with dysregulated modulatory inputs to the respiratory network would be expected to increase progressively as these systems play increasingly important roles in shaping mature respiratory behavior. Therefore, the complexity, developmental progression and state-dependence of respiratory dysfunction in RTT might be explained by defects in neurochemical signaling mechanisms important for acute and adaptive modulation of respiratory motor output.
7. SUMMARY
The complexity of the RTT breathing phenotype may well be related to the diversity of neurochemical changes caused by loss of MeCP2 function, as all of the affected neurotransmitter and neuromodulatory systems described thus far are important for expression, modulation and/or adaptation of respiratory motor output. Further advances in understanding cardiorespiratory pathophysiology in RTT will benefit from integrative experimental strategies that are able to link specific neurochemical deficits at the molecular and cellular level to specific features of respiratory network dysfunction. Such an approach will also facilitate further development of therapeutic modalities for the treatment of breathing disorders in RTT.
Acknowledgments
The authors gratefully acknowledge funding support from the following organizations: DMK (United States National Institutes of Health/National Institute of Neurological Diseases and Stroke, Rett Syndrome Research Foundation and the International Rett Syndrome Foundation (IRSF)); MD (IRSRF and the Deutsche Forschungsgemeinschaft (DFG) Research Center for Molecular Physiology of the Brain (CMPB Göttingen/Germany)); JMR (United States National Institutes of Health (NIH)/National Heart, Lung, Blood Institute (NHLBI)); GH (Centre National de la Recherche Scientifique (CNRS), the French Ministry for Research (ACI NIC No. 054) and IRSF fellowships to Jean-Christophe Roux, Sebastien Zanella and Nicolas Voituron).
ABBREVIATIONS
The following abbreviations are used for the gene encoding methyl-CpG-binding protein 2 and its respective protein product:
- MECP2
human gene
- Mecp2
mouse gene
- MeCP2
protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbarian S, Rios M, Liu RJ, Gold SJ, Fong HF, Zeiler S, Coppola V, Tessarollo L, Jones KR, Nestler EJ, Aghajanian GK, Jaenisch R. Brain-derived neurotrophic factor is essential for opiate-induced plasticity of noradrenergic neurons. J Neurosci. 2002;22:4153–4162. doi: 10.1523/JNEUROSCI.22-10-04153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Andaku DK, Mercadante MT, Schwartzman JS. Buspirone in Rett syndrome respiratory dysfunction. Brain Dev. 2005;27:437–438. doi: 10.1016/j.braindev.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis. 2006;21:217–227. doi: 10.1016/j.nbd.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Cellular mechanisms of activity-dependent release of native BDNF from hippocampal neurons. J Neurosci. 2002;22:10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Kunze D, Katz DM. Brain-derived neurotrophic factor acutely inhibits AMPA currents in developing sensory relay neurons. J Neurosci. 2000;20:1094–1911. doi: 10.1523/JNEUROSCI.20-05-01904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Brain-derived neurotrophic factor is required for normal development of the central respiratory rhythm in mice. J Physiol. 1998;510:527–533. doi: 10.1111/j.1469-7793.1998.527bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette J, Hilaire G. Possible role of bioaminergic systems in the respiratory disorders of Rett syndrome. In: Gaultier C, editor. Genetic Basis for Respiratory Control Disorders. Springer Publishers; 2008. pp. 271–289. [Google Scholar]
- Bissonnette JM, Knopp SJ. Separate respiratory phenotypes in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Pediatr Res. 2006;59:513–518. doi: 10.1203/01.pdr.0000203157.31924.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette JM, Knopp SJ, Maylie J, Thong T. Autonomic cardiovascular control in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Auton Neurosci. 2007;136:82–89. doi: 10.1016/j.autneu.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette JM, Knopp SJ. Effect of inspired oxygen on periodic breathing in methy-CpG-binding protein 2 (Mecp2) deficient mice. J Appl Physiol. 2008;104:198–204. doi: 10.1152/japplphysiol.00843.2007. [DOI] [PubMed] [Google Scholar]
- Blue ME, Naidu S, Johnston MV. Altered development of glutamate and GABA receptors in the basal ganglia of girls with Rett syndrome. Exp Neurol. 1999;156:345–352. doi: 10.1006/exnr.1999.7030. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Lajard AM, Monteau R, De Maeyer E, Seif I, Lanoir J, Hilaire G. Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A-deficient transgenic mice: possible role of a serotonin excess. J Neurosci. 2000;20:4646–4656. doi: 10.1523/JNEUROSCI.20-12-04646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J, Autran S, Dehorter N, Katz DM, Champagnat J, Fortin G, Thoby-Brisson M. Brain-derived neurotrophic factor enhances fetal respiratory rhythm frequency in the mouse preBötzinger complex in vitro. Eur J Neurosci. 2008;28:510–20. doi: 10.1111/j.1460-9568.2008.06345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bras H, Gaytán SP, Portalier P, Zanella S, Pásaro R, Coulon P, Hilaire G. Prenatal activation of 5-HT2A receptor induces expression of 5-HT1B receptor in phrenic motoneurons and alters the organization of their premotor network in newborn mice. Eur J Neurosci. 2008;28:1097–1107. doi: 10.1111/j.1460-9568.2008.06407.x. [DOI] [PubMed] [Google Scholar]
- Brücke T, Sofic E, Killian W, Rett A, Riederer P. Reduced concentrations and increased metabolism of biogenic amines in a single case of Rett-syndrome: a postmortem brain study. J Neural Transm. 1987;68:315–324. doi: 10.1007/BF02098506. [DOI] [PubMed] [Google Scholar]
- Burnet H, Bévengut M, Chakri F, Bou-Florès C, Coulon P, Gaytan S, Pásaro R, Hilaire G. Altered respiratory activity and respiratory regulations in adult monoamine oxidase A-deficient mice. J Neurosci. 2001;21:5212–5221. doi: 10.1523/JNEUROSCI.21-14-05212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, Gaspar P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Gardette M, Hilaire G. Locomotor network maturation is transiently delayed in the MAOA-deficient mouse. J Neurophysiol. 2000;83:2468–2470. doi: 10.1152/jn.2000.83.4.2468. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, David Sweatt J, Zoghbi HY. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet. 2004;13:2679–2689. doi: 10.1093/hmg/ddh282. [DOI] [PubMed] [Google Scholar]
- Copray JC, Bastiaansen M, Gibbons H, van Roon WM, Corner AM, Lipski J. Neurotrophic requirements of rat embryonic catecholaminergic neurons from the rostral ventrolateral medulla. Brain Res Dev Brain Res. 1999;116:217–222. doi: 10.1016/s0165-3806(99)00085-1. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Mörschel M, Reuter J, Zhang W, Gestreau C, Stettner GM, Kron M. Postnatal emergence of synaptic plasticity associated with dynamic adaptation of the respiratory motor pattern. Respir Physiol Neurobiol. 2008;164:72–79. doi: 10.1016/j.resp.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Elian M, Rudolf ND. EEG and respiration in Rett syndrome. Acta Neurol Scand. 1991;83:123–128. doi: 10.1111/j.1600-0404.1991.tb04660.x. [DOI] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J Neurosci. 1996;16:5361–5371. doi: 10.1523/JNEUROSCI.16-17-05361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rill E, Charlesworth A, Heister D, Ye M, Hayar A. The developmental decrease in REM sleep: the role of transmitters and electrical coupling. Sleep. 2008;31:673–690. doi: 10.1093/sleep/31.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargaglioni LH, Bícegoa KC, Branco LG. Brain monoaminergic neurons and ventilatory control in vertebrates. Respir Physiol Neurobiol. 2008;164:112–122. doi: 10.1016/j.resp.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Dutschmann M, Obled S, Bianchi AL. Activation of XII motoneurons and premotor neurons during various oropharyngeal behaviors. Respir Physiol Neurobiol. 2005;147:159–176. doi: 10.1016/j.resp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Giacometti E, Luikenhuis S, Beard C, Jaenisch R. Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc Natl Acad Sci U S A. 2007;104:1931–1936. doi: 10.1073/pnas.0610593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guideri F, Acampa M, Blardi P, de Lalla A, Zappella M, Hayek Y. Cardiac dysautonomia and serotonin plasma levels in Rett syndrome. Neuropediatrics. 2004;35:36–38. doi: 10.1055/s-2004-815789. [DOI] [PubMed] [Google Scholar]
- Guideri F, Acampa M, DiPerri T, Zappella M, Hayek Y. Progressive cardiac dysautonomia observed in patients affected by classic Rett syndrome and not in the preserved speech variant. J Child Neurol. 2001;16:370–373. doi: 10.1177/088307380101600512. [DOI] [PubMed] [Google Scholar]
- Guideri F, Acampa M, Hayek G, Zappella M, Di Perri T. Reduced heart rate variability in patients affected with Rett syndrome. A possible explanation for sudden death. Neuropediatrics. 1999;30:146–148. doi: 10.1055/s-2007-973480. [DOI] [PubMed] [Google Scholar]
- Guo H, Hellard DT, Huang L, Katz DM. Development of pontine noradrenergic A5 neurons requires brain-derived neurotrophic factor. Eur J Neurosci. 2005;21:2019–2023. doi: 10.1111/j.1460-9568.2005.04016.x. [DOI] [PubMed] [Google Scholar]
- Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- Hilaire G. Endogenous noradrenaline affects the maturation and function of the respiratory network: Possible implication for SIDS. Auton Neurosci. 2006;127:320–331. doi: 10.1016/j.autneu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Viemari JC, Coulon P, Simonneau M, Bévengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respir Physiol Neurobiol. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Ide S, Itoh M, Goto Y. Defect in normal developmental increase of the brain biogenic amine concentrations in the Mecp2-null mouse. Neurosci Lett. 2005;386:14–17. doi: 10.1016/j.neulet.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, Schehr RS, Lynch G. Enhancement by an ampakine of memory encoding in humans. Exp Neurol. 1997;146:553–559. doi: 10.1006/exnr.1997.6581. [DOI] [PubMed] [Google Scholar]
- Jönsson EG, Saetre P, Edman-Ahlbom B, Sillén A, Gunnar A, Andreou D, Agartz I, Sedvall G, Hall H, Terenius L. Brain-derived neurotrophic factor gene variation influences cerebrospinal fluid 3-methoxy-4-hydroxyphenylglycol concentrations in healthy volunteers. J Neural Transm. 2008;115:1695–1699. doi: 10.1007/s00702-008-0113-9. [DOI] [PubMed] [Google Scholar]
- Julu PO, Kerr AM, Hansen S, Apartopoulos F, Jamal GA. Functional evidence of brain stem immaturity in Rett syndrome. Eur Child Adolesc Psychiatry. 1997;6 Suppl 1:47–54. [PubMed] [Google Scholar]
- Julu PO, Kerr AM, Apartopoulos F, Al-Rawas S, Engerström IW, Engerström L, Jamal GA, Hansen S. Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Arch Dis Child. 2001;85:29–37. doi: 10.1136/adc.85.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julu PO, Witt Engerström I. Assessment of the maturity-related brainstem functions reveals the heterogeneous phenotypes and facilitates clinical management of Rett syndrome. Brain Dev. 2005;27:S43–S53. doi: 10.1016/j.braindev.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Katz DM. Neurotrophic and transcriptional regulation of respiratory neuron development. Respiratory Physiology and Neurobiology. 2005;149(1–3):99–109. doi: 10.1016/j.resp.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Kerr AM, Armstrong DD, Prescott RJ, Doyle D, Kearney DL. Rett syndrome: analysis of deaths in the British survey. Eur Child Adolesc Psychiatry. 1997;6 Suppl 1:71–4. [PubMed] [Google Scholar]
- Kline DD, Hasser EM, Clark CG, Kunze DL, 2, Katz DM. Mecp2 null mice exhibit enhanced excitatory postsynaptic currents (EPSCs) in the brainstem nucleus tractus solitarius (NTS) Soc for Neuroscience Abstracts. 2008:382.1. [Google Scholar]
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Kron M, Morschel M, Reuter J, Zhang W, Dutschmann M. Developmental changes in brain-derived neurotrophic factor-mediated modulations of synaptic activities in the pontine Kolliker-Fuse nucleus of the rat. J Physiol. 2007a;583:315–327. doi: 10.1113/jphysiol.2007.134726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron M, Reuter J, Gerhardt E, Manzke T, Zhang W, Dutschmann M. Emergence of BDNF induced postsynaptic potentiation of NMDA currents during the postnatal maturation of the Kölliker-Fuse nucleus of rat. J Physiol. 2008;586:2331–2343. doi: 10.1113/jphysiol.2007.148916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Truong GS, Baudry M, Bi X, Lynch G, Gall CM. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Pharmacol Exp Ther. 2003;307:297–305. doi: 10.1124/jpet.103.053694. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim W, Ham BJ, Chen W, Bear MF, Yoon BJ. Activity-dependent NR2B expression is mediated by MeCP2-dependent epigenetic regulation. Biochem Biophys Res Commun. 2008;377:930–934. doi: 10.1016/j.bbrc.2008.10.082. [DOI] [PubMed] [Google Scholar]
- Lekman A, Witt-Engerström I, Gottfries J, Hagberg BA, Percy AK, Svennerholm L. Rett syndrome: biogenic amines and metabolites in postmortem brain. Pediatr Neurol. 1989;5:357–362. doi: 10.1016/0887-8994(89)90049-0. [DOI] [PubMed] [Google Scholar]
- Lekman A, Witt-Engerström I, Holmberg B, Percy A, Svennerholm L, Hagberg B. CSF and urine biogenic amine metabolites in Rett syndrome. Clin Genet. 1990;37:173–178. doi: 10.1111/j.1399-0004.1990.tb03499.x. [DOI] [PubMed] [Google Scholar]
- Lynch G. Glutamate-based therapeutic approaches: ampakines. Curr Opin Pharmacol. 2006;6:82–88. doi: 10.1016/j.coph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Mannari C, Origlia N, Scatena A, Del Debbio A, Catena M, Dell’agnello G, Barraco A, Giovannini L, Dell’osso L, Domenici L, Piccinni A. BDNF level in the rat prefrontal cortex increases following chronic but not acute treatment with duloxetine, a dual acting inhibitor of noradrenaline and serotonin re-uptake. Cell Mol Neurobiol. 2008;28:457–468. doi: 10.1007/s10571-007-9254-x. [DOI] [PubMed] [Google Scholar]
- Manzke T, Bidon O, Dutschmann M, Richter DW. Mecp2-deficiency causes developmental changes in the serotonergic system. Act Physiol. 2008;192:PT03–PT05. [Google Scholar]
- Marcus CL, Carroll JL, McColley SA, Loughlin GM, Curtis S, Pyzik P, Naidu S. Polysomnographic characteristics of patients with Rett syndrome. J Pediatr. 1994;125:218–224. doi: 10.1016/s0022-3476(94)70196-2. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- Medrihan L, Tantalaki E, Aramuni G, Sargsyan V, Dudanova I, Missler M, Zhang W. Early Defects of GABAergic Synapses in the Brain Stem of a MeCP2 Mouse Model of Rett Syndrome. J Neurophysiol. 2008;99:112–121. doi: 10.1152/jn.00826.2007. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Kavalali ET. Rett syndrome and the impact of MeCP2 associated transcriptional mechanisms on neurotransmission. Biol Psychiatry. 2009;65:204–210. doi: 10.1016/j.biopsych.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A. 2009;106:1199–1204. doi: 10.1073/pnas.0811018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB, Lou HC, Andresen J. Biochemical and clinical effects of tyrosine and tryptophan in the Rett syndrome. Brain Dev. 1990;12:143–147. doi: 10.1016/s0387-7604(12)80197-5. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Segawa M, Higurashi M. Rett syndrome--an early catecholamine and indolamine deficient disorder? Brain Dev. 1985;7:334–341. doi: 10.1016/s0387-7604(85)80040-1. [DOI] [PubMed] [Google Scholar]
- Ogier M, Katz DM. Breathing dysfunction in Rett Syndrome: Understanding epigenetic regulation of the respiratory network. Resp Physiol Neurobiol. 2008;164:55–63. doi: 10.1016/j.resp.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier M, Wang H, Hong E, Wang Q, Greenberg ME, Katz DM. Brain Derived Neurotrophic Factor and respiratory function improve after ampakine treatment in a mouse model of Rett Syndrome. JNeurosci. 2007;27:10912–10917. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DS, Thompson EG, Belliveau RA, Antalffy BA, Trachtenberg FL, Armstrong DD, Kinney HC. Serotonin transporter abnormality in the dorsal motor nucleus of the vagus in Rett syndrome: potential implications for clinical autonomic dysfunction. J Neuropathol Exp Neurol. 2005;64:1018–1027. doi: 10.1097/01.jnen.0000187054.59018.f2. [DOI] [PubMed] [Google Scholar]
- Pelka GJ, Watson CM, Radziewic T, Hayward M, Lahooti H, Christodoulou J, Tam PP. Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain. 2006;129:887–898. doi: 10.1093/brain/awl022. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Daunais JB, Rogers GA, Hampson RE, Deadwyler SA. Facilitation of task performance and removal of the effects of sleep deprivation by an ampakine (CX717) in nonhuman primates. PLoS Biol. 2005;3:e299. doi: 10.1371/journal.pbio.0030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin CY, Kramar EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med. 2003;9:542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Riederer P, Brücke T, Sofic E, Kienzl E, Schnecker K, Schay V, Kruzik P, Killian W, Rett A. Neurochemical aspects of the Rett syndrome. Brain Dev. 1985;7:351–360. doi: 10.1016/s0387-7604(85)80044-9. [DOI] [PubMed] [Google Scholar]
- Riederer P, Weiser M, Wichart I, Schmidt B, Killian W, Rett A. Preliminary brain autopsy findings in progredient Rett syndrome. Am J Med Genet Suppl. 1986;1:305–315. doi: 10.1002/ajmg.1320250530. [DOI] [PubMed] [Google Scholar]
- Roux JC, Dura E, Moncla A, Mancini J, Villard L. Treatment with desipramine improves breathing and survival in a mouse model for Rett syndrome. Eur J Neurosci. 2007;25:1915–1922. doi: 10.1111/j.1460-9568.2007.05466.x. [DOI] [PubMed] [Google Scholar]
- Samaco RC, Fryer JD, Ren J, Fyffe S, Chao HT, Sun Y, Greer JJ, Zoghbi HY, Neul JL. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum Mol Genet. 2008;17:1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sarup A, Bak LK, Waagepetersen HS, Larsson OM. Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochem Int. 2004;45:521–527. doi: 10.1016/j.neuint.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Sekul EA, Moak JP, Schultz RJ, Glaze DG, Dunn JK, Percy AK. Electrocardiographic findings in Rett syndrome: an explanation for sudden death? J Pediatr. 1994;125:80–82. doi: 10.1016/s0022-3476(94)70128-8. [DOI] [PubMed] [Google Scholar]
- Shahbazian M, Young J, Yuva-Paylor L, Spencer C, Antalffy B, Noebels J, Armstrong D, Paylor R, Zoghbi H. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Huppke P, Gärtner J, Richter DW, Dutschmann M. Disturbances of breathing in Rett syndrome: results from patients and animal models. Adv Exp Med Biol. 2008;605:503–507. doi: 10.1007/978-0-387-73693-8_88. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Huppke P, Brendel C, Richter DW, Gärtner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2−/y knockout mice. J Physiol. 2007;579:863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temudo T, Rios M, Prior C, Carrilho I, Santos M, Maciel P, Sequeiros J, Fonseca M, Monteiro J, Cabral P, Vieira JP, Ormazabal A, Artuch R. Evaluation of CSF neurotransmitters and folate in 25 patients with Rett disorder and effects of treatment. Brain Dev. 2009;31:46–51. doi: 10.1016/j.braindev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Cauli B, Champagnat J, Fortin G, Katz DM. Expression of functional tyrosine kinase B receptors by rhythmically active respiratory neurons in the pre-Bötzinger complex of neonatal mice. J Neurosci. 2003;23:7685–7689. doi: 10.1523/JNEUROSCI.23-20-07685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- Upton AL, Salichon N, Lebrand C, Ravary A, Blakely R, Seif I, Gaspar P. Excess of serotonin (5-HT) alters the segregation of ispilateral and contralateral retinal projections in monoamine oxidase A knock-out mice: possible role of 5-HT uptake in retinal ganglion cells during development. J Neurosci. 1999;19:7007–7024. doi: 10.1523/JNEUROSCI.19-16-07007.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdinguio RG, Lopez-Serra L, Lopez-Nieva P, Alaminos M, Diaz-Uriarte R, Fernandez AF, Esteller M. MeCP2-null mice provide new neuronal targets for Rett syndrome. PLoS ONE. 2008;3:e3669. doi: 10.1371/journal.pone.0003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari J-C, Tryba A. Bioaminergic neuromodulation of respiratory rhythm. Respir Physiol Neurobiol. 2009;x:yy–zz. doi: 10.1016/j.resp.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Roux JC, Tryba AK, Saywell V, Bumet H, Pena F, Zanella S, Bévengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola A, Saywell V, Villard L, Cozzone PJ, Lutz NW. Metabolic fingerprints of altered brain growth, osmoregulation and neurotransmission in a Rett syndrome model. PLoS ONE. 2007;2:e157. doi: 10.1371/journal.pone.0000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voituron N, Zanella S, Menuet C, Dutschmann M, Hilaire G. Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett Syndrome. Resp Physiol Neurobiol. 2009;x:yy–zz. doi: 10.1016/j.resp.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Vorsanova SG, Iourov IY, Yurov YB. Neurological, genetic and epigenetic features of Rett syndrome. J Pediatr Neurol. 2004;2:179–190. [Google Scholar]
- Wang H, Chan SA, Ogier M, Hellard D, Wang Q, Smith C, Katz DM. Dysregulation of brain-derived neurotrophic factor expression and neurosecretory function in Mecp2 null mice. J Neurosci. 2006;26:10911–10915. doi: 10.1523/JNEUROSCI.1810-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RE, Desesso JM, Hurtt ME, Cappon GD. Postnatal growth and morphological development of the brain: a species comparison. Birth Defects Res B Dev Reprod Toxicol. 2006;77:471–84. doi: 10.1002/bdrb.20090. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Silvestri JM, Ramirez JM. Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr Res. 2006;60:443–449. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Ramirez JM. Autonomic dysregulation in young girls with Rett Syndrome during nighttime in-home recordings. Pediatr Pulmonol. 2008;43:1045–1060. doi: 10.1002/ppul.20866. [DOI] [PubMed] [Google Scholar]
- Wezenberg E, Verkes RJ, Ruigt GS, Hulstijn W, Sabbe BG. Acute effects of the ampakine farampator on memory and information processing in healthy elderly volunteers. Neuropsychopharmacology. 2007;32:1272–1283. doi: 10.1038/sj.npp.1301257. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MT, Liu Q. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir Physiol Neurobiol. 2008;164:28–37. doi: 10.1016/j.resp.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir Physiol Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Zanella S, Mebarek S, Lajard AM, Picard N, Dutschmann M, Hilaire G. Oral treatment with desipramine improves breathing and life span in Rett syndrome mouse model. Respir Physiol Neurobiol. 2008;160:116–121. doi: 10.1016/j.resp.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Zanella S, Roux JC, Viemari JC, Hilaire G. Possible modulation of the mouse respiratory rhythm generator by A1/C1 neurones. Respir Physiol Neurobiol. 2006;153:126–138. doi: 10.1016/j.resp.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, Percy AK, Glaze DG, Butler IJ, Riccardi VM. Reduction of biogenic amine levels in the Rett syndrome. N Engl J Med. 1985;313:921–924. doi: 10.1056/NEJM198510103131504. [DOI] [PubMed] [Google Scholar]