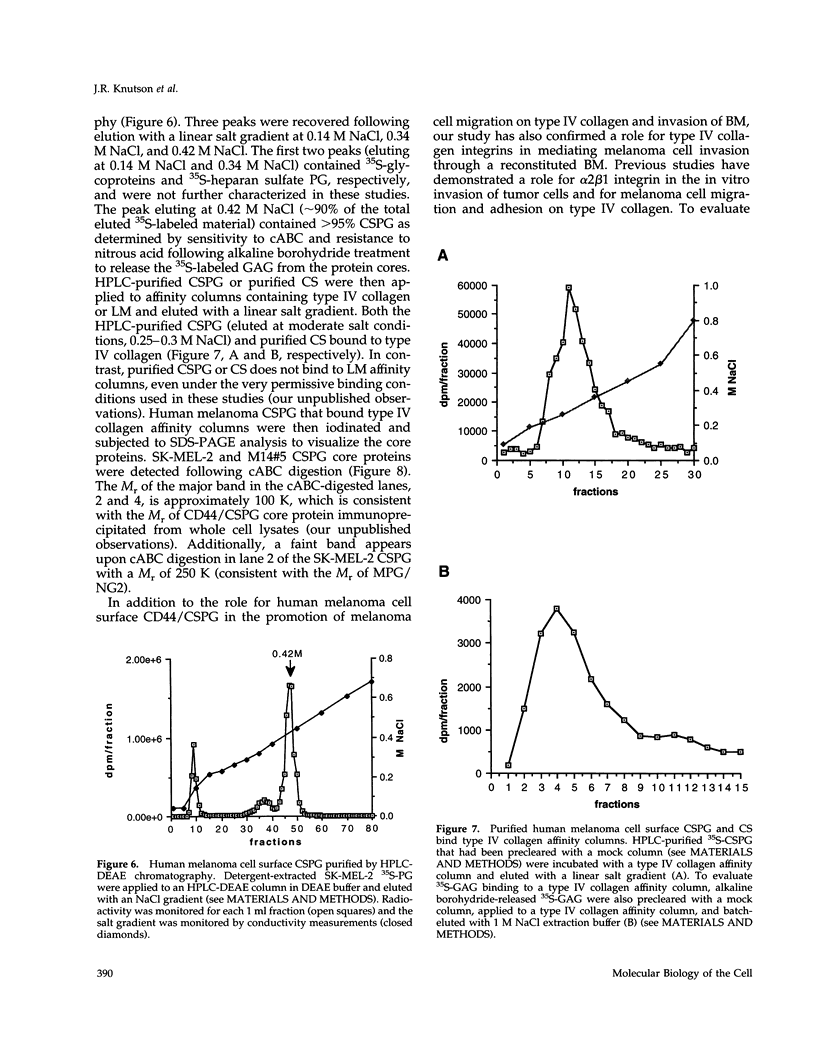
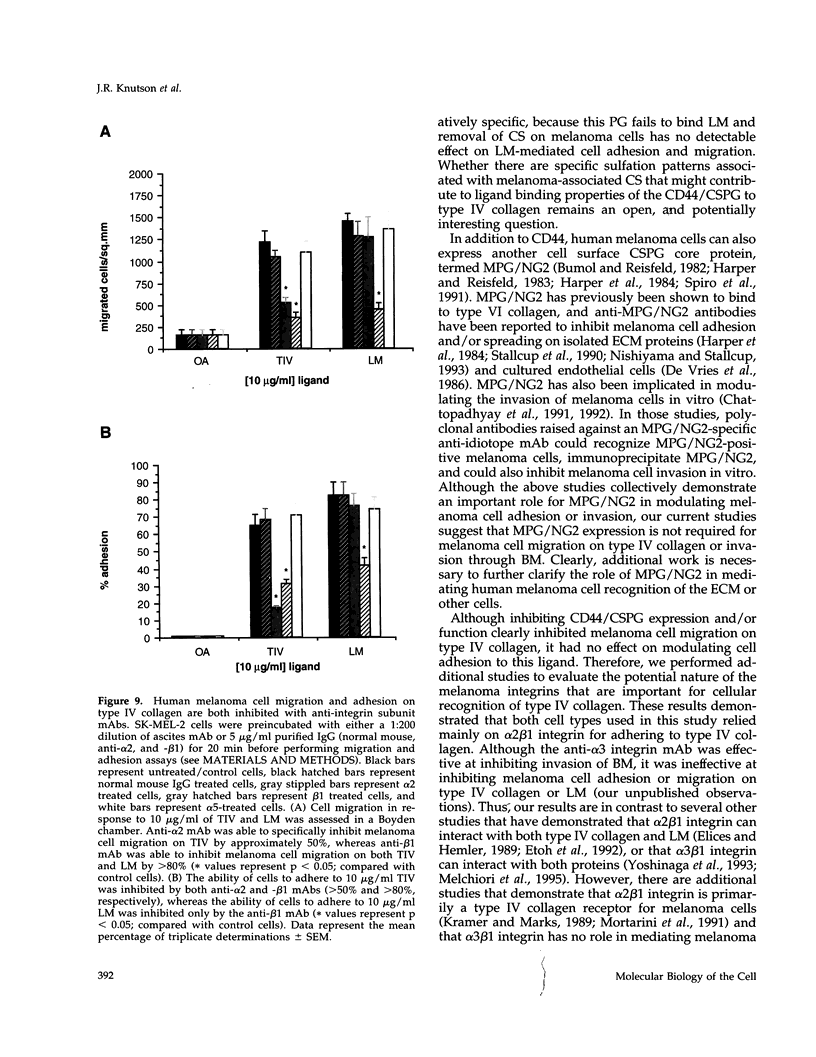
Abstract
Tumor cell invasion of basement membranes (BM) represents one of the critical steps in the metastatic process. Tumor cell recognition of individual BM matrix components may involve individual cell adhesion receptors, such as integrins or cell surface proteoglycans, or may involve a coordinate action of both types of receptors. In this study, we have focused on the identification of a cell surface CD44/chondroitin sulfate proteoglycan (CSPG) and alpha 2 beta 1 integrin on human melanoma cells that are both directly involved in the in vitro invasion of reconstituted BM via a type IV collagen-dependent mechanism. Interfering with cell surface expression of human melanoma CSPG with either p-nitro-phenyl-beta-D-xylopyranoside treatment or anti-CD44 monoclonal antibody (mAb) preincubation (mAb) preincubation inhibits melanoma cell invasion through reconstituted BM. These treatments also strongly inhibit melanoma cell migration on type IV collagen, however, they are ineffective at inhibiting cell adhesion to type IV collagen. Purified melanoma cell surface CD44/CSPG, or purified chondroitin sulfate, bind to type IV collagen affinity columns, consistent with a role for CD44/CSPG-type IV collagen interactions in mediating tumor cell invasion. In contrast, melanoma cell migration on laminin (LM) does not involve CD44/CSPG, nor does CD44/CSPG bind to LM, suggesting that CD44/CSPG-type IV collagen interactions are specific in nature. Additionally, anti-alpha 2 and anti-beta 1 integrin mAbs are capable of blocking melanoma cell invasion of reconstituted BM. Both of these anti-integrin mAbs inhibit melanoma cell adhesion and migration on type IV collagen, whereas only anti-beta 1 mAb inhibits cell adhesion to LM. Collectively, these results indicate that melanoma cell adhesion to type IV collagen is an important consideration in invasion of reconstituted BM in vitro, and suggest that CD44/CSPG and alpha 2 beta 1 integrin may collaborate to promote human melanoma cell adhesion, migration, and invasion in vivo.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Iwamoto Y., Kleinman H. K., Martin G. R., Aaronson S. A., Kozlowski J. M., McEwan R. N. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987 Jun 15;47(12):3239–3245. [PubMed] [Google Scholar]
- Birch M., Mitchell S., Hart I. R. Isolation and characterization of human melanoma cell variants expressing high and low levels of CD44. Cancer Res. 1991 Dec 15;51(24):6660–6667. [PubMed] [Google Scholar]
- Bosman F. T. Integrins: cell adhesives and modulators of cell function. Histochem J. 1993 Jul;25(7):469–477. doi: 10.1007/BF00159282. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Lokeshwar V. B., He J., Chen X., Bourguignon G. J. A CD44-like endothelial cell transmembrane glycoprotein (GP116) interacts with extracellular matrix and ankyrin. Mol Cell Biol. 1992 Oct;12(10):4464–4471. doi: 10.1128/mcb.12.10.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumol T. F., Reisfeld R. A. Unique glycoprotein-proteoglycan complex defined by monoclonal antibody on human melanoma cells. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1245–1249. doi: 10.1073/pnas.79.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. D., Skubitz A. P., Furcht L. T. Type IV collagen and corneal epithelial adhesion and migration. Effects of type IV collagen fragments and synthetic peptides on rabbit corneal epithelial cell adhesion and migration in vitro. Invest Ophthalmol Vis Sci. 1991 Sep;32(10):2766–2773. [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A. Characterization of the class III collagen receptor, a phosphorylated, transmembrane glycoprotein expressed in nucleated human cells. J Biol Chem. 1988 Mar 25;263(9):4193–4201. [PubMed] [Google Scholar]
- Chan B. M., Hemler M. E. Multiple functional forms of the integrin VLA-2 can be derived from a single alpha 2 cDNA clone: interconversion of forms induced by an anti-beta 1 antibody. J Cell Biol. 1993 Jan;120(2):537–543. doi: 10.1083/jcb.120.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B. M., Kassner P. D., Schiro J. A., Byers H. R., Kupper T. S., Hemler M. E. Distinct cellular functions mediated by different VLA integrin alpha subunit cytoplasmic domains. Cell. 1992 Mar 20;68(6):1051–1060. doi: 10.1016/0092-8674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- Chan B. M., Matsuura N., Takada Y., Zetter B. R., Hemler M. E. In vitro and in vivo consequences of VLA-2 expression on rhabdomyosarcoma cells. Science. 1991 Mar 29;251(5001):1600–1602. doi: 10.1126/science.2011740. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay P., Starkey J., Morrow W. J., Raychaudhuri S. Murine monoclonal anti-idiotope antibody breaks unresponsiveness and induces a specific antibody response to human melanoma-associated proteoglycan antigen in cynomolgus monkeys. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2684–2688. doi: 10.1073/pnas.89.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay P., Starkey J., Raychaudhuri S. Analysis of anti-tumor antibodies in mice and rabbits induced by monoclonal anti-idiotope antibodies. J Immunol. 1991 Sep 15;147(6):2055–2062. [PubMed] [Google Scholar]
- Chelberg M. K., McCarthy J. B., Skubitz A. P., Furcht L. T., Tsilibary E. C. Characterization of a synthetic peptide from type IV collagen that promotes melanoma cell adhesion, spreading, and motility. J Cell Biol. 1990 Jul;111(1):261–270. doi: 10.1083/jcb.111.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelberg M. K., Tsilibary E. C., Hauser A. R., McCarthy J. B. Type IV collagen-mediated melanoma cell adhesion and migration: involvement of multiple, distinct domains of the collagen molecule. Cancer Res. 1989 Sep 1;49(17):4796–4802. [PubMed] [Google Scholar]
- Danen E. H., van Muijen G. N., van de Wiel-van Kemenade E., Jansen K. F., Ruiter D. J., Figdor C. G. Regulation of integrin-mediated adhesion to laminin and collagen in human melanocytes and in non-metastatic and highly metastatic human melanoma cells. Int J Cancer. 1993 May 8;54(2):315–321. doi: 10.1002/ijc.2910540225. [DOI] [PubMed] [Google Scholar]
- Dedhar S., Saulnier R., Nagle R., Overall C. M. Specific alterations in the expression of alpha 3 beta 1 and alpha 6 beta 4 integrins in highly invasive and metastatic variants of human prostate carcinoma cells selected by in vitro invasion through reconstituted basement membrane. Clin Exp Metastasis. 1993 Sep;11(5):391–400. doi: 10.1007/BF00132982. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Hemler M. E. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9906–9910. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etoh T., Byers H. R., Mihm M. C., Jr Integrin expression in malignant melanoma and their role in cell attachment and migration on extracellular matrix proteins. J Dermatol. 1992 Nov;19(11):841–846. doi: 10.1111/j.1346-8138.1992.tb03794.x. [DOI] [PubMed] [Google Scholar]
- Etoh T., Thomas L., Pastel-Levy C., Colvin R. B., Mihm M. C., Jr, Byers H. R. Role of integrin alpha 2 beta 1 (VLA-2) in the migration of human melanoma cells on laminin and type IV collagen. J Invest Dermatol. 1993 May;100(5):640–647. doi: 10.1111/1523-1747.ep12472299. [DOI] [PubMed] [Google Scholar]
- Faassen A. E., Mooradian D. L., Tranquillo R. T., Dickinson R. B., Letourneau P. C., Oegema T. R., McCarthy J. B. Cell surface CD44-related chondroitin sulfate proteoglycan is required for transforming growth factor-beta-stimulated mouse melanoma cell motility and invasive behavior on type I collagen. J Cell Sci. 1993 Jun;105(Pt 2):501–511. doi: 10.1242/jcs.105.2.501. [DOI] [PubMed] [Google Scholar]
- Faassen A. E., Schrager J. A., Klein D. J., Oegema T. R., Couchman J. R., McCarthy J. B. A cell surface chondroitin sulfate proteoglycan, immunologically related to CD44, is involved in type I collagen-mediated melanoma cell motility and invasion. J Cell Biol. 1992 Jan;116(2):521–531. doi: 10.1083/jcb.116.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields C. G., Mickelson D. J., Drake S. L., McCarthy J. B., Fields G. B. Melanoma cell adhesion and spreading activities of a synthetic 124-residue triple-helical "mini-collagen". J Biol Chem. 1993 Jul 5;268(19):14153–14160. [PubMed] [Google Scholar]
- Furcht L. T., McCarthy J. B., Palm S. L., Basara M. L., Enenstein J. Peptide fragments of laminin and fibronectin promote migration (haptotaxis and chemotaxis) of metastatic cells. Ciba Found Symp. 1984;108:130–145. doi: 10.1002/9780470720899.ch9. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T. The extended family of proteoglycans: social residents of the pericellular zone. Curr Opin Cell Biol. 1989 Dec;1(6):1201–1218. doi: 10.1016/s0955-0674(89)80072-9. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Wayner E. A., Hoffman P. A., St John T., Butcher E. C., Carter W. G. Structural homology between lymphocyte receptors for high endothelium and class III extracellular matrix receptor. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4654–4658. doi: 10.1073/pnas.86.12.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlsen K. R., Davis G. E., Sriramarao P. Integrin expression in human melanoma cells with differing invasive and metastatic properties. Clin Exp Metastasis. 1992 Mar;10(2):111–120. doi: 10.1007/BF00114587. [DOI] [PubMed] [Google Scholar]
- Gehlsen K. R., Sriramarao P., Furcht L. T., Skubitz A. P. A synthetic peptide derived from the carboxy terminus of the laminin A chain represents a binding site for the alpha 3 beta 1 integrin. J Cell Biol. 1992 Apr;117(2):449–459. doi: 10.1083/jcb.117.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Association of various T cell-surface molecules with the cytoskeleton. Effect of cross-linking and activation. J Immunol. 1991 May 15;146(10):3298–3305. [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Harper J. R., Bumol T. F., Reisfeld R. A. Characterization of monoclonal antibody 155.8 and partial characterization of its proteoglycan antigen on human melanoma cells. J Immunol. 1984 Apr;132(4):2096–2104. [PubMed] [Google Scholar]
- Harper J. R., Reisfeld R. A. Inhibition of anchorage-independent growth of human melanoma cells by a monoclonal antibody to a chondroitin sulfate proteoglycan. J Natl Cancer Inst. 1983 Aug;71(2):259–263. [PubMed] [Google Scholar]
- Honn K. V., Tang D. G. Adhesion molecules and tumor cell interaction with endothelium and subendothelial matrix. Cancer Metastasis Rev. 1992 Nov;11(3-4):353–375. doi: 10.1007/BF01307187. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y., Robey F. A., Graf J., Sasaki M., Kleinman H. K., Yamada Y., Martin G. R. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987 Nov 20;238(4830):1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- Jacobson K., O'Dell D., Holifield B., Murphy T. L., August J. T. Redistribution of a major cell surface glycoprotein during cell movement. J Cell Biol. 1984 Nov;99(5):1613–1623. doi: 10.1083/jcb.99.5.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalomiris E. L., Bourguignon L. Y. Mouse T lymphoma cells contain a transmembrane glycoprotein (GP85) that binds ankyrin. J Cell Biol. 1988 Feb;106(2):319–327. doi: 10.1083/jcb.106.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto T., Martin G. R., Hamilton T. C., Fridman R. Effects of synthetic peptides and protease inhibitors on the interaction of a human ovarian cancer cell line (NIH:OVCAR-3) with a reconstituted basement membrane (Matrigel). Invasion Metastasis. 1991;11(2):84–92. [PubMed] [Google Scholar]
- Kapron-Bras C., Fitz-Gibbon L., Jeevaratnam P., Wilkins J., Dedhar S. Stimulation of tyrosine phosphorylation and accumulation of GTP-bound p21ras upon antibody-mediated alpha 2 beta 1 integrin activation in T-lymphoblastic cells. J Biol Chem. 1993 Oct 5;268(28):20701–20704. [PubMed] [Google Scholar]
- Karecla P. I., Timpl R., Watt F. M. Adhesion of human epidermal keratinocytes to laminin. Cell Adhes Commun. 1994 Aug;2(4):309–318. doi: 10.3109/15419069409014206. [DOI] [PubMed] [Google Scholar]
- Kawasaki K., Murakami T., Namikawa M., Mizuta T., Iwai Y., Yamashiro Y., Hama T., Yamamoto S., Mayumi T. Amino acids and peptides. XXI. Laminin-related peptide analogs including poly(ethylene glycol) hybrids and their inhibitory effect on experimental metastasis. Chem Pharm Bull (Tokyo) 1994 Apr;42(4):917–921. doi: 10.1248/cpb.42.917. [DOI] [PubMed] [Google Scholar]
- Kramer R. H., Marks N. Identification of integrin collagen receptors on human melanoma cells. J Biol Chem. 1989 Mar 15;264(8):4684–4688. [PubMed] [Google Scholar]
- Lacy B. E., Underhill C. B. The hyaluronate receptor is associated with actin filaments. J Cell Biol. 1987 Sep;105(3):1395–1404. doi: 10.1083/jcb.105.3.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallier T., Deutzmann R., Perris R., Bronner-Fraser M. Neural crest cell interactions with laminin: structural requirements and localization of the binding site for alpha 1 beta 1 integrin. Dev Biol. 1994 Apr;162(2):451–464. doi: 10.1006/dbio.1994.1101. [DOI] [PubMed] [Google Scholar]
- Larjava H., Lyons J. G., Salo T., Mäkelä M., Koivisto L., Birkedal-Hansen H., Akiyama S. K., Yamada K. M., Heino J. Anti-integrin antibodies induce type IV collagenase expression in keratinocytes. J Cell Physiol. 1993 Oct;157(1):190–200. doi: 10.1002/jcp.1041570125. [DOI] [PubMed] [Google Scholar]
- Leavesley D. I., Schwartz M. A., Rosenfeld M., Cheresh D. A. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993 Apr;121(1):163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J., Hyman R., Kincade P. W. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- Lynch S. A., Bouchard B. N., Vijayasaradhi S., Yuasa H., Houghton A. N. Antigens of melanocytes and melanoma. Cancer Metastasis Rev. 1991 Jun;10(2):141–150. doi: 10.1007/BF00049411. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Tarin D. Significance of CD44 gene products for cancer diagnosis and disease evaluation. Lancet. 1992 Oct 31;340(8827):1053–1058. doi: 10.1016/0140-6736(92)93077-z. [DOI] [PubMed] [Google Scholar]
- Matter M. L., Laurie G. W. A novel laminin E8 cell adhesion site required for lung alveolar formation in vitro. J Cell Biol. 1994 Mar;124(6):1083–1090. doi: 10.1083/jcb.124.6.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchiori A., Mortarini R., Carlone S., Marchisio P. C., Anichini A., Noonan D. M., Albini A. The alpha 3 beta 1 integrin is involved in melanoma cell migration and invasion. Exp Cell Res. 1995 Jul;219(1):233–242. doi: 10.1006/excr.1995.1223. [DOI] [PubMed] [Google Scholar]
- Merzak A., Koocheckpour S., Pilkington G. J. CD44 mediates human glioma cell adhesion and invasion in vitro. Cancer Res. 1994 Aug 1;54(15):3988–3992. [PubMed] [Google Scholar]
- Miyamoto S., Akiyama S. K., Yamada K. M. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995 Feb 10;267(5199):883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Moczar M., Caux F., Bailly M., Berthier O., Doré J. F. Accumulation of heparan sulfate in the culture of human melanoma cells with different metastatic ability. Clin Exp Metastasis. 1993 Nov;11(6):462–471. doi: 10.1007/BF00054937. [DOI] [PubMed] [Google Scholar]
- Mortarini R., Anichini A., Parmiani G. Heterogeneity for integrin expression and cytokine-mediated VLA modulation can influence the adhesion of human melanoma cells to extracellular matrix proteins. Int J Cancer. 1991 Feb 20;47(4):551–559. doi: 10.1002/ijc.2910470413. [DOI] [PubMed] [Google Scholar]
- Nakai M., Mundy G. R., Williams P. J., Boyce B., Yoneda T. A synthetic antagonist to laminin inhibits the formation of osteolytic metastases by human melanoma cells in nude mice. Cancer Res. 1992 Oct 1;52(19):5395–5399. [PubMed] [Google Scholar]
- Nishiyama A., Stallcup W. B. Expression of NG2 proteoglycan causes retention of type VI collagen on the cell surface. Mol Biol Cell. 1993 Nov;4(11):1097–1108. doi: 10.1091/mbc.4.11.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomizu M., Yamamura K., Kleinman H. K., Yamada Y. Multimeric forms of Tyr-Ile-Gly-Ser-Arg (YIGSR) peptide enhance the inhibition of tumor growth and metastasis. Cancer Res. 1993 Aug 1;53(15):3459–3461. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Eisenstein R. Characterization of bovine aorta proteoglycan extracted with guanidine hydrochloride in the presence of protease inhibitors. J Biol Chem. 1979 Feb 25;254(4):1312–1318. [PubMed] [Google Scholar]
- Paulsson M. Basement membrane proteins: structure, assembly, and cellular interactions. Crit Rev Biochem Mol Biol. 1992;27(1-2):93–127. doi: 10.3109/10409239209082560. [DOI] [PubMed] [Google Scholar]
- Paulus W., Tonn J. C. Basement membrane invasion of glioma cells mediated by integrin receptors. J Neurosurg. 1994 Mar;80(3):515–519. doi: 10.3171/jns.1994.80.3.0515. [DOI] [PubMed] [Google Scholar]
- Perris R., Syfrig J., Paulsson M., Bronner-Fraser M. Molecular mechanisms of neural crest cell attachment and migration on types I and IV collagen. J Cell Sci. 1993 Dec;106(Pt 4):1357–1368. doi: 10.1242/jcs.106.4.1357. [DOI] [PubMed] [Google Scholar]
- Perschl A., Lesley J., English N., Trowbridge I., Hyman R. Role of CD44 cytoplasmic domain in hyaluronan binding. Eur J Immunol. 1995 Feb;25(2):495–501. doi: 10.1002/eji.1830250228. [DOI] [PubMed] [Google Scholar]
- Riikonen T., Westermarck J., Koivisto L., Broberg A., Kähäri V. M., Heino J. Integrin alpha 2 beta 1 is a positive regulator of collagenase (MMP-1) and collagen alpha 1(I) gene expression. J Biol Chem. 1995 Jun 2;270(22):13548–13552. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- Rudy W., Hofmann M., Schwartz-Albiez R., Zöller M., Heider K. H., Ponta H., Herrlich P. The two major CD44 proteins expressed on a metastatic rat tumor cell line are derived from different splice variants: each one individually suffices to confer metastatic behavior. Cancer Res. 1993 Mar 15;53(6):1262–1268. [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Seftor R. E., Seftor E. A., Stetler-Stevenson W. G., Hendrix M. J. The 72 kDa type IV collagenase is modulated via differential expression of alpha v beta 3 and alpha 5 beta 1 integrins during human melanoma cell invasion. Cancer Res. 1993 Jul 15;53(14):3411–3415. [PubMed] [Google Scholar]
- Seltzer J. L., Lee A. Y., Akers K. T., Sudbeck B., Southon E. A., Wayner E. A., Eisen A. Z. Activation of 72-kDa type IV collagenase/gelatinase by normal fibroblasts in collagen lattices is mediated by integrin receptors but is not related to lattice contraction. Exp Cell Res. 1994 Aug;213(2):365–374. doi: 10.1006/excr.1994.1211. [DOI] [PubMed] [Google Scholar]
- Simon-Assmann P., Leberquier C., Molto N., Uezato T., Bouziges F., Kedinger M. Adhesive properties and integrin expression profiles of two colonic cancer populations differing by their spreading on laminin. J Cell Sci. 1994 Mar;107(Pt 3):577–587. doi: 10.1242/jcs.107.3.577. [DOI] [PubMed] [Google Scholar]
- Spiro R. C., Freeze H. H., Sampath D., Garcia J. A. Uncoupling of chondroitin sulfate glycosaminoglycan synthesis by brefeldin A. J Cell Biol. 1991 Dec;115(5):1463–1473. doi: 10.1083/jcb.115.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup W. B., Dahlin K., Healy P. Interaction of the NG2 chondroitin sulfate proteoglycan with type VI collagen. J Cell Biol. 1990 Dec;111(6 Pt 2):3177–3188. doi: 10.1083/jcb.111.6.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Aznavoorian S., Liotta L. A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Sánchez-Mateos P., Arroyo A. G., Balboa M. A., Sánchez-Madrid F. Post-receptor occupancy events in leukocytes during beta 1 integrin-ligand interactions. Eur J Immunol. 1993 Oct;23(10):2642–2648. doi: 10.1002/eji.1830231038. [DOI] [PubMed] [Google Scholar]
- Tamura R. N., Cooper H. M., Collo G., Quaranta V. Cell type-specific integrin variants with alternative alpha chain cytoplasmic domains. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10183–10187. doi: 10.1073/pnas.88.22.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarone G., Ferracini R., Galetto G., Comoglio P. A cell surface integral membrane glycoprotein of 85,000 mol wt (gp85) associated with triton X-100-insoluble cell skeleton. J Cell Biol. 1984 Aug;99(2):512–519. doi: 10.1083/jcb.99.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L., Etoh T., Stamenkovic I., Mihm M. C., Jr, Byers H. R. Migration of human melanoma cells on hyaluronate is related to CD44 expression. J Invest Dermatol. 1993 Feb;100(2):115–120. doi: 10.1111/1523-1747.ep12462776. [DOI] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M. S., Skubitz A. P. Human keratinocytes adhere to multiple distinct peptide sequences of laminin. J Invest Dermatol. 1991 Jul;97(1):141–146. doi: 10.1111/1523-1747.ep12479311. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W., Yamada S. S., Gralnick H., Chen W. T., Akiyama S. K. Monoclonal antibody and synthetic peptide inhibitors of human tumor cell migration. Cancer Res. 1990 Aug 1;50(15):4485–4496. [PubMed] [Google Scholar]
- Yamamura K., Kibbey M. C., Kleinman H. K. Melanoma cells selected for adhesion to laminin peptides have different malignant properties. Cancer Res. 1993 Jan 15;53(2):423–428. [PubMed] [Google Scholar]
- Yoshinaga I. G., Vink J., Dekker S. K., Mihm M. C., Jr, Byers H. R. Role of alpha 3 beta 1 and alpha 2 beta 1 integrins in melanoma cell migration. Melanoma Res. 1993 Dec;3(6):435–441. doi: 10.1097/00008390-199311000-00006. [DOI] [PubMed] [Google Scholar]
- Zhao M., Kleinman H. K., Mokotoff M. Synthetic laminin-like peptides and pseudopeptides as potential antimetastatic agents. J Med Chem. 1994 Sep 30;37(20):3383–3388. doi: 10.1021/jm00046a023. [DOI] [PubMed] [Google Scholar]
- de Vries J. E., Keizer G. D., te Velde A. A., Voordouw A., Ruiter D., Rümke P., Spits H., Figdor C. G. Characterization of melanoma-associated surface antigens involved in the adhesion and motility of human melanoma cells. Int J Cancer. 1986 Oct 15;38(4):465–473. doi: 10.1002/ijc.2910380403. [DOI] [PubMed] [Google Scholar]