Summary
High-throughput identification of small molecules that selectively modulate molecular, cellular or systems-level properties of the mammalian brain is a significant challenge. Here we report the chemical genetic identification of the orphan ligand phosphoserine (P-Ser) as an enhancer of neurogenesis. P-Ser inhibits neural stem cell/progenitor proliferation and self-renewal, enhances neurogenic fate commitment, and improves neuronal survival. We further demonstrate that the effects of P-Ser are mediated by the group III metabotropic glutamate receptor 4 (mGluR4). siRNA-mediated knockdown of mGluR4 abolished the effects of P-Ser and increased neurosphere proliferation, at least in part through upregulation of mTOR pathway activity. We also found that P-Ser increases neurogenesis in human embryonic stem (ES) cell-derived neural progenitors. This work highlights the tremendous potential of developing effective small molecule drugs for use in regenerative medicine or transplantation therapy.
Introduction
The use of small molecule probes in biological studies has a long, rich history. Such compounds, though often found by chance, are increasingly sought after using high-throughput screening (HTS) efforts (Schreiber, 2005). Complicated experimental systems, e.g., the mammalian brain, remain recalcitrant to systematic approaches (Koh and Crews, 2002; Stockwell, 2002). Potentially, cell-based chemical genetic screens, which can be used to identify compounds that produce a phenotype of interest, provide a simple, rapid, and content-rich format for identifying such small molecules (Mayer et al., 1999).
Neural stem cells (NSCs) have the capacity to self-renew and ultimately produce the three major cell types of the brain, i.e. neurons, astrocytes and oligodendrocytes. The discovery of neural stem cells has tremendous importance not only for understanding the generation of this cellular diversity, but also has vast practical implications for repair of the central nervous system (CNS) following injury. The initial isolation of neural stem cells has led to large, systematic efforts to identify and manipulate molecular mechanisms of NSC proliferation, cell fate determination, and differentation. While many of the genetic and biochemical pathways underlying these processes are beginning to be understood, currently there are few known small molecule probes for specifically studying and controlling these mechanisms.
The need for such tools has been recognized for some time (reviewed by (Ding and Schultz, 2004)), and previous studies have utilized cell lines to screen for small molecules that specifically induce differentiation of stem cells of neuroepithelial, and other, origin (Wu et al., 2002; Ding et al., 2003; Kawazoe et al., 2004). However, small molecule probes for examining the intricately related processes of stem cell proliferation and cell fate choice have remained unidentified, and the advantages of using primary culture stem cells have not been exploited. In the current study, we developed a reliable primary cell screen using neurospheres, ex vivo cultures containing neural stem cells (Reynolds and Weiss, 1992). Neurospheres derived from embryonic day 11.5 (E11.5) mouse telencephalon were screened against libraries of bioactive small molecules (following mitogen withdrawal), including kinase/phosphatase inhibitors and orphan ligands (see Methods). Using these primary cultures as a screening platform, we identified phosphoserine (P-Ser), a member of the orphan ligand library and an endogenous serine metabolite, as a regulator of neural progenitor proliferation, fate commitment, and differentiation. Subsequently, we determined that these effects are mediated through activation of metabotropic glutamate receptor 4. Broadly, these studies demonstrate the utility of relatively simple screening assays for identifying highly useful small molecule probes which can be used to elucidate novel roles for cell-intrinsic and extrinsic factors in neural stem cell biology.
Results
Primary neurospheres were passaged one time to expand the limited amount of starting material and to ensure a self-renewing progenitor population while minimizing effects of any differentiated cells that remain after dissection. Secondary neurospheres were differentiated in 96-well plates with one library compound per well for three days. Cultures were then processed for immunoreactivity against a commonly used neuron-specific marker, βIII tubulin (Tuj1), using chemiluminescent detection (Fig. 1a). Approximately 7-9% of cells normally differentiate towards a neuronal lineage and express Tuj1; this assay reliably detects molecules which enhance expression of this marker, as shown by the addition of the neurotrophin BDNF (Supplementary Fig. 1a online). Importantly, this enhancement in Tuj1 immunoreactivity need not be due to increased neurogenesis; compounds which influence neurite branching or axonal outgrowth would also register as positive hits. Any potential candidates need to be tested in secondary assays to validate functional effects on the phenotype of interest.
Figure 1. P-Ser enhances neuronal differentiation of neural stem cells.

(a) Scheme of small molecule screen. The SEM micrograph is from the embryo images collection at www.med.unc.edu/embryo_images, used with permission. (b) Secondary E12.5 spheres differentiated with or without P-Ser and stained for Tuj1 and propidium iodide, scale bar = 300μm. (c) Neuron counts of differentiated secondary E12.5 spheres, p=0.0013. (d) Neuron counts of differentiated secondary P1 cortical spheres, p=0.0169. (e) Dose-response of P-Ser concentration vs. number of neurons generated from differentiated E12.5 spheres. Data is normalized so that untreated is set to 100%. (f) RT-PCR analysis of differentiated E12.5 spheres. –RT, no reverse transcriptase control. For densitometry, data is normalized so that untreated is set to 100%. 10μM P-Ser was used in all experiments. Data are represented as mean ± SEM in c-e.
For the primary screen using Tuj1 immunoreactivity as a readout, we assayed several hundred bioactive molecules (including kinase/phosphatase inhibitors and orphan ligands, see Methods) and identified 64 compounds which were classified into two groups: “enhancers” which increased Tuj1 signals, and “inhibitors” which decreased Tuj1 signals (Supplementary Table 1 online). Most of the “inhibitors” exhibited general cytotoxicity and were not studied further; others have been described as exerting negative effects on neuronal function, e.g., p38 MAP kinase inhibitors (Xia et al., 1995; Dougherty et al., 2005). Among the “enhancers”, several are known to improve neuronal survival, e.g., tobacco smoke constituents (Soto-Otero et al., 1998). Fourteen compounds were chosen for secondary screening, of which five (four “enhancers” and one “inhibitor”, Supplementary Fig. 1b-d online) were confirmed to modulate neuronal differentiation. A novel enhancer identified from the orphan ligand library is L-serine-O-phosphate (phosphoserine or P-Ser). P-Ser increased the number of Tuj1+ cells in differentiating E12.5 cortical cultures (Fig. 1b,c) in a dose-dependent manner (Fig. 1e). Similar effects were observed in cultures derived from mouse postnatal day one (P1) cortex (Fig. 1d). These results suggest that the effects of P-Ser are not limited to a single developmental stage. The increased neuronal identity of P-Ser treated cultures was further confirmed by elevated expression of the neurogenic bHLH transcription factor NeuroD in differentiated E12.5 neurospheres, as determined by RT-PCR (Fig. 1f).
The neurogenic phenotype of P-Ser could be due to effects on progenitor proliferation, cell fate commitment, and/or neuronal survival. We first assayed the effects of P-Ser on progenitor proliferation using the neurosphere assay. P-Ser treatment of secondary neurospheres (see below) reduced proliferation, based on cell number measurements (Fig. 2a) and neurosphere size (Fig. 2b). This finding was confirmed by a 33% decrease in BrdU labeling of P-Ser treated attached progenitor cultures (Fig. 2c).
Figure 2. P-Ser slows progenitor proliferation and enhances neurogenic commitment.
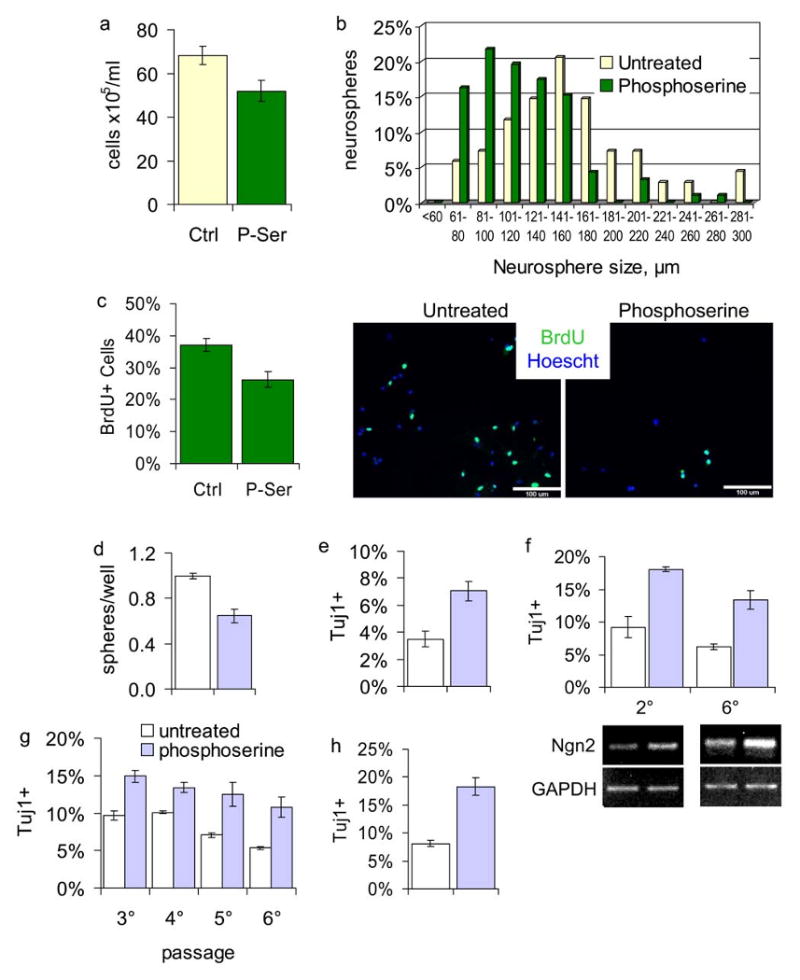
(a) Cells were plated at 1k/mL and grown with or without 10μM P-Ser for one week, then trypsinized and counted by trypan blue exclusion, p=0.0293. (b) Histogram of clonal neurosphere size after one week in culture. (c) BrdU labeling index; dissociated primary neurospheres plated were plated on poly-ornithine-fibronectin-coated coverslips and grown for four days with or without 10μM P-Ser, then pulsed with 5μM BrdU for two hours and fixed, p=0.009, scale bar = 100μm. (d) Number of tertiary clonal neurospheres derived from P-Ser treated (10μM) or non-treated secondary neurospheres. Data is normalized to untreated (100%), p=1.91×10-5. (e) Analysis of Tuj1+ cells present in acutely dissociated neurospheres grown with or without 10μM P-Ser, p=0.00137. (f) Neurospheres were grown continually with or without 20μM P-Ser for the indicated number of passages. Neurospheres were then harvested for RNA for RT-PCR analysis of NGN2 expression, or differentiated and stained for Tuj1, p(2°)=0.0031, p(6°)=0.00031. (g) Neurospheres were continually grown with or without 20μM P-Ser over multiple passages. Aliquots of each passage were differentiated and stained for Tuj1, p(3°)=.00295, p(4°)=0.0163, p(5°)=0.0111, p(6°)=0.0075. Multiple comparisons across passages were not made. (h) Tertiary neurospheres were derived from P-Ser treated (20μM) or non-treated secondary spheres, differentiated for five days, and stained for Tuj1, p=2.86×10-5. Data are represented as mean ± SEM in b, d-h.
The findings above could represent an effect on neural stem cells, other progenitors within the culture, or both. Therefore, we also analyzed production of new stem cells during P-Ser treatment. Primary neurospheres can be dissociated and replated, as single cells, to form secondary neurospheres. Neurospheres form aggregates at cell densities >5,000 cells/mL, which can confound analysis of the true proliferative potential of the plated cells (Singec et al., 2006). In contrast, at a cell density of 1,000 cells/mL nearly all newly formed neurospheres are clonal in origin, i.e. derived from a single plated cell ((Groszer et al., 2001); T.K.K. and H.I.K. unpublished observations). Thus, at this cell density, the neurosphere assay can be used as a reliable estimate of stem cell proliferation (see Discussion). Secondary neurospheres were grown under clonal conditions with or without P-Ser. These spheres were then dissociated to single cells and allowed to form tertiary neurospheres under optimal conditions. The number of tertiary neurospheres formed is a direct measure of stem cell proliferation. We found that there were fewer tertiary spheres formed from P-Ser treated secondary spheres than from control secondary spheres (Fig. 2d). Nearly all spheres were tripotent, giving rise to neurons, astrocytes and oligodendrocytes (Supplementary Fig. 2a online), suggesting that P-Ser inhibits proliferation of multipotent sphere-forming progenitors.
We next measured the effects of P-Ser on neurogenesis in proliferating neurospheres. We found that while P-Ser decreased cell number and neurosphere size (Fig. 2a, b), there was an increase in the percentage of Tuj1+ cells within the proliferating spheres (Fig. 2e, and Supplementary Fig. 2b online). Upon differentiation, secondary spheres grown in P-Ser generated more Tuj1+ cells than control cultures (Fig. 2f). As these P-Ser treated spheres were passaged continually, the relative percentages of Tuj1+ cells generated upon differentiation increased compared to controls (Fig. 2g). Furthermore, spheres grown in the presence of P-Ser contained elevated levels of the neuronal fate-determining bHLH transcription factor Neurogenin2 (Ngn2, Fig. 2f), as determined by RT-PCR. Together, these results strongly suggest that P-Ser directly enhances commitment of NSCs towards a neurogenic fate.
This possibility was confirmed via clonal analysis. If P-Ser instructs commitment of NSCs towards a particular lineage, then tertiary neurospheres derived from treated secondary spheres should show a predisposition towards generating that fate. Therefore, secondary neurospheres were grown at clonal density in the absence or presence of P-Ser. These neurospheres were then passaged to form tertiary neurospheres at clonal density, in the absence of P-Ser, and subsequently differentiated to determine their neurogenic capacity. We found that P-Ser treated spheres gave rise to tertiary spheres that were twice as neurogenic as control-derived tertiary spheres (Fig. 2h).
We also tested whether P-Ser affects neuronal survival. To this end, we derived neuronal cultures from E14.5 mouse cortex, treated with P-Ser or water, and quantified the neurons at multiple time points. Compared to control conditions in which the number of Tuj1+ cells with neuronal morphology decreased over time (Fig. 3a, b), P-Ser treatment caused an increase in the number of these cells remaining in the cultures at both time points assayed (Fig. 3b). This rescue was especially pronounced by three days post-plating, suggesting that P-Ser has neuroprotective effects under these conditions (Fig. 3a, b). Consistent with this possibility, P-Ser treatment resulted in a substantial decrease in the total number of apoptotic nuclei as indicated by TUNEL assay (Fig. 3a, c), in agreement with the percentage of Tuj1+ cells undergoing apoptosis after three days (Fig. 3d).
Figure 3. P-Ser improves survival in differentiating neuron cultures.
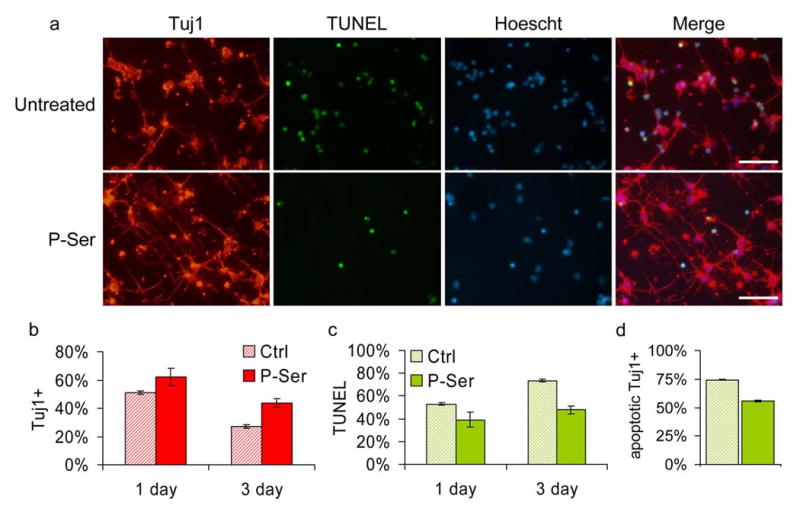
(a) Three-day old E14.5 cortical neurons cultured with or without 10μM P-Ser. TUNEL assay (green) was performed on cultures, followed by staining for Tuj1 (red), scale bar = 25μm. (b) Number of Tuj1+ cells in one or three day E14 cortical neuron cultures untreated or with 10μM P-Ser. 1dayCtrl vs. 1dayP-Ser p=0.0931, 3dayCtrl vs. 3dayPSer p=0.0346, 1dayCtrl vs. 3dayCtrl p=0.0136, 1dayPSer vs. 3dayPSer p=0.0284. (c) Number of TUNEL+ cells in one or three day E14 cortical neuron cultures untreated or with 10μM P-Ser. 1dayCtrl vs. 1dayP-Ser p=0.0719, 3dayCtrl vs. 3dayP-Ser p=0.0138, 1dayCtrl vs. 3dayCtrl p=0.025, 1dayPSer vs. 3dayPSer p=0.1877. (d) Number of TUNEL+Tuj1 double-positive cells as a percentage of total Tuj1+ cells in three day E14 cortical neuron cultures untreated or with 10μM P-Ser, p=0.0013. All data are presented as mean ± SEM.
We then investigated potential molecular mechanisms through which P-Ser could act to modulate these phenotypes. One possibility is that P-Ser influences the serine biosynthesis pathway, which has been postulated to be important for neurogenesis (Furuya et al., 2000; Yoshida et al., 2004). Another possibility is that the neurogenic effects of P-Ser may be mediated by group III metabotropic glutamate receptors, for which it is an agonist (Conn and Pin, 1997). The latter hypothesis is supported by our findings that another group III receptor agonist (L-AP4, (Conn and Pin, 1997)) produced similar effects as P-Ser on neurosphere size, whereas a group III receptor antagonist (UBP1112, (Conway et al., 2001)) reversed the effects of P-Ser (Fig. 4a).
Figure 4. The effects of P-Ser are mediated by metabotropic glutamate receptor 4.
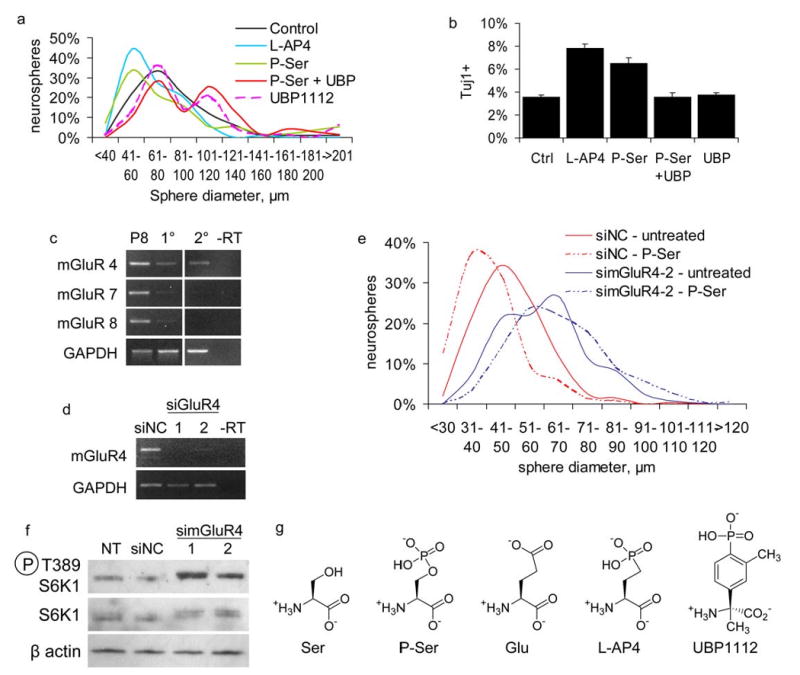
(a) Histogram of secondary sphere sizes, cells plated at 1k/mL and grown untreated or with 10μM P-Ser, 20μM L-AP4, 15μM UBP1112, or 10μM P-Ser + 15μM UBP1112. (b) P1 secondary cortical spheres differentiated untreated or with 20μM L-AP4, 10μM P-Ser, 10μM P-Ser + 15μM UBP1112, or 15μM UBP1112, then stained for Tuj1 expression. Ctrl vs. L-AP4 p=0.0034, Ctrl vs. P-Ser p=0.0041, P-Ser vs Pser+UBP p=0.0065. Presented as mean±SEM. (c) RT-PCR analysis of group III metabotropic glutamate receptor expression in primary (1°) and secondary (2°) neurospheres, and postnatal day 8 mouse brain (P8, positive control). The retina-specific mGluR6 was not detected in any experiments (data not shown). (d) RT-PCR analysis of cells transfected with negative control siRNA (siNC) or duplexes targeting mGluR4 (simGluR4). (e) Histogram of neurosphere size following transfection with siNC or simGluR4 and treatment with water or 10μM P-Ser. Similar results were obtained with both duplexes; for clarity, only results from duplex #2 (simGluR4-2) are shown. –RT, no reverse transcriptase. (f) Western blot analysis of E12.5 adherent progenitors cultured for three days post-transfection with either siNC, simGluR4-1, or simGluR4-2, or no transfection (NT) (g) Chemical structures of L-Serine, P-Ser, L-Glutamate, L-AP4, and UBP1112.
The effects of these compounds were also observed on differentiating secondary neurospheres derived from P1 cortex (Fig. 4b). Here, treatment with either P-Ser or L-AP4 caused a >2-fold increase in the number of Tuj1+ cells generated (Fig. 4b). UBP1112 again abolished this increase (Fig. 4b). No enhancement of neuronal differentiation was observed in E12 neurospheres following treatment with either 10μM of D-serine-O-phosphate or D-AP4 (Supplementary Fig. 3a online), which are >300-fold less potent at this class of receptors than their L enantiomers (Davies and Watkins, 1982; Thomsen and Suzdak, 1993). Thus, group III metabotropic receptors appear to mediate the effects of P-Ser in both proliferating and differentiating neurosphere cultures.
In order to examine the physiologically relevant receptor species, we analyzed the expression of the various members of the group III mGluRs by RT-PCR. In primary neurospheres, mGluR 7 and 8 were present at very low levels, whereas mGluR4 was moderately abundant (Fig. 4c). In secondary neurospheres, only mGluR4 was detected (Fig. 4c). The primer sets used were equally capable of detecting equimolar quantities of appropriate template in control reactions (Supplementary Fig. 3b), suggesting that observed differences in mRNA quantity were not due to inefficient amplification by different primer sets. mGluR4 mRNA was present in the positive and negative fractions of secondary neurospheres FAC-sorted for the NSC marker LeX (Capela and Temple, 2002) (Supplementary Fig. 3c online), indicating the receptor is present in progenitors as well as more differentiated or committed cells. As a control, the progenitor-specific marker nucleostemin (Tsai and McKay, 2002) was correctly detected only in the LeX(+) fraction (Supplementary Fig. 3c online). According to the Allen Brain Atlas (http://www.brain-map.org) (Lein et al., 2007), mGluR4 was expressed by a subset of cells within the subventricular zone proximal to the lateral ventricles (one of two neurogenic germinal zones within the adult brain (Doetsch, 2003), Supplementary Fig. 3d online), as shown by non-isotopic in situ hybridization. A specific probe for mGluR8 did not detect any positive cells within this region (Supplementary Fig. 3d online). Thus, mGluR4 expression patterns suggest an important role for this receptor in mediating neural progenitor proliferation.
To confirm that mGluR4 is the target of P-Ser for progenitor proliferation, we used a loss-of-function approach by RNA interference. Two siRNAs (Methods online) were designed to target mGluR4; both effectively reduced mGluR4 mRNA levels (Fig. 4d). Dissociated primary spheres were transfected with mGluR4 siRNA vs. control siRNA and replated to form secondary spheres in the presence of either P-Ser or vehicle. As shown in figure 4e, although P-Ser inhibits proliferation of cells transfected with control siRNA (as expected), this inhibition by P-Ser is lost in mGluR4 knockdown cells, indicating that the effect of P-Ser is indeed mediated though mGluR4. Interestingly, we found that mGluR4 knockdown produced larger spheres than control cells (Fig. 4e), suggesting that mGluR4 may serve to restrict neural progenitor growth. Consistent with this hypothesis, mGluR4 knockdown led to hyperactivation of mTOR, a major regulator of cell growth and proliferation (Wullschleger et al., 2006), as indicated by the greatly elevated levels of phospho-S389 S6K1 (Fig. 4f).
We then tested the effects of mGluR4 knockdown on neuronal differentiation. Following transfection with control or receptor-specific siRNA duplexes, progenitors were differentiated in the absence or presence of P-Ser. As expected, cells transfected with control siRNA generated more Tuj1+ cells when treated with P-Ser (Fig. 5a). Consistent with previous results, knockdown of mGluR4 abrogated the effect of P-Ser on neuronal differentiation (Fig. 5a). Interestingly, we consistently observed an increase in O4+ cells in the receptor knockdown conditions, regardless of P-Ser treatment (Fig. 5b, c). These data show that mGluR4 mediates the effects of P-Ser on neuronal differentiation and also suggests an unexpected role for mGluR4 in oligodendrocyte differentiation.
Figure 5. mGluR4 mediates the effects of P-Ser on differentiation.
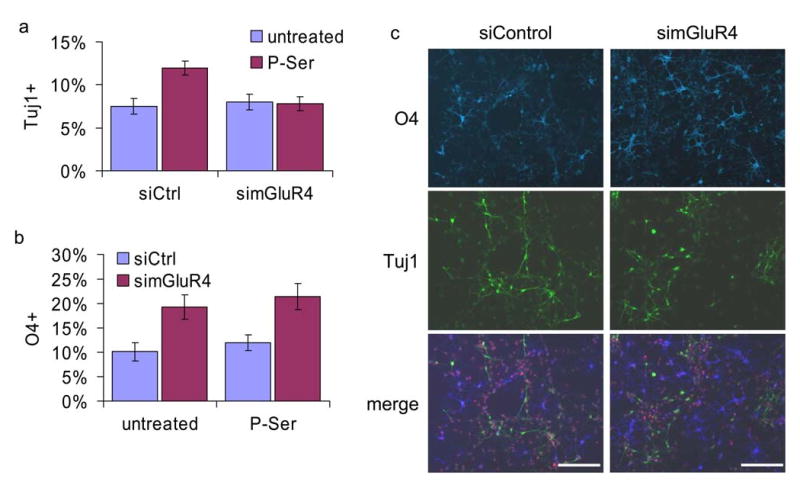
(a) mGluR4 knockdown abolishes P-Ser-induced neuronal differentiation. E12 progenitors were transfected with negative control siRNA (siControl) or duplex targeting mGluR4 (simGluR4) and differentiated for three days in the absence or presence of 20μM P-Ser, and stained for Tuj1. siCtrl UT vs. siCtrl P-Ser p=0.0018, siCtrl P-Ser vs simGluR4 Pser p=0.0021. (b) mGluR4 knockdown increases oligodendrocyte differentiation. Cells were treated as in (a) and stained for the oligodendrocyte marker O4. siCtrl UT vs. simGluR4 UT p=0.0374, siCtrl P-Ser vs. simGluR4 P-Ser p=0.0184. (c) E12 progenitors transfected with siControl or simGluR4, differentiated in the absence of P-Ser, and stained for Tuj1 (green) and O4 (blue), counterstained with propidium iodide (red). Scale bar = 50 μm.
The experiments described above suggest that treatment with P-ser provides a simple method to enhance neuronal production from mouse neural progenitors. To determine whether such is also the case for human neural progenitors, and to investigate potential therapeutic application of these studies, we determined the effects of P-Ser on differentiating neural progenitors derived from human embryonic stem (hES) cells. As shown in figure 6a-c, treatment with P-Ser doubled the number of neurons produced from two hES cell lines, HSF-1 (NIH UC01; karyotype XX) and HSF-6 (NIH UC06; karyotype XY). These results demonstrate that the neurogenic effects of P-Ser are neither species- nor gender- specific, and thus should prove broadly useful in directing hES cells for neural regeneration.
Figure 6. P-Ser increases neuronal differentiation of human embryonic stem cells.
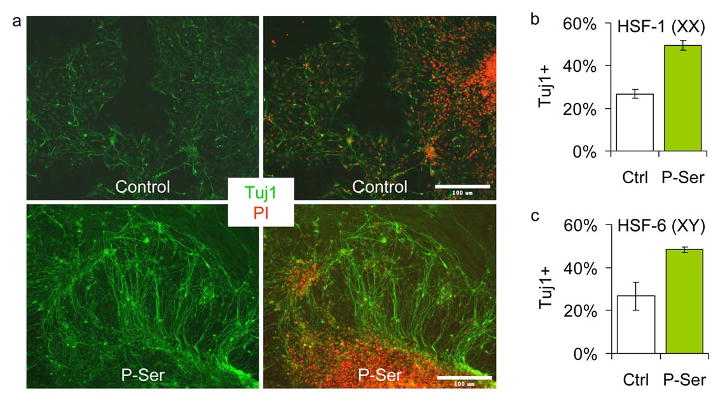
(a) Human embryonic stem cell-derived neural progenitors (hES-NPCs) derived from line HSF-1 were differentiated with or without 10μM P-Ser for six days, then stained for Tuj1 expression. Left panels, Tuj1 (green); right panels, Tuj1 (green) and propidium iodide (red). Scale bar = 100μm. (b) hES-NPCs derived from line HSF-1 were differentiated as above and the percentage of Tuj1+ cells was determined, p=0.000862. (c) hES-NPS derived from line HSF-6 were differentiated as above and the percentage of Tuj1+ cells was determined, p=0.0158. Data are represented as mean ± SEM.
Discussion
In this paper, we describe high-throughput screening using primary cells to identify small molecules that regulate complex phenotypes. There are clear advantages in being able to use primary cells from animals of defined genetic makeup and developmental stage, as well as patient-derived cells, as opposed to cell lines that may contain unknown genetic abnormalities or adaptations. We utilized a simple method for the propagation of multipotent neural progenitors that yielded reliable and reproducible data. This culture system has proven invaluable for determining the roles of both cell-intrinsic and extrinsic regulators (i.e. PTEN, LIF, and BMPs) of NSC proliferation, commitment, and differentiation (Shimazaki et al., 2001; Hitoshi et al., 2002; Nakano et al., 2005; Groszer et al., 2006).
When dissected neural tissue is plated as single cells under non-adherent, permissive conditions, floating balls of cells termed neurospheres (Reynolds and Weiss, 1992) are formed. These highly heterogenous structures contain neural stem cells as well as more restricted (i.e. bi- and uni-potent) progenitors and differentiated progeny. The presence of stem cells can be confirmed by dissociating these neurospheres into single cells and replating them. A small percentage (generally ∼3%) of these cells will re-form neurospheres, demonstrating self-renewal. Multipotency of all of these spheres can be determined post-hoc, by differentiating them and performing immunocytochemistry for common markers of all three lineages.
To truly test the self-renewal potential of cells within the neurosphere cultures, dissociated single cells need to be plated at a density at which a single cell gives rise to a single neurosphere. This is termed clonal analysis, and recent reports (Singec et al., 2006; Jessberger et al., 2007) have demonstrated that, when cultured together under medium to high cell densities, small neurospheres can adhere to each other and combine to form larger neurospheres. Clonal analysis is impossible under these conditions, and the true proliferative capacity of the cells cannot be determined.
We have previously determined culture conditions which allow for true clonal analysis (Groszer et al., 2001). Neural stem cells from GFP+ animals were dissected and mixed with comparable cells from a wild-type animal, and the resulting mixture was plated at varying cell densities. These different cell densities were then analyzed for the presence of chimeric neurospheres. Under high density conditions, nearly all spheres contained both GFP+ and GFP- spheres, mirroring the results of Singec et al and Jessberger et al. However, no chimeric neurospheres were observed at a low cell density of 1,000 cells/mL. We have recently re-confirmed this data, observing <5% chimeric neurospheres when cells were plated at 1,000 cells/mL in 96-well plates (T.K.K. and H.I.K., unpublished observations). These results are important for proper interpretation of the clonal analysis and sphere size data reported here (although not for the primary screen, which was performed at high density).
In our initial screen, we utilized neurospheres passaged one time to assay for cell differentiation and/or fate commitment effects. These neurospheres contain very few differentiated cells, as judged by TuJ1 immunocytochemistry (Fig. 2e and data not shown), at the time they were exposed to the chemical libraries, ensuring that observed effects were not due to the presence of neurons that were present at the time of initial culturing. It is important to note that this screen does not require that each neurosphere is derived from a single stem or progenitor cell. Furthermore, the general methodology described here is widely applicable. As effects on differentiation were determined via antibody-based detection (Stockwell et al., 1999), this assay should be easily adaptable to studying any process of interest so long as one has a suitable antigenic marker. This methodology is highly advantageous for screens utilizing delicate cell types such as primary culture stem cells (although nearly any cell type may be used); specifically, no genetic manipulation, such as introduction of a reporter gene, is necessary.
Here, we show that our method has great potential for drug discovery and functional studies. Specifically, we identified the orphan ligand P-Ser as a neurogenic enhancer, and established group III metabotropic glutamate receptor 4 as its target. Metabotropic glutamate receptors are generally located pre-synaptically, where they act to modulate neuronal excitability and play roles in learning and memory, depression, and other behaviors (Swanson et al., 2005). Recent reports have demonstrated a role for groups I (Gq-linked) and II (Gs-linked) receptors in stem cell biology in response to glutamate; mGluR3 and 5 have been shown to promote proliferation of embryonic stem cells (Cappuccio et al., 2005) and neural stem cells (Di Giorgi Gerevini et al., 2004; 2005), and a positive role in neural stem cell proliferation has been suggested for group II receptors in response to high extracellular concentrations of glutamate (Brazel et al., 2005). We found a negative role for group III receptors (Gi-linked) in inhibiting neural stem cell/progenitor proliferation and in enhancing neuronal survival. mGluR4 has previously been shown to inhibit proliferation of unipotent cerebellar granule cell precursors (Canudas et al., 2004); in contrast to this report, we highlight effects of activation of this receptor by an endogenous ligand, which act together to regulate proliferation, self-renewal, and neuronal lineage commitment of multipotent progenitors, as well as survival of their differentiated progeny, derived from multiple developmental timepoints.
Our data support the hypothesis that neural progenitor proliferation, cell fate, and terminal cell differentiation and survival are coupled. For example, we and others have demonstrated that the phosphatase PTEN (phosphatase and tensin homologe deleted on chromosome 10) negatively regulates neural progenitor proliferation, as well as neuronal differentiation and survival (Groszer et al., 2001; 2006), for review see (Ohnuma and Harris, 2003)). Others have shown that the length of the neural progenitor cell cycle is directly coupled to cell fate choices, as factors that shorten the cell cycle inhibit differentiative divisions, whereas those that lengthen the cell cycle promote differentiative divisions (Lukaszewicz et al., 2002). Thus, the data presented here are consistent with a model whereby mGluR4 activation by P-Ser promotes neurogenesis through enhanced neuronogenic commitment (possibly a result of increased expression of bHLH transcription factors (Fig. 1f and 2f)) by slowing progenitor proliferation.
As mentioned above, BMP, LIF, Wnt, PTEN/PI3K-Akt, and other signaling events act to regulate NSC proliferation and differentiation. Known downstream effectors of these regulators include the MAPK, STAT, and mTOR pathways (which display extensive crosstalk) (Rajan et al., 2003; Shamji et al., 2003; Shaw and Cantley, 2006). PTEN is a negative regulator of the PI3K-Akt-mTOR pathway (Shaw and Cantley, 2006). Activation of Akt results in enhanced neural progenitor proliferation (Sinor and Lillien, 2004), neuronal differentiation (Vojtek et al., 2003), and neuronal survival (Dudek et al., 1997). Treatment with rapamycin, an mTOR inhibitor, results in decreased neurosphere formation (Sinor and Lillien, 2004). Consistent with these observations, we found that, in addition to abrogating the effects of P-Ser, siRNA knockdown of mGluR4 also results in a large enhancement of mTOR activity (Fig. 4f). This is likely due to loss of constitutive (ligand-dependent) activity upon receptor knockdown. Although P-Ser enhances neuronal differentiation and survival, activation of mGluR4 by P-Ser moderately inhibits Akt and mTOR activity (data not shown). The nature of this complexity could arise from differential activation thresholds for these processes, disparate effects of divergent downstream effectors, modulation by unexamined pathways, or other possibilities.
Although the neurogenic activity of P-ser was discovered in vitro, this molecule likely has direct relevance for in vivo neurogenesis. The “phosphorylated” serine biosynthetic pathway seems to be the main mechanism through which the brain manufactures L-serine. The enzyme 3-phosphoglycerate dehydrogenase (3-PGDH) catalyzes the unidirectional production of a pyruvate intermediate, which is further metabolized to P-Ser by phosphohydroxypyruvate aminotransferase. P-Ser is then irreversibly dephosphorylated by phosphoserine phosphatase (PSP) to form L-serine (for discussion, see (de Koning et al., 2003)). 3-PDGH is specifically expressed by radial glia and the astrocytes derived from them (Yamasaki et al., 2001). Futhermore, genetic deletion of 3-PGDH results in mid-embryonic lethality accompanied by severe neurodevelopmental defects (Yoshida et al., 2004). PSP was identified by a genetic screen to be differentially expressed in neural progenitors versus their differentiated progeny (Geschwind et al., 2001), and is expressed in the germinal zones during embryonic and post-natal development (Easterday et al., 2003).
Provocatively, PSP is specifically expressed by GFAP-expressing progenitors within the subventricular zone (a region of ongoing adult neurogenesis (Doetsch, 2003)) and is present in only a subset of 3-PGDH-expressing cells within this area (Nakano et al., 2007). This suggests that while many cells within the neurogenic regions may synthesize P-Ser, only some of these cells are capable of further metabolizing it to L-serine and removing P-Ser from their local microenvironment. Following along this reasoning, P-Ser may be released and then act in an autocrine or paracrine manner, whereas PSP-expressing cells may be able to “avoid” P-Ser's effects by converting it to L-serine. Furthermore, since P-Ser has 10- to 100-fold higher potency at mGluR4 than glutamate (Johansen et al., 1995) and is active only at group III (but not at groups I or II) receptors (Conn and Pin, 1997), a model can be envisioned where interplay between P-Ser and glutamate contributes to drive neural progenitor behavior in vivo.
The identification of this P-Ser/mGluR4 pathway has implications not only for neural development, but also for neural repair. A great deal of effort has been invested in identifying methods of efficient neuronal production from both embryonic and neural stem cells, in vitro and in vivo. In the current study, we demonstrate that P-ser treatment dramatically enhances neuronal production from human embryonic stem cells, as well as from murine CNS-derived neural progenitors, making it a highly cost-effective reagent to potentially ”prime” transplantable cells for differentiation towards a neuronal cell fate. These studies also raise the possibility that P-ser or other mGluR4 effectors could be used to promote neurogenesis following stroke or other focal CNS injury.
Surprisingly, we also found a potential role for mGluR4 in oligodendrocyte differentiation. In mGluR4 knockdown cells, western blot analysis of Stat3 (another mTor substrate (Androutsellis-Theotokis et al., 2006)) revealed the presence of lower molecular weight bands (Supplementary Fig. 4 online), similar to those previously shown to arise through proteolytic cleavage of Stat3 mediated by non-apoptotic caspase activity (Darnowski et al., 2006; Matthews et al., 2007). As Stat3 signaling is important in astrogliogenesis (Bonni et al., 1997), regulated degradation of Stat3 in mGluR4-knockdown cells may promote generation of oligodendrocytes at the expense of astrocytes, although the details of this mechanism are currently under investigation.
In summary, we have demonstrated a powerful, easily adaptable, and robust method to screen molecular libraries for compounds that enhance neuronal production from ex vivo stem and progenitor cells. This method was used to identify P-Ser (phosphoserine), an endogenous serine metabolite, as a regulator of neural stem cell proliferation and differentiation. Use of P-Ser as a tool to probe these processes at the molecular level led to the identification of metabotropic glutamate receptor 4 as the mediator of P-Ser effects, and revealed a novel role for this receptor in neural stem cell biology.
Significance
Neural stem cells (NSCs) are capable of producing new neurons, and are thus of great interest to those studying the means to repair the central nervous system. Enhancing the generation of neurons from NSCs is an important therapeutic goal, and an understanding of the mechanisms by which neural stem cells proliferate and produce neurons will be critical to these efforts. Small molecules that regulate neural stem cell proliferation and differentiation would be highly useful as tools for exploring and controlling the mechanisms underlying these processes, and could serve as candidate lead compounds for therapy of a variety of disorders including stroke and spinal cord injury. Here, we report the development of a chemical genetic screen using cells isolated from embryonic mouse forebrain. We used this screen to identify the orphan ligand phosphoserine (P-Ser) as a regulator of NSC differentiation. P-ser inhibits NSC proliferation and promotes survival of nascent neurons. P-Ser also promotes neuronal differentiation of human embryonic stem cell-derived neural stem cells. Thus, P-Ser has potential therapeutic value in addition to its basic utility as a probe for dissecting molecular mechanisms underlying neurogenesis.
This endogenous amino acid metabolite is a ligand for group III metabotropic glutamate receptors (mGluRs), and we show that the group III mGluR4 subtype mediates the effects of P-Ser on NSC proliferation and differentiation (a previously unknown role for mGluR4). Furthermore, we found that the effects of mGluR4 are mediated, at least in part, via the mTor pathway, a pathway known to mediate neural stem cell proliferation and neuronal survival. The current findings not only specifically establish a role for P-Ser and mGluR4 in neural stem cell biology, but also demonstrate the potential usefulness of cell-based chemical genetic screens to identify molecules for use in both basic and translational neural stem cell research.
Methods
Small molecule screen
Four days after passaging primary spheres as single cells, the resultant secondary spheres were resuspended in differentiation media (750 spheres in 200μL) and cultured in white 96-well plates (Corning, Corning NY) coated with 10μg/mL poly-L-lsyine (Sigma-Aldrich, St. Louis MO). Libraries of orphan ligands and kinase/phosphatase inhibitors were obtained from Biomol (Plymouth Meeting PA). Compounds were transferred to assay plates using 96-pin replicators (Genetix Limited, Hampshire U.K.). Spheres were incubated for three days and used for antibody probing. Antigen detection was similar to cytoblotting protocols (Stockwell et al., 1999; Koeller et al., 2003), with the following modifications. Following incubation with Tuj1 antibody (1:500, Covance USA) overnight at 4°C, HRP-conjugated goat anti-mouse secondary antibody (Pierce, Rockford IL) was added for 2 hrs at room temperature, and chemiluminescence was developed with Supersignal ELISA Pico substrate (Pierce). Signal was quantified on a plate reader (Analyst HT, Molecular Devices, Sunnyvale CA).
Human Embryonic Stem Cell Culture
hESC lines HSF6 (46, XX) and HSF1 (46, XY) were maintained on irradiated CF1 MEF cells in high-glucose DMEM supplemented with 1mM L-glutamine, 1% non-essential amino acids, 0.1mM β-mercaptoethanol, and 20% knockout serum replacement (all from Invitrogen, Carlsbad CA). Culture medium was changed and supplemented with 10ng/mL bFGF daily. Confluent hESC cultures were passaged weekly by incubation in 1mg/mL dispase/collogenase IV (Invitrogen) for 20 minutes at 37°C.
For directed differentiation into neural progenitor cells (NPCs), hESCs were passaged onto dishes coated with Matrigel (1:10 dilution, BD Biosciences, San Jose CA) in the absence of irradiated MEFs. hESC medium was replaced with NPC medium (DMEM/F12 with B27 supplement and 500U/mL penicillin/streptomycin, all from Invitrogen) four days after passaging. Partially differentiated hESCs were cultured for an additional four days, on Matrigel, in NPC medium with daily addition of 10ng/mL bFGF. hESC-NPCs were then continuously passaged mechanically and cultured on PO/FN-coated culture dishes (poly-ornithine and fibronectin, Sigma-Aldrich). For differentiation of hESC-NPCs, cells were washed with Dulbecco's PBS and fresh NPC medium, without bFGF, was added. Cells were incubated for six days, with one intermediate medium change. 20μM P-Ser was added to appropriate wells. The UCLA Institutional Review Board (IRB) has approved all experiments involving hESCs.
Supplementary Material
Acknowledgments
The authors wish to thank Kathleen Sulik for use of the SEM micrograph in figure 1a, and Owen Witte for helping to establish the UCLA Molecular Screening Center. J.P.S. was a recipient of an NIH Research Training in Pharmacological Sciences pre-doctoral training grant and UCLA dissertation year fellowship, and T.K.K. was supported by the Adelson Program in Neural Repair Research. This work was partly supported by the Singleton Developmental Grant (to J.H.) and NIMH RO165756 (to H.I.K.).
Footnotes
Other Methods are described in Supplementary Information online.
The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD, Greenberg ME. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- Brazel CY, Nunez JL, Yang Z, Levison SW. Glutamate enhances survival and proliferation of neural progenitors derived from the subventricular zone. Neuroscience. 2005;131:55–65. doi: 10.1016/j.neuroscience.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Canudas AM, Di Giorgi-Gerevini V, Iacovelli L, Nano G, D'Onofrio M, Arcella A, Giangaspero F, Busceti C, Ricci-Vitiani L, Battaglia G, et al. PHCCC, a specific enhancer of type 4 metabotropic glutamate receptors, reduces proliferation and promotes differentiation of cerebellar granule cell neuroprecursors. J Neurosci. 2004;24:10343–10352. doi: 10.1523/JNEUROSCI.3229-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/s0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Cappuccio I, Spinsanti P, Porcellini A, Desiderati F, De Vita T, Storto M, Capobianco L, Battaglia G, Nicoletti F, Melchiorri D. Endogenous activation of mGlu5 metabotropic glutamate receptors supports self-renewal of cultured mouse embryonic stem cells. Neuropharmacology. 2005;49 1:196–205. doi: 10.1016/j.neuropharm.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Miller JC, Howson PA, Clark BP, Jane DE. Synthesis of phenylglycine derivatives as potent and selective antagonists of group III metabotropic glutamate receptors. Bioorg Med Chem Lett. 2001;11:777–780. doi: 10.1016/s0960-894x(01)00052-x. [DOI] [PubMed] [Google Scholar]
- Darnowski JW, Goulette FA, Guan YJ, Chatterjee D, Yang ZF, Cousens LP, Chin YE. Stat3 cleavage by caspases: impact on full-length Stat3 expression, fragment formation, and transcriptional activity. J Biol Chem. 2006;281:17707–17717. doi: 10.1074/jbc.M600088200. [DOI] [PubMed] [Google Scholar]
- Davies J, Watkins JC. Actions of D and L forms of 2-amino-5-phosphonovalerate and 2-amino-4-phosphonobutyrate in the cat spinal cord. Brain Res. 1982;235:378–386. doi: 10.1016/0006-8993(82)91017-4. [DOI] [PubMed] [Google Scholar]
- de Koning TJ, Snell K, Duran M, Berger R, Poll-The BT, Surtees R. L-serine in disease and development. Biochem J. 2003;371:653–661. doi: 10.1042/BJ20021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giorgi Gerevini VD, Caruso A, Cappuccio I, Ricci Vitiani L, Romeo S, Della Rocca C, Gradini R, Melchiorri D, Nicoletti F. The mGlu5 metabotropic glutamate receptor is expressed in zones of active neurogenesis of the embryonic and postnatal brain. Brain Res Dev Brain Res. 2004;150:17–22. doi: 10.1016/j.devbrainres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Di Giorgi-Gerevini V, Melchiorri D, Battaglia G, Ricci-Vitiani L, Ciceroni C, Busceti CL, Biagioni F, Iacovelli L, Canudas AM, Parati E, et al. Endogenous activation of metabotropic glutamate receptors supports the proliferation and survival of neural progenitor cells. Cell Death Differ. 2005;12:1124–1133. doi: 10.1038/sj.cdd.4401639. [DOI] [PubMed] [Google Scholar]
- Ding S, Schultz PG. A role for chemistry in stem cell biology. Nat Biotechnol. 2004;22:833–840. doi: 10.1038/nbt987. [DOI] [PubMed] [Google Scholar]
- Ding S, Wu TY, Brinker A, Peters EC, Hur W, Gray NS, Schultz PG. Synthetic small molecules that control stem cell fate. Proc Natl Acad Sci U S A. 2003;100:7632–7637. doi: 10.1073/pnas.0732087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F. The glial identity of neural stem cells. Nat Neurosci. 2003;6:1127–1134. doi: 10.1038/nn1144. [DOI] [PubMed] [Google Scholar]
- Dougherty JD, Garcia AD, Nakano I, Livingstone M, Norris B, Polakiewicz R, Wexler EM, Sofroniew MV, Kornblum HI, Geschwind DH. PBK/TOPK, a proliferating neural progenitor-specific mitogen-activated protein kinase kinase. J Neurosci. 2005;25:10773–10785. doi: 10.1523/JNEUROSCI.3207-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Easterday MC, Dougherty JD, Jackson RL, Ou J, Nakano I, Paucar AA, Roobini B, Dianati M, Irvin DK, Weissman IL, et al. Neural progenitor genes. Germinal zone expression and analysis of genetic overlap in stem cell populations. Dev Biol. 2003;264:309–322. doi: 10.1016/j.ydbio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Furuya S, Tabata T, Mitoma J, Yamada K, Yamasaki M, Makino A, Yamamoto T, Watanabe M, Kano M, Hirabayashi Y. L-serine and glycine serve as major astroglia-derived trophic factors for cerebellar Purkinje neurons. Proc Natl Acad Sci U S A. 2000;97:11528–11533. doi: 10.1073/pnas.200364497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Ou J, Easterday MC, Dougherty JD, Jackson RL, Chen Z, Antoine H, Terskikh A, Weissman IL, Nelson SF, Kornblum HI. A genetic analysis of neural progenitor differentiation. Neuron. 2001;29:325–339. doi: 10.1016/s0896-6273(01)00209-4. [DOI] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Dougherty JD, Le Belle J, Zack JA, Geschwind DH, Liu X, Kornblum HI, Wu H. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci U S A. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, Kornblum HI, Liu X, Wu H. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Clemenson GD, Jr, Gage FH. Spontaneous fusion and nonclonal growth of adult neural stem cells. Stem Cells. 2007;25:871–874. doi: 10.1634/stemcells.2006-0620. [DOI] [PubMed] [Google Scholar]
- Johansen PA, Chase LA, Sinor AD, Koerner JF, Johnson RL, Robinson MB. Type 4a metabotropic glutamate receptor: identification of new potent agonists and differentiation from the L-(+)-2-amino-4-phosphonobutanoic acid-sensitive receptor in the lateral perforant pathway in rats. Mol Pharmacol. 1995;48:140–149. [PubMed] [Google Scholar]
- Kawazoe Y, Tanaka S, Uesugi M. Chemical genetic identification of the histamine H1 receptor as a stimulator of insulin-induced adipogenesis. Chem Biol. 2004;11:907–913. doi: 10.1016/j.chembiol.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Koeller KM, Haggarty SJ, Perkins BD, Leykin I, Wong JC, Kao MC, Schreiber SL. Chemical genetic modifier screens: small molecule trichostatin suppressors as probes of intracellular histone and tubulin acetylation. Chem Biol. 2003;10:397–410. doi: 10.1016/s1074-5521(03)00093-0. [DOI] [PubMed] [Google Scholar]
- Koh B, Crews CM. Chemical genetics: a small molecule approach to neurobiology. Neuron. 2002;36:563–566. doi: 10.1016/s0896-6273(02)01059-0. [DOI] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, Kennedy H, Dehay C. Contrasting effects of basic fibroblast growth factor and neurotrophin 3 on cell cycle kinetics of mouse cortical stem cells. J Neurosci. 2002;22:6610–6622. doi: 10.1523/JNEUROSCI.22-15-06610.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JR, Watson SM, Tevendale MC, Watson CJ, Clarke AR. Caspase-dependent proteolytic cleavage of STAT3alpha in ES cells, in mammary glands undergoing forced involution and in breast cancer cell lines. BMC Cancer. 2007;7:29. doi: 10.1186/1471-2407-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- Nakano I, Dougherty JD, Kim K, Klement I, Geschwind DH, Kornblum HI. Phosphoserine phosphatase is expressed in the neural stem cell niche and regulates neural stem and progenitor cell proliferation. Stem Cells. 2007 doi: 10.1634/stemcells.2007-0046. [DOI] [PubMed] [Google Scholar]
- Nakano I, Paucar AA, Bajpai R, Dougherty JD, Zewail A, Kelly TK, Kim KJ, Ou J, Groszer M, Imura T, et al. Maternal embryonic leucine zipper kinase (MELK) regulates multipotent neural progenitor proliferation. J Cell Biol. 2005;170:413–427. doi: 10.1083/jcb.200412115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma S, Harris WA. Neurogenesis and the cell cycle. Neuron. 2003;40:199–208. doi: 10.1016/s0896-6273(03)00632-9. [DOI] [PubMed] [Google Scholar]
- Rajan P, Panchision DM, Newell LF, McKay RD. BMPs signal alternately through a SMAD or FRAP-STAT pathway to regulate fate choice in CNS stem cells. J Cell Biol. 2003;161:911–921. doi: 10.1083/jcb.200211021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Schreiber SL. Small molecules: the missing link in the central dogma. Nat Chem Biol. 2005;1:64–66. doi: 10.1038/nchembio0705-64. [DOI] [PubMed] [Google Scholar]
- Shamji AF, Nghiem P, Schreiber SL. Integration of growth factor and nutrient signaling: implications for cancer biology. Mol Cell. 2003;12:271–280. doi: 10.1016/j.molcel.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Shimazaki T, Shingo T, Weiss S. The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J Neurosci. 2001;21:7642–7653. doi: 10.1523/JNEUROSCI.21-19-07642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singec I, Knoth R, Meyer RP, Maciaczyk J, Volk B, Nikkhah G, Frotscher M, Snyder EY. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 2006;3:801–806. doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- Sinor AD, Lillien L. Akt-1 expression level regulates CNS precursors. J Neurosci. 2004;24:8531–8541. doi: 10.1523/JNEUROSCI.1470-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Otero R, Mendez-Alvarez E, Riguera-Vega R, Quinoa-Cabana E, Sanchez-Sellero I, Lopez-Rivadulla Lamas M. Studies on the interaction between 1,2,3,4-tetrahydro-beta-carboline and cigarette smoke: a potential mechanism of neuroprotection for Parkinson's disease. Brain Res. 1998;802:155–162. doi: 10.1016/s0006-8993(98)00538-1. [DOI] [PubMed] [Google Scholar]
- Stockwell BR. Chemical genetic screening approaches to neurobiology. Neuron. 2002;36:559–562. doi: 10.1016/s0896-6273(02)01056-5. [DOI] [PubMed] [Google Scholar]
- Stockwell BR, Haggarty SJ, Schreiber SL. High-throughput screening of small molecules in miniaturized mammalian cell-based assays involving post-translational modifications. Chem Biol. 1999;6:71–83. doi: 10.1016/S1074-5521(99)80004-0. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Suzdak PD. Serine-O-phosphate has affinity for type IV, but not type I, metabotropic glutamate receptor. Neuroreport. 1993;4:1099–1101. [PubMed] [Google Scholar]
- Tsai RY, McKay RD. A nucleolar mechanism controlling cell proliferation in stem cells and cancer cells. Genes Dev. 2002;16:2991–3003. doi: 10.1101/gad.55671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek AB, Taylor J, DeRuiter SL, Yu JY, Figueroa C, Kwok RP, Turner DL. Akt regulates basic helix-loop-helix transcription factor-coactivator complex formation and activity during neuronal differentiation. Mol Cell Biol. 2003;23:4417–4427. doi: 10.1128/MCB.23.13.4417-4427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Ding S, Ding Q, Gray NS, Schultz PG. A small molecule with osteogenesis-inducing activity in multipotent mesenchymal progenitor cells. J Am Chem Soc. 2002;124:14520–14521. doi: 10.1021/ja0283908. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Yamada K, Furuya S, Mitoma J, Hirabayashi Y, Watanabe M. 3-Phosphoglycerate dehydrogenase, a key enzyme for l-serine biosynthesis, is preferentially expressed in the radial glia/astrocyte lineage and olfactory ensheathing glia in the mouse brain. J Neurosci. 2001;21:7691–7704. doi: 10.1523/JNEUROSCI.21-19-07691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Furuya S, Osuka S, Mitoma J, Shinoda Y, Watanabe M, Azuma N, Tanaka H, Hashikawa T, Itohara S, Hirabayashi Y. Targeted disruption of the mouse 3-phosphoglycerate dehydrogenase gene causes severe neurodevelopmental defects and results in embryonic lethality. J Biol Chem. 2004;279:3573–3577. doi: 10.1074/jbc.C300507200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


