Abstract
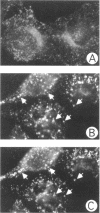
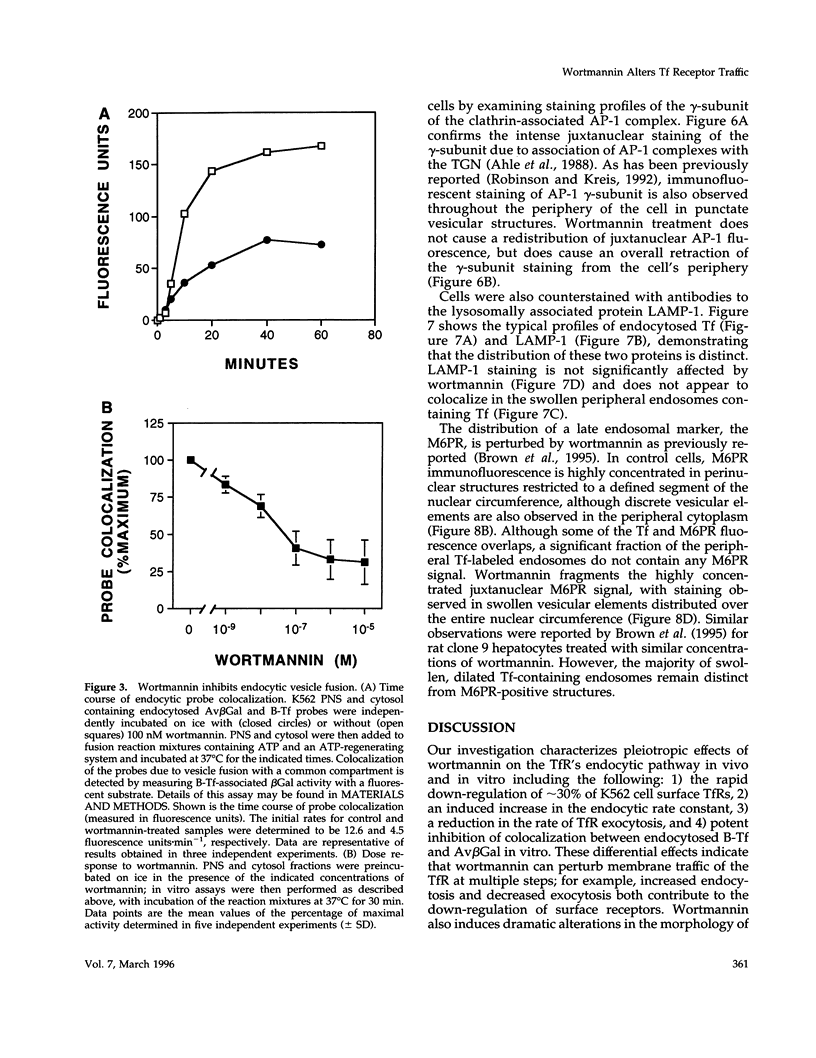
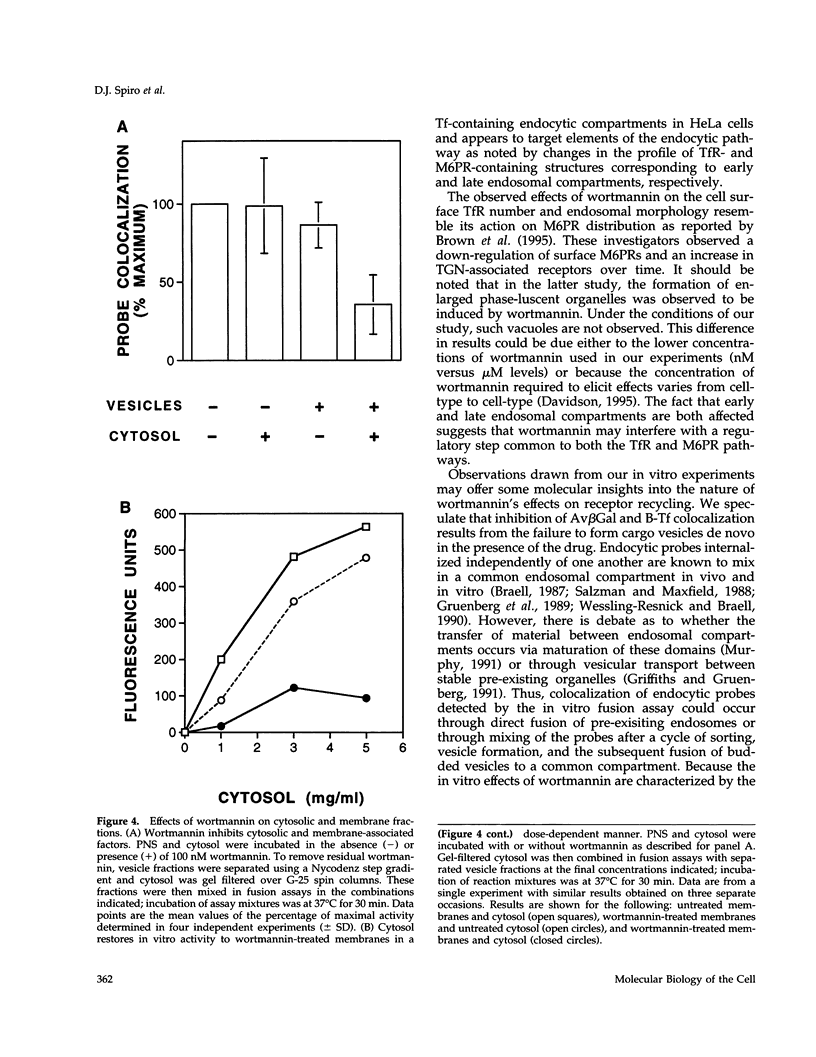
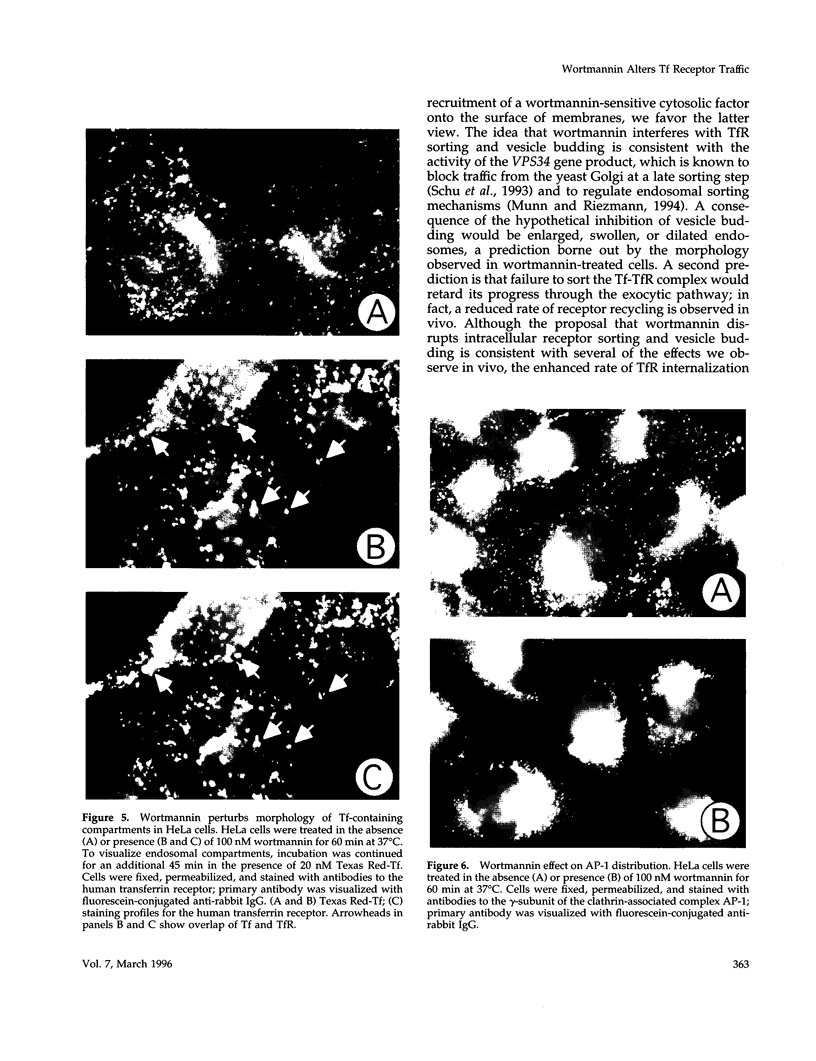
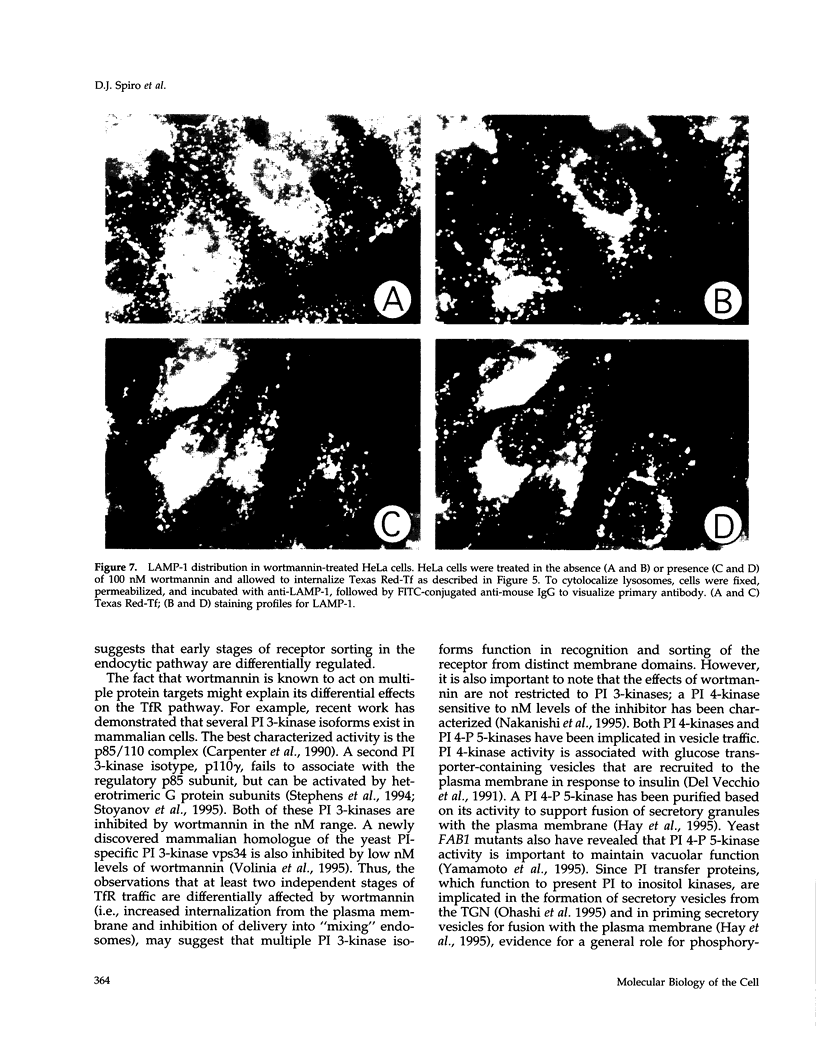
Treatment with the phosphatidylinositol 3-kinase inhibitor wortmannin promotes approximately 30% decrease in the steady-state number of cell-surface transferrin receptors. This effect is rapid and dose dependent, with maximal down-regulation elicited with 30 min of treatment and with an IC50 approximately 25 nM wortmannin. Wortmannin-treated cells display an increased endocytic rate constant for transferrin internalization and decreased exocytic rate constants for transferrin recycling. In addition to these effects in vivo, wortmannin is a potent inhibitor (IC50 approximately 15 nM) of a cell-free assay that detects the delivery of endocytosed probes into a common compartment. Inhibition of the in vitro assay involves the inactivation of a membrane-associated factor that can be recruited onto the surface of vesicles from the cytosol. Its effects on the cell-free assay suggest that wortmannin inhibits receptor sorting and/or vesicle budding required for delivery of endocytosed material to "mixing" endosomes. This idea is consistent with morphological changes induced by wortmannin, which include the formation of enlarged transferrin-containing structures and the disruption of the perinuclear endosomal compartment. However, the differential effects of wortmannin, specifically increased transferrin receptor internalization and inhibition of receptor recycling, implicate a role for phosphatidylinositol 3-kinase activity in multiple sorting events in the transferrin receptor's membrane traffic pathway.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahle S., Mann A., Eichelsbacher U., Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988 Apr;7(4):919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcaro A., Wymann M. P. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993 Dec 1;296(Pt 2):297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell W. A. Fusion between endocytic vesicles in a cell-free system. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1137–1141. doi: 10.1073/pnas.84.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. J., DeWald D. B., Emr S. D., Plutner H., Balch W. E. Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J Cell Biol. 1995 Aug;130(4):781–796. doi: 10.1083/jcb.130.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. L., Duckworth B. C., Auger K. R., Cohen B., Schaffhausen B. S., Cantley L. C. Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem. 1990 Nov 15;265(32):19704–19711. [PubMed] [Google Scholar]
- Clague M. J., Thorpe C., Jones A. T. Phosphatidylinositol 3-kinase regulation of fluid phase endocytosis. FEBS Lett. 1995 Jul 3;367(3):272–274. doi: 10.1016/0014-5793(95)00576-u. [DOI] [PubMed] [Google Scholar]
- Clarke J. F., Young P. W., Yonezawa K., Kasuga M., Holman G. D. Inhibition of the translocation of GLUT1 and GLUT4 in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor, wortmannin. Biochem J. 1994 Jun 15;300(Pt 3):631–635. doi: 10.1042/bj3000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M. J., Stewart A., Hodgkin M. N., Kerr D. J., Wakelam M. J. Wortmannin and its structural analogue demethoxyviridin inhibit stimulated phospholipase A2 activity in Swiss 3T3 cells. Wortmannin is not a specific inhibitor of phosphatidylinositol 3-kinase. J Biol Chem. 1995 Oct 27;270(43):25352–25355. doi: 10.1074/jbc.270.43.25352. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson H. W. Wortmannin causes mistargeting of procathepsin D. evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J Cell Biol. 1995 Aug;130(4):797–805. doi: 10.1083/jcb.130.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Vecchio R. L., Pilch P. F. Phosphatidylinositol 4-kinase is a component of glucose transporter (GLUT 4)-containing vesicles. J Biol Chem. 1991 Jul 15;266(20):13278–13283. [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Peppard J., von Figura K., Hasilik A., Schwartz A. L. Intracellular receptor sorting during endocytosis: comparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984 May;37(1):195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Gruenberg J. The arguments for pre-existing early and late endosomes. Trends Cell Biol. 1991 Jul;1(1):5–9. doi: 10.1016/0962-8924(91)90047-d. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Griffiths G., Howell K. E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989 Apr;108(4):1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. H., Olsson A., Casanova J. E. Wortmannin, an inhibitor of phosphoinositide 3-kinase, inhibits transcytosis in polarized epithelial cells. J Biol Chem. 1995 Nov 24;270(47):28425–28432. doi: 10.1074/jbc.270.47.28425. [DOI] [PubMed] [Google Scholar]
- Hay J. C., Fisette P. L., Jenkins G. H., Fukami K., Takenawa T., Anderson R. A., Martin T. F. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995 Mar 9;374(6518):173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- Herman P. K., Emr S. D. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Dec;10(12):6742–6754. doi: 10.1128/mcb.10.12.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- Joly M., Kazlauskas A., Corvera S. Phosphatidylinositol 3-kinase activity is required at a postendocytic step in platelet-derived growth factor receptor trafficking. J Biol Chem. 1995 Jun 2;270(22):13225–13230. doi: 10.1074/jbc.270.22.13225. [DOI] [PubMed] [Google Scholar]
- Joly M., Kazlauskas A., Fay F. S., Corvera S. Disruption of PDGF receptor trafficking by mutation of its PI-3 kinase binding sites. Science. 1994 Feb 4;263(5147):684–687. doi: 10.1126/science.8303278. [DOI] [PubMed] [Google Scholar]
- Jones A. T., Clague M. J. Phosphatidylinositol 3-kinase activity is required for early endosome fusion. Biochem J. 1995 Oct 1;311(Pt 1):31–34. doi: 10.1042/bj3110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Van Renswoude J., Ashwell G., Kempf C., Schechter A. N., Dean A., Bridges K. R. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983 Apr 25;258(8):4715–4724. [PubMed] [Google Scholar]
- Li G., D'Souza-Schorey C., Barbieri M. A., Roberts R. L., Klippel A., Williams L. T., Stahl P. D. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga L. S., Colombo M. I., Lennartz M., Brown E. J., Rahman K. H., Weiss R., Lennon P. J., Stahl P. D. Inhibition of endosome fusion by phospholipase A2 (PLA2) inhibitors points to a role for PLA2 in endocytosis. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10255–10259. doi: 10.1073/pnas.90.21.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn A. L., Riezman H. Endocytosis is required for the growth of vacuolar H(+)-ATPase-defective yeast: identification of six new END genes. J Cell Biol. 1994 Oct;127(2):373–386. doi: 10.1083/jcb.127.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. F. Maturation models for endosome and lysosome biogenesis. Trends Cell Biol. 1991 Oct;1(4):77–82. doi: 10.1016/0962-8924(91)90022-2. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Catt K. J., Balla T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi M., Jan de Vries K., Frank R., Snoek G., Bankaitis V., Wirtz K., Huttner W. B. A role for phosphatidylinositol transfer protein in secretory vesicle formation. Nature. 1995 Oct 12;377(6549):544–547. doi: 10.1038/377544a0. [DOI] [PubMed] [Google Scholar]
- Okada T., Kawano Y., Sakakibara T., Hazeki O., Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994 Feb 4;269(5):3568–3573. [PubMed] [Google Scholar]
- Powis G., Bonjouklian R., Berggren M. M., Gallegos A., Abraham R., Ashendel C., Zalkow L., Matter W. F., Dodge J., Grindey G. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994 May 1;54(9):2419–2423. [PubMed] [Google Scholar]
- Robinson M. S., Kreis T. E. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell. 1992 Apr 3;69(1):129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Salzman N. H., Maxfield F. R. Fusion accessibility of endocytic compartments along the recycling and lysosomal endocytic pathways in intact cells. J Cell Biol. 1989 Nov;109(5):2097–2104. doi: 10.1083/jcb.109.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhorn J. E., Wessling-Resnick M. Brefeldin A down-regulates the transferrin receptor in K562 cells. Mol Cell Biochem. 1994 Jun 29;135(2):159–169. doi: 10.1007/BF00926519. [DOI] [PubMed] [Google Scholar]
- Schu P. V., Takegawa K., Fry M. J., Stack J. H., Waterfield M. D., Emr S. D. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993 Apr 2;260(5104):88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Shepherd P. R., Soos M. A., Siddle K. Inhibitors of phosphoinositide 3-kinase block exocytosis but not endocytosis of transferrin receptors in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1995 Jun 15;211(2):535–539. doi: 10.1006/bbrc.1995.1846. [DOI] [PubMed] [Google Scholar]
- Snider M. D., Rogers O. C. Intracellular movement of cell surface receptors after endocytosis: resialylation of asialo-transferrin receptor in human erythroleukemia cells. J Cell Biol. 1985 Mar;100(3):826–834. doi: 10.1083/jcb.100.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack J. H., DeWald D. B., Takegawa K., Emr S. D. Vesicle-mediated protein transport: regulatory interactions between the Vps15 protein kinase and the Vps34 PtdIns 3-kinase essential for protein sorting to the vacuole in yeast. J Cell Biol. 1995 Apr;129(2):321–334. doi: 10.1083/jcb.129.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack J. H., Emr S. D. Vps34p required for yeast vacuolar protein sorting is a multiple specificity kinase that exhibits both protein kinase and phosphatidylinositol-specific PI 3-kinase activities. J Biol Chem. 1994 Dec 16;269(50):31552–31562. [PubMed] [Google Scholar]
- Stack J. H., Herman P. K., Schu P. V., Emr S. D. A membrane-associated complex containing the Vps15 protein kinase and the Vps34 PI 3-kinase is essential for protein sorting to the yeast lysosome-like vacuole. EMBO J. 1993 May;12(5):2195–2204. doi: 10.1002/j.1460-2075.1993.tb05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B. S., Sussman H. H. Demonstration of two distinct transferrin receptor recycling pathways and transferrin-independent receptor internalization in K562 cells. J Biol Chem. 1986 Aug 5;261(22):10319–10331. [PubMed] [Google Scholar]
- Stephens L., Smrcka A., Cooke F. T., Jackson T. R., Sternweis P. C., Hawkins P. T. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 1994 Apr 8;77(1):83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Stoyanov B., Volinia S., Hanck T., Rubio I., Loubtchenkov M., Malek D., Stoyanova S., Vanhaesebroeck B., Dhand R., Nürnberg B. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science. 1995 Aug 4;269(5224):690–693. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- Volinia S., Dhand R., Vanhaesebroeck B., MacDougall L. K., Stein R., Zvelebil M. J., Domin J., Panaretou C., Waterfield M. D. A human phosphatidylinositol 3-kinase complex related to the yeast Vps34p-Vps15p protein sorting system. EMBO J. 1995 Jul 17;14(14):3339–3348. doi: 10.1002/j.1460-2075.1995.tb07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessling-Resnick M., Braell W. A. The sorting and segregation mechanism of the endocytic pathway is functional in a cell-free system. J Biol Chem. 1990 Jan 15;265(2):690–699. [PubMed] [Google Scholar]
- Wiley H. S., Cunningham D. D. The endocytotic rate constant. A cellular parameter for quantitating receptor-mediated endocytosis. J Biol Chem. 1982 Apr 25;257(8):4222–4229. [PubMed] [Google Scholar]
- Yamamoto A., DeWald D. B., Boronenkov I. V., Anderson R. A., Emr S. D., Koshland D. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol Biol Cell. 1995 May;6(5):525–539. doi: 10.1091/mbc.6.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro D. J., Maxfield F. R. Acidification of morphologically distinct endosomes in mutant and wild-type Chinese hamster ovary cells. J Cell Biol. 1987 Dec;105(6 Pt 1):2723–2733. doi: 10.1083/jcb.105.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro D. J., Tycko B., Fluss S. R., Maxfield F. R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984 Jul;37(3):789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]