Abstract
Innate immune responses involve the production of antimicrobial peptides (AMPs), chemokines, and cytokines. We report here the identification of B-cell leukemia (Bcl)-3 as a modulator of innate immune signaling in keratinocytes. In this study, it is shown that Bcl-3 is inducible by the Th2 cytokines IL-4 and IL-13 and is overexpressed in lesional skin of atopic dermatitis (AD) patients. Bcl-3 was shown to be important to cutaneous innate immune responses as silencing of Bcl-3 by small-interfering RNA (siRNA) reversed the downregulatory effect of IL-4 on the HBD3 expression. Bcl-3 silencing enhanced vitamin D3 (1,25D3)-induced gene expression of cathelicidin AMP in keratinocytes, suggesting a negative regulatory function on cathelicidin transcription. Furthermore, 1,25D3 suppressed Bcl-3 expression in vitro and in vivo. This study identified Bcl-3 as an important modulator of cutaneous innate immune responses and its possible therapeutic role in AD.
INTRODUCTION
The innate immune system encompasses all mechanisms that enable defense against microbial threats without prior exposure to the pathogen. As key components of the innate immune response, epithelial and non-epithelial cells express several antimicrobial peptides (AMPs) and proteins, including cathelicidins, β-defensins, and several S100 proteins (reviewed in Kolls et al., 2008). These AMPs and antimicrobial proteins share a broad-spectrum antimicrobial activity, but are differently regulated in epidermal keratinocytes. Recent studies have shown that the Th2 type cytokines, IL-4, IL-10, and IL-13, can downregulate AMP expression in atopic dermatitis (AD), and that this phenomena may account for the observed propensity toward skin infections in these patients (Howell et al., 2005, 2006a). Conversely, Th1 type cytokines, including tumor necrosis factor-α (TNF-α), IL-1β and Toll-like receptor (TLR) ligands, predominantly enhance innate immune responses.
Bcl-3 was originally identified as a proto-oncogene in a subgroup of B-cell leukemia (McKeithan et al., 1990), but was later found to be expressed in various cell types, including B and T lymphocytes, erythroblasts, hepatocytes, and keratinocytes (Bours et al., 1993; Zhang et al., 1994, 1998; Brasier et al., 2001; Nishikori et al., 2003; Massoumi et al., 2006). Subsequently, it was shown that Bcl-3 associates with NF-κB p50 or p52 homodimers and is involved in cell proliferation, oncogenesis, and TLR signaling (Westerheide et al., 2001; Rocha et al., 2003; Carmody et al., 2007). Therefore, we hypothesized that Bcl-3 may play a critical role in controlling cutaneous inflammation and host defense responses.
In this study, we show that Bcl-3 acts as a transcriptional modulator of innate immune function of keratinocytes by modulating expression of the AMPs HBD3 and cathelicidin, as well of as the cytokines IL-8 and IL-6. We show that Bcl-3 is inducible by the Th2 type cytokines, IL-4 and IL-13, and is overexpressed in lesional skin from AD patients. Silencing Bcl-3 by siRNA in keratinocytes reversed the downregulatory effect of IL-4 on HBD3 expression. Furthermore, Bcl-3 silencing enhanced 1,25D3-induced cathelicidin AMP expression in keratinocytes, suggesting a negative regulatory function on cathelicidin transcription. 1,25D3 suppressed Bcl-3 expression in vitro and in vivo, suggesting an autoregulatory role of 1,25D3 on Bcl-3 function. Taken together, these observations suggest a regulatory role for Bcl-3 in cutaneous innate host defense function and reveal a possible explanation for altered innate immune responses in AD and other inflammatory skin diseases.
RESULTS
Bcl-3 is induced by IL-4 and IL-13 and is overexpressed in lesional AD skin
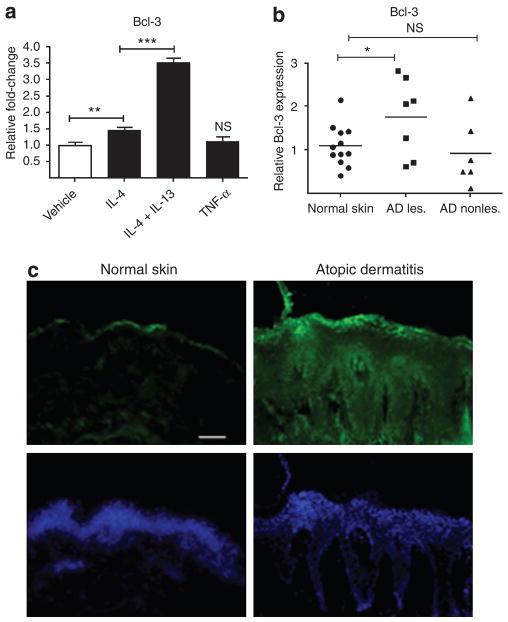
Bcl-3 was recently identified as an important factor of innate immune responses in macrophages (Carmody et al., 2007). Furthermore, Th2 type cytokines have been shown to induce Bcl-3 expression in mast cells, macrophages, and B and T lymphocytes (Richard et al., 1999; Kuwata et al., 2003). On the basis of these findings, we investigated Bcl-3 expression, regulation, and function in human epidermal keratinocytes. In initial studies, the expression of Bcl-3 in cultured primary keratinocytes was evaluated using quantitative real-time PCR and immunofluorescence staining (Figure 1 and data not shown). To investigate whether Th2 type cytokines influence Bcl-3 expression also in human keratinocytes and to test whether this effect is unique to Th2 type cytokines, keratinocytes were exposed to IL-4, the combination of IL-4 and IL-13, or the Th1 type cytokine TNF-α (Figure 1a). Gene expression analyses revealed that Bcl-3 was weakly induced by IL-4, but expression was further enhanced by the combination of the Th2 type cytokines, IL-4 and IL-13, whereas TNF-α had no effect. Immunohistochemistry of cultured keratinocytes revealed that Bcl-3 was abundantly expressed and localized mainly in the cytoplasm of keratinocytes and expression was weakly enhanced upon IL-4 and IL-4/IL-13 treatment. Cytoplasmic localization of Bcl-3 did not significantly change upon IL-4 or TNF-α treatment (data not shown).
Figure 1. Bcl-3 is induced by IL-4 and IL-13 and is expressed in the lesional skin of atopic dermatitis.
(a) Cells were stimulated for 18 hours with IL-4 (50 ng ml−1) and IL-4 (50 ng ml−1) in combination with IL-13 (50 ng ml−1) or TNF-α (20 ng ml−1), and Bcl-3 mRNA abundance was determined by quantitative RT-PCR. (b) Skin biopsy samples from lesional and non-lesional atopic dermatitis (AD) were collected and analyzed for expression levels of Bcl-3 by qPCR. Values of Bcl-3 were normalized to the housekeeping gene GAPDH and data plotted are presented as relative Bcl-3 expression compared with normal skin. (c) Skin biopsy samples from lesional AD and healthy donors were collected and analyzed by immunofluorescence staining. Bcl-3 expression was visualized by an FITC-labelled antibody. AD, atopic dermatitis; les., lesional; non-les., non-lesional. *P<0.05, **P<0.01; ***P<0.001, NS, not significant; unpaired Student’s t-test. Bar=50 μm.
On the basis of the finding that Bcl-3 is inducible by IL-4/IL-13, cytokines that are both elevated in AD, we next sought to investigate whether Bcl-3 levels were changed in AD versus healthy skin. Skin explants were collected from lesional and non-lesional skin from AD patients and the normal skin of healthy subjects and were evaluated for the expression of Bcl-3. As shown in Figure 1b, Bcl-3 mRNA expression was significantly elevated in lesional skin from AD patients compared with the skin from healthy subjects. There was no difference between levels of Bcl-3 in non-lesional AD skin compared with the skin of healthy controls.
To evaluate the protein expression of Bcl-3 in healthy and diseased skin, skin biopsy samples from lesional areas of AD patients and those from healthy subjects were compared by immunohistochemistry (Figure 1c). Data obtained here showed that the protein expression of Bcl-3 was more pronounced in the skin from lesional AD compared with healthy skin. In healthy skin, staining was only observed in the uppermost layers of the epidermis, whereas in AD skin strong fluorescent intensity was found throughout the whole epidermis.
Bcl-3 modifies keratinocyte innate immune responses
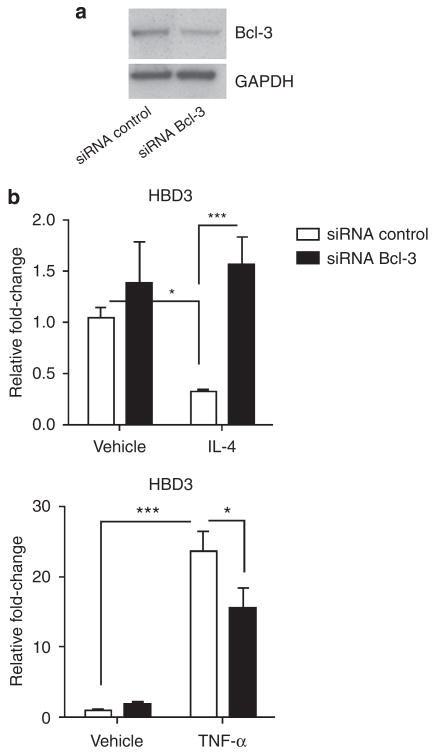
Integrating the findings that AD skin is relatively deficient in AMP expression (Ong et al., 2002), together with our finding that Bcl-3 is overexpressed in AD skin, we next hypothesized that Bcl-3 may directly affect the expression of AMPs and proteins and tested this hypothesis by silencing Bcl-3 by siRNA. Effectiveness of Bcl-3 knockdown was determined by quantitative Real-Time RT-polymerase chain reaction (qPCR) and confirmed at the protein level by western blot analysis and was consistently 50–65% (Figure 2a and data not shown). We first analyzed HBD3, because this AMP is known to be effectively downregulated by Th2 cytokines and has been shown to be upregulated by proinflammatory stimuli, such as TNF-α (Howell et al., 2006b). Suppressing Bcl-3 by siRNA did not significantly affect the basal expression of AMPs (Figure 2b and data not shown). To test whether the suppression of HBD3 gene transcription requires Bcl-3, keratinocytes silenced for Bcl-3 were stimulated with IL-4 or TNF-α, and transcript abundance of HBD3 was evaluated by qPCR (Figure 2b). The HBD3 expression was suppressed in keratinocytes treated with IL-4 in the presence of control siRNA, but this effect was abrogated when Bcl-3 was partially silenced by siRNA, thus indicating that Bcl-3 is required for IL-4-mediated suppression of HBD3 (Figure 2b, upper graph). To test whether the effect of Bcl-3 on HBD3 expression is unique or may be dependent on the cytokine milieu, we also tested whether Bcl-3 was required for TNF-α-induced HBD3 expression. Silencing Bcl-3 resulted in a moderate down-regulation of TNF-α-induced HBD3 expression (Figure 2b, lower graph), thus showing that the effect of Bcl-3 on HBD3 expression is dependent on external stimuli.
Figure 2. Bcl-3 is required for the IL-4-mediated downregulation of HBD3.
(a) Efficiency of siRNA silencing was evaluated by western blot analysis of Bcl-3 expressed by keratinocytes. (b) Stimulation with IL-4 (50 ng ml−1) significantly reduced basal human HBD3 expression in keratinocytes. Silencing of Bcl-3 by siRNA decreased this effect (upper graph). TNF-α induced (20 ng ml−1) HBD3 expression is moderately suppressed by siBcl-3. *P<0.05, ***P<0.001, unpaired Student’s t-test. Data shown are means (±SEM) of results from one representative experiment performed in triplicates.
Next, we investigated whether cathelicidin AMP expression is regulated by Bcl-3. Cathelicidin is expressed at low levels in healthy skin but is highly upregulated in states of injury, inflammation, or infection. Similar to HBD3, cathelicidin expression is lower in skin from AD patients compared with that from psoriatic patients. Recently, 1,25D3 has been identified as a potent inducer of cathelicidin in human keratinocytes, whereas stimulation with proinflammatory cytokines alone, such as TNF-α or interferons, is not capable of upregulating cathelicidin transcription in keratinocytes. On account of these findings, the role of vitamin D as an important target for therapy for cutaneous diseases has been revisited (Hata and Gallo, 2008).
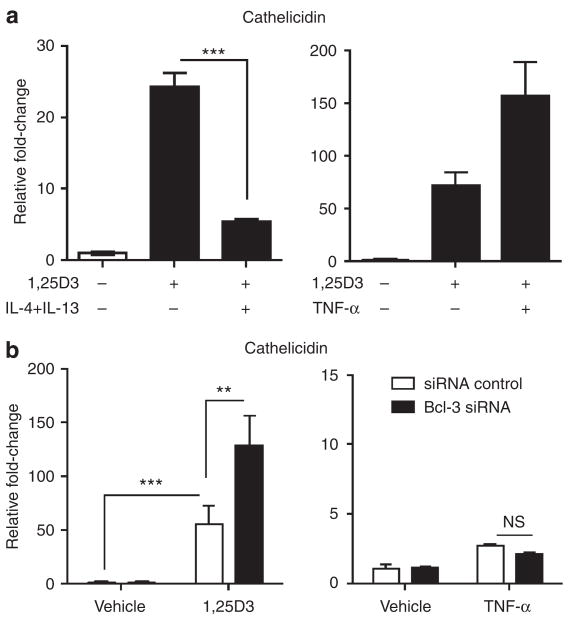
We sought to investigate whether IL-4/IL-13 pretreatment of keratinocytes, leading to an increase in Bcl-3, would subsequently alter the capacity of 1,25D3 to induce cathelicidin (Figure 3a). Keratinocytes were pretreated with IL-4/IL-13 for 24 hours before exposure to 1,25D3. Keratinocytes that were pretreated with IL-4/IL-13 showed impaired upregulation of cathelicidin gene expression by 1,25D3 compared with vehicle-pretreated keratinocytes (Figure 3a, left graph). Conversely, pretreatment of keratinocytes with TNF-α did not reduce 1,25D3-induced cathelicidin expression but rather enhanced its expression (Figure 3a, right graph). To define the role of Bcl-3 signaling in cathelicidin regulation, keratinocytes were silenced for Bcl-3 by siRNA and were stimulated with 1,25D3. Silencing Bcl-3 did not alter basal cathelicidin expression (Figure 3b). As shown earlier, 1,25D3 strongly induced cathelicidin gene transcription and this induction was enhanced by Bcl-3 silencing, suggesting that Bcl-3 acts as a negative regulator of 1,25D3-mediated cathelicidin expression in human keratinocytes (Figure 3b, left graph). TNF-α alone did not induce cathelicidin transcription and this was not altered by silencing Bcl-3 (Figure 3b, right graph).
Figure 3. Bcl-3 silencing enhances cathelicidin expression induced by 1,25D3.
(a) Cultured human keratinocytes were exposed to IL-4 (50 ng ml−1) and IL-13 (50 ng ml−1) for 24 hours before stimulation with 1,25D3 (10 nM). After 6 hours of 1,25D3 stimulation, cells were harvested and mRNA expression of cathelicidin was determined by qPCR (left graph). As control, keratinocytes were also pretreated with TNF-α (20 ng ml−1) and stimulated with 1,25D3 (10 nM, right graph). Data shown are means (±SD) of results from single experiments performed in triplicates and are representative of at least two independent experiments. (b) Treatment of keratinocytes with siRNA to Bcl-3, enhances cathelicidin mRNA expression after induction by 1,25D3 (10 nM, left graph), whereas Bcl-3 siRNA has no effect on cathelicidin expression with or without TNF-α (20 ng ml−1, right graph). Data shown are means (±SEM) of results from single experiments performed in triplicates.
p50 decoy enhances 1,25D3-mediated cathelicidin expression
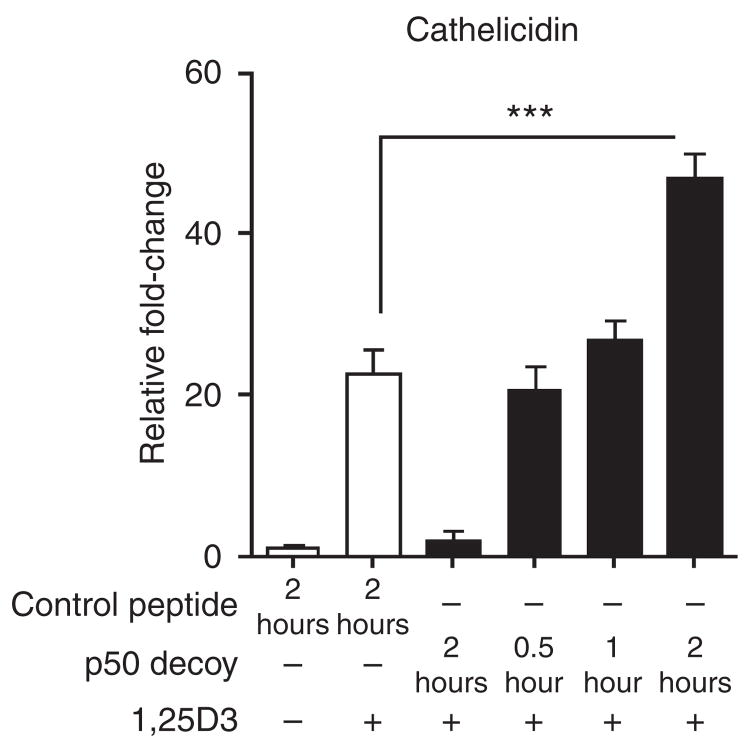
Earlier it has been shown that Bcl-3 interacts with p50 homodimers of the NF-κB pathway. To test whether p50 blocking results in similar effects compared with Bcl-3 silencing, and would thereby mimic the mechanism used by Bcl-3 to regulate cathelicidin expression, we used p50 decoy and control peptides. Keratinocytes were pretreated for 0.5, 1, or 2 hours with 1 μM of p50 decoy or control peptides before stimulation with 1,25D3. After 2 hours of pretreatment with a p50 decoy, 1,25D3-mediated cathelicidin induction was significantly enhanced (Figure 4). These results show that blocking of p50 enhances 1,25D3-mediated cathelicidin induction, thus further implying that Bcl-3 may suppress the capacity of 1,25D3 to increase AMP expression in keratinocytes.
Figure 4. p50 decoy enhances 1,25D3-mediated cathelicidin expression.
Keratinocytes were treated with p50 decoy (1 μM) or a control peptide (1 μM) for 0.5, 1, or 2 hours and were then stimulated with 1,25D3 (1 nM) for 4 hours. Cathelicidin expression was measured by qPCR. ***P<0.001, unpaired Student’s t-test.
Vitamin D decreases the expression of Bcl-3
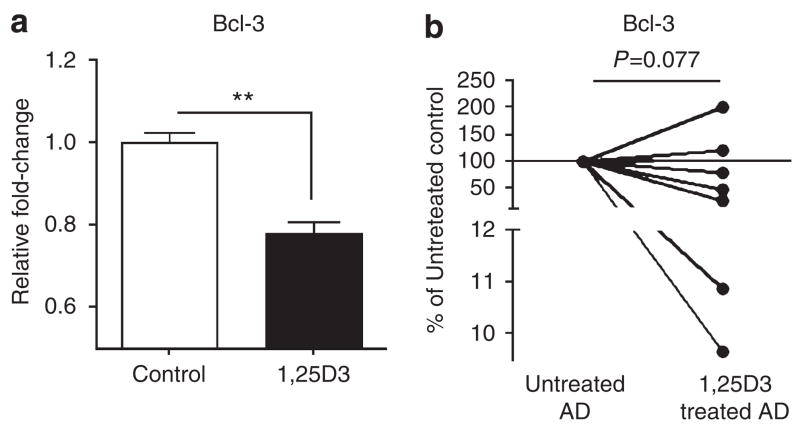
It was recently shown that oral vitamin D3 administration enhances cutaneous cathelicidin expression in lesional skin of individuals with AD (Hata and Gallo, 2008). Therefore, we next analyzed whether 1,25D3 in vitro, or oral administration of vitamin D to patients, could also affect the Bcl-3 expression. Stimulation of keratinocytes with 1,25D3 led to a moderate but statistically significant decrease of the Bcl-3 expression (Figure 5a). Furthermore, oral administration of 1,25D3 also caused a decrease of Bcl-3 in lesional AD in most patients tested (Figure 5b).
Figure 5. 1,25D3 suppresses Bcl-3 expression in keratinocytes.
(a) Cultured keratinocytes treated with 1,25D3 (10 nM) for 24 hours show decreased Bcl-3 expression. **P<0.01, NS, not significant, unpaired Student’s t-test. (b) Atopic dermatitis patients (AD) received orally 1,25D3 as described in the Materials and Methods section. Bcl-3 transcript expression was measured in skin biopsy samples taken from lesional skin from AD patients. Each dot represents one patient. The mean values of untreated AD (n=7) were set as 100% and Bcl-3 levels in 1,25D3-treated AD are presented as percentage (%) of the untreated control. P=0.077, paired Student’s t-test.
Bcl-3 expression enhances stimulation of cytokine production
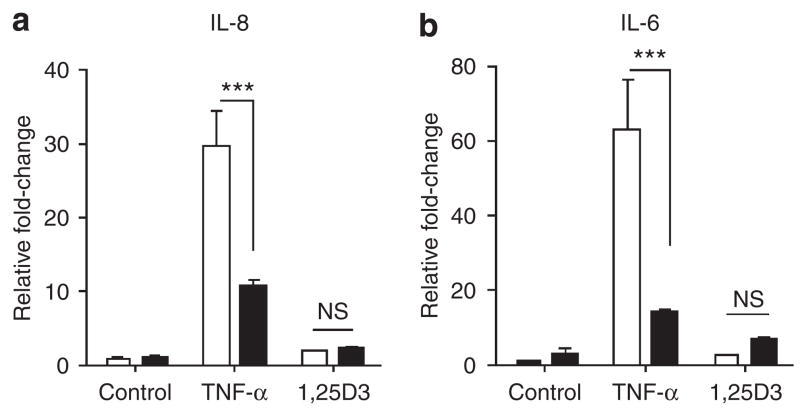
As AD is a complex chronic inflammatory skin disease, it was also of interest to analyze how Bcl-3 influences the production of inflammatory cytokines. To test this, the expressions of CXCL8/IL-8 and IL-6 were measured in keratinocytes that were stimulated with TNF-α or 1,25D3 and also silenced for Bcl-3. TNF-α-induced IL-8 and IL-6 expressions were significantly downregulated by Bcl-3 silencing, thus showing that this pathway is positively controlled by Bcl-3 abundance (Figure 6). As expected, stimulation with 1,25D3 did not alter IL-8 or IL-6 levels, and this did not change upon treatment with siRNA targeting Bcl-3.
Figure 6. Bcl-3 regulates the expressions of IL-6 and IL-8.
Keratinocytes transfected with siRNA oligonucleotides for Bcl-3 were stimulated with (a) TNF-α (20 ng ml−1) or (b) 1,25D3 (10 nM), and IL-8 and IL-6 expressions was measured using real-time RT-PCR. Data shown are means (±SEM) of results from a representative experiment performed in triplicates. ***P<0.001, NS, not significant, unpaired Student’s t-test. Open bar, siRNA control; solid bar, Bcl-3 siRNA.
DISCUSSION
The epidermis is an active immunological barrier providing powerful protection against the invasion of microbes. AMPs are an integral part of this innate immune response, and they are effective in killing bacterial, viral, and other pathogens. In normal skin, expression of these peptides is relatively low, but they accumulate during wound healing and infectious or inflammatory diseases (Ong et al., 2002; Schauber et al., 2007; Yamasaki et al., 2007). Unfortunately, expression of the AMPs, cathelicidin and HBD3, is significantly less in lesional skin from patients with AD compared with other inflammatory skin disorders such as psoriasis (Ong et al., 2002). This phenomenon is believed to contribute to the higher susceptibility to severe skin infections seen in AD patients, most commonly caused by Staphylococcus aureus and vaccinia virus, whereas psoriasis patients rarely suffer from cutaneous superinfections. It is not clear why patients with AD express lower AMPs than expected, but the Th2 type cytokines, such as IL-4, IL-10, and IL-13, which are abundant in AD skin, have been shown to inhibit expression of some AMPs (reviewed in Bieber, 2008). Here, we provide further explanation for the potential decrease in AMP expression in AD and show that Bcl-3, a recently identified element of the NF-κB signaling pathway, is increased in lesional skin from AD patients compared with normal skin and induced by Th-2 cytokines.
The transcriptional regulation of Bcl-3 itself is currently poorly understood. Studies on the human Bcl-3 promoter are very limited. However, sequencing of the murine Bcl-3 promoter revealed IL-4 specifically induced AP1 and AP1-like binding activity and mutation of these binding sites abolished IL-4-induced Bcl-3 promoter activity, suggesting that these transcription factors are important in Bcl-3 promoter transactivation (Rebollo et al., 2000). To test whether the same transcription factors are responsible for Th2 cytokine-induced Bcl-3 expression in humans, further studies are needed. Here, we have shown that IL-4 and IL-13 appear to act together to induce Bcl-3. This synergy may arise from receptor interaction: in non-lymphoid cells, the IL-4 receptor (IL-4R) is composed of the IL-4Rα chain and the IL-13Rα1 or IL-13Rα2 chains, explaining, at least in part, why IL-13 shares several biological activities with IL-4 (Vita et al., 1995). Moreover, it has been shown that IL-13 and IL-4 can induce IL-13Rα2 (David et al., 2001), suggesting an autocrine loop for IL-4/IL-13 effects.
In our study, we showed that Bcl-3 acts as a potential transcriptional modulator of keratinocyte immune responses. Bcl-3 was required for IL-4-mediated downregulation of HBD3 expression, and Bcl-3 negatively regulated TNF-α-induced HBD3, IL-8, and IL-6 expressions. The effects on the expression of cathelicidin by keratinocytes was more complex, as classical activators of the innate immune response, such as TNF-α and TLR ligands, are alone ineffective in stimulating cathelicidin AMP expression in keratinocytes. In contrast, we and others have recently identified 1,25D3 as a strong inducer of cathelicidin expression in humans. 1,25D3 induces cathelicidin owing to a vitamin D-responsive element in the human cathelicidin promoter and through the modification of keratinocyte TLR2 and CD14 expression levels (Schauber et al., 2007). Silencing Bcl-3 resulted in the enhancement of 1,25D3-induced cathelicidin expression, thus implying a negative regulatory role for Bcl-3 in cathelicidin expression. 1,25D3 stimulation also led to a modest decrease of Bcl-3 transcripts in cultured keratinocytes, suggesting that an autoregulatory mechanism of Bcl-3 function may be involved. Furthermore, we show here that TNF-α together with 1,25D3 led to a higher expression of cathelicidin than 1,25D3 stimulation alone. As silencing of Bcl-3 and stimulation with TNF-α alone did not induce cathelicidin transcription, this suggests that activation of the cathelicidin vitamin D-responsive element by 1,25D3 is crucial for cathelicidin induction in human keratinocytes.
Mechanisms accounting for the activities of Bcl-3 are just beginning to be understood. Bcl-3 interacts with p50 NF-κB homodimers; however, its specific action is still controversial. It has been reported that Bcl-3 acts as a negative regulator of TLR signaling in macrophages through p50 ubiquitination blockade (Carmody et al. 2007), whereas in other studies, it has been shown that Bcl-3 facilitates NF-κB-mediated transactivation by removing inhibitory p50 homodimers from selected κB sites and thereby enhancing NF-κB activity (Franzoso et al., 1992; Bours et al., 1993; Fujita et al., 1993; Hayden and Ghosh, 2008). In this study using primary keratinocytes, effects mediated by Bcl-3 appear to be dependent on both the target gene tested and stimuli used to induce antimicrobial and cytokine responses. Furthermore, using a p50 decoy, effects similar to Bcl-3 silencing were observed in keratinocytes treated with 1,25D3, indicating that regulation of cathelicidin expression may involve blocking p50 subunits. A transcription activation domain necessary for the positive regulation of gene expression is present only in p65, c-Rel, and RelB. As they lack transcription activation domains, p50 and p52 may repress transcription unless associated with a transcription activation domain-containing NF-κB family member or other proteins capable of co-activator recruitment (Chen and Greene, 2004). Constitutive binding of p50 or p52 homodimers to κB sites on NF-κB-responsive promoters may thus act to check NF-κB transactivation until displaced by transcriptionally competent NF-κB dimers. In addition, negative regulatory elements within promoter regions, as have been discovered for human cathelicidin, may impact the tight regulation of gene transcription (Elloumi and Holland, 2008). Their study showed that the cathelicidin promoter region contains a negative regulatory element; however, the exact element was not identified (Elloumi and Holland, 2008). Our initial studies investigating the human cathelicidin promoter region identified various putative NF-κB binding/p50 sites (data not shown).
Several studies recently uncovered an important role for 1,25D3 in innate host defense: 1,25D3 is a potent inducer of the cathelicidin AMP expression (Wang et al., 2004). In response to TLR2/6 or TLR2/1 activation or injury, vitamin D3 metabolism is altered, and subsequently the active form of 1,25D3 is generated to serve as a signaling molecule in innate immune responses in keratinocytes and monocytes, respectively (Liu et al., 2007; Schauber et al., 2007). 1,25D3 directly affects NF-κB activity by inhibiting de novo synthesis of the NF-κB p50 protein and its precursor p105 in human lymphocytes (Yu et al., 1995) and by downregulating c-Rel and RelB in dendritic cells (Xing et al., 2002). It is not known yet whether this action of 1,25D3 is important for cathelicidin induction, but it is intriguing to speculate that silencing NF-κB signaling molecules may enhance cathelicidin transcription. Interestingly, inducers of cathelicidin AMP in colonic cells, the histone deacetylate inhibitor butyrate and trichostatin A, also inhibit NF-κB (Schauber et al., 2003, 2004). Moreover, calcineurin inhibitors, shown to suppress p105/p50 nuclear translocation, enhance the 1,25D3-induced cathelicidin expression in keratinocytes (Buchau et al., 2008).
Bcl-3 associates exclusively with NF-κB p50 or p52 homodimers. In this study, we found that Bcl-3 silencing enhanced the 1,25D3-induced cathelicidin expression, thus showing that Bcl-3 signaling is important to 1,25D3-mediated cathelicidin regulation in human keratinocytes. Blocking p50 by a p50 decoy resulted in an increase in the 1,25D3-mediated cathelicidin expression, thus suggesting that it is blocking p50 that enhances cathelicidin expression and may explain a possible mechanism used by Bcl-3. However, further studies are needed to confirm this hypothesis.
Interestingly, orally administered vitamin D resulted in the downregulation of Bcl-3 expression in lesional AD skin in five out of seven patients tested. In light of the observation that Bcl-3 can decrease the cathelicidin expression, these data are consistent with our recent finding that oral administration of vitamin D3 increases the cathelicidin expression in lesional skin from AD patients (Hata and Gallo, 2008). Taken together, these data indicate that Bcl-3 signaling plays a crucial role in 1,25D3-associated immune responses in AD, and that the effects observed with vitamin D may be due to direct action on the cathelicidin promoter or indirect action by suppressing the inhibitory action of Bcl-3. Further clinical trials are needed to confirm the efficacy of vitamin D as a therapeutic agent, its effects on the Bcl-3 expression, and to determine to what extent it may also be acting to inhibit Th2 cytokines. Nevertheless, our findings presented here reveal an important and complex role for Bcl-3 in regulating innate immune responses in the skin.
Bcl-3 and its deubiquitinase Cyld play important roles in chemical-induced skin carcinogenesis and cylindromatosis (Massoumi et al., 2006). To our knowledge it is previously unreported that, Bcl-3 is upregulated in AD, one of the most common chronic inflammatory skin diseases. Whether other inflammatory skin diseases or skin malignancies show altered levels of Bcl-3 is currently unknown and will be vital information for defining the role of Bcl-3 in a variety of cutaneous diseases.
Taken together, the study presented here defines a previously unknown element regulating immune function of keratinocytes and establishes Bcl-3 as a potential therapeutic target in the treatment of AD. On the basis of these observations, it will be important to investigate whether disturbed antimicrobial defense in the skin of patients with other disorders characterized by altered innate immune responses show changes in Bcl-3, and may therefore benefit from therapy targeting the Bcl-3 pathway.
MATERIALS AND METHODS
Cell culture and stimuli
Normal human epidermal keratinocytes were grown in serum-free EpiLife cell culture media (Cascade Biologics, Portland, OR) containing 0.06mM Ca2+ and 1 × EpiLife Defined Growth Supplement at 37 °C under standard tissue culture conditions. The cultures were maintained for up to four passages in this media with the addition of 50Uml−1 penicillin and 50 μgml−1 streptomycin. Cells at 60–80% confluence were stimulated with IL-4 (50 ng ml−1, R&D Systems, Minneapolis, MN), IL-13 (50 ng ml−1, R&D Systems), TNF-α (20 ng ml−1, R&D Systems), 1,25D3 (1 or 10 nM, Sigma, St. Louis, MO). NF-κB p50 (NLS) inhibitor and control peptide were purchased from Imgenex (San Diego, CA) and used at a concentration of 1 μM. P50 NLS sequence: DRQIKIWFQNRRMKWKKVQRKRQKLM (p50 NLS sequence is underlined). The inhibitory mechanism relies on the function as a p50 decoy by blocking the intracellular recognition mechanisms of p50 NLS. These peptides are cell permeable through a protein transduction sequence from antennapedia.
Skin samples
All studies were approved by the Human Research Protection program at the University of California, San Diego, and were in adherence to the Declaration of Helsinki Principles. For all procedures, written informed consent from donors was obtained. Skin biopsy samples were obtained from lesional and non-lesional skin of AD patients and from matching sites of healthy donors. For a subsequent experiment, AD patients received 4000 IU day−1 over 21 days, and skin biopsy samples were taken as described earlier (Hata and Gallo, 2008). All samples were stored at −80 °C until use.
Real-time RT-PCR (qPCR)
Total RNA from skin samples and cultured keratinocytes was extracted using Trizol (Invitrogen, Carlsbad, CA) and 1 μg RNA was reverse-transcribed using iScript (Bio-Rad, Hercules, CA). The expression of cathelicidin was evaluated using a FAM-CAGAGGATTGTGACTTCA-MGB probe with primers 5′-CTTCACCAGCCCGTCCTTC-3′ and 5′-CCAGGACGACACAGCAGTCA-3′. For GAPDH expression, a VIC-CATCCATGACAACTTTGGTA-MGB probe with primers 5′-CTTAGCACCCCTGGCCAAG-3′ and 5′-TGGTCATGAGTCCTTCCACG-3′ was used as described (Schauber et al., 2006). qPCR efficiencies for cathelicidin and GAPDH primers and probes were optimized as published earlier (Schauber et al., 2006). Predeveloped Taqman assay probes (Applied Biosystems ABI, Foster City, CA) were used for the analyses of the expressions of Bcl-3, HBD3, IL-6, and IL-8. All analyses were performed in triplicate from two to five independent cell stimulation experiments in an ABI Prism 7000 Sequence detection system. Fold induction relative to the control was calculated using the 2(−ΔΔCt) method, where ΔCt is the ΔCt(stimulant/diseased skin) − ΔCt(vehicle/control skin), ΔCt is Ct(target) − Ct(GAPDH), and Ct is the cycle at which the detection threshold is crossed. Results were considered significant when statistical analysis revealed P<0.05.
Fluorescence immunohistochemistry
Keratinocytes were grown on chamber slides. After methanol fixation and subsequent washings in phosphate-buffered saline (PBS), the slides were blocked in 3% BSA in PBS for 30 minutes at room temperature and stained with a rabbit anti-Bcl-3 antibody or preimmune serum. After washings in PBS, the slides were reprobed with a FITC-labelled goat anti-rabbit antibody. After subsequent washings with PBS, the slides were mounted in ProLong Anti-Fade reagent containing 4′,6-diamidino-2-phenylindole (Molecular Probes, Eugene, OR) and evaluated with an Olympus BX41 microscope (Olympus, Melville, NY) at original magnification of × 400.
siRNA and transfection
Second or third passage keratinocytes were maintained in serum-free keratinocyte growth medium (Cascade Biologics) containing 10 ng ml−1 human recombinant epidermal growth factor (half the dose compared with Cascade Biologics’s standard composition). Keratinocytes were transfected with 20 nM siRNA oligonucleotides using siLentFect (Bio-Rad) for 18–22 hours. siRNA oligonucleotides for Bcl-3 (Santa Cruz Biotechnology, Santa Cruz, CA) and a non-targeted control siRNA (Santa Cruz Biotechnology) were used. Bcl-3 siRNA is a pool of three target specific 20–25 nt siRNAs designed to knock down gene expression (sequences: sense strand (A): AGACACGCCUCUCCAUAUUTT mRNA loc. 422, sense strand (B): ACAUGCACCUACCCAUACATT mRNA loc. 1519, and sense strand (C): ACAUCUUCCAUUUCCAUGUTT mRNA loc. 1635). The efficiency of blockage was evaluated by real-time RT-PCR and western blot analysis. Blockage efficiency in all experiments was 50–65% for siBcl-3 compared with that for baseline. After transfection, cells were washed with PBS and then stimulated. These experiments were repeated using at least two different batches of primary keratinocytes.
Western blot
Cultured keratinocytes were subsequently lysed in ice-cold RIPA buffer containing proteinase inhibitors (Roche, Indianapolis, IN). After centrifugation, equal amounts of protein were mixed with loading buffer (0.25M Tris-HCl, 10% SDS, 10% glycerol, 5% β-mercaptoethanol) and loaded onto a 16% Tris-Tricine gel (GeneMate, Kaysville, UT). After separation, proteins were blotted onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA) and blocked in 5% milk (Bio-Rad) in TBS 0.1% Tween 20 for 1 hour at room temperature. After washings in tris buffered saline (TBS) 0.1% Tween 20, the membranes were stained with a rabbit anti-Bcl-3 antibody, washed again in TBS 0.1% Tween 20, and reprobed with a horseradish peroxidase-coupled goat anti-rabbit antibody (DakoCytomation, Glostrup, Denmark). Stained protein was visualized using the Western Lightning system (PerkinElmer, Boston, MA). Densitometric analyses were performed using ImageJ (version 1.37r).
Acknowledgments
We thank Yuko Oda from the Department of Medicine (Endocrine Unit, Veterans Affairs Medical Centre, University of California San Francisco) for advice in siRNA technology. We thank Dr Jürgen Schauber, Dr Mark Peric, and Sarah Koglin from the Department of Dermatology (Ludwig-Maximilians-University-Munich, Munich, Germany) for help and technical assistance. This work was supported by a VA Merit award and NIH grants, AI052453, and AR45676 to R.L.G. and a grant from The Deutsche Forschungsgemeinschaft (DFG) to A.S.B (Bu 2212/1-1).
Abbreviations
- (Bcl)-3
B-cell leukemia
- 1,25D3
vitamin D3
- AD
atopic dermatitis
- AMP
antimicrobial peptide
- HBD
human β-defensin
- PBS
phosphate-buffered saline
- siRNA
small-interfering RNA
- TLR
Toll-like receptor
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Bieber T. Atopic Dermatitis. N Engl J Med. 2008;358:1483–94. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- Bours V, Franzoso G, Azarenko V, Park S, Kanno T, Brown K, et al. The oncoprotein Bcl-3 directly transactivates through kappa B motifs via association with DNA-binding p50B homodimers. Cell. 1993;72:729–39. doi: 10.1016/0092-8674(93)90401-b. [DOI] [PubMed] [Google Scholar]
- Brasier AR, Lu M, Hai T, Lu Y, Boldogh I. NF-kappa B-inducible BCL-3 expression is an autoregulatory loop controlling nuclear p50/NF-kappa B1 residence. J Biol Chem. 2001;276:32080–93. doi: 10.1074/jbc.M102949200. [DOI] [PubMed] [Google Scholar]
- Buchau AS, Schauber J, Hultsch T, Stuetz A, Gallo RL. Pimecrolimus enhances TLR2/6-induced expression of antimicrobial peptides in keratinocytes. J Invest Dermatol. 2008;128:2646–54. doi: 10.1038/jid.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody RJ, Ruan Q, Palmer S, Hilliard B, Chen YH. Negative regulation of toll-like receptor signaling by NF-kappaB p50 ubiquitination blockade. Science. 2007;317:675–8. doi: 10.1126/science.1142953. [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- David M, Ford D, Bertoglio J, Maizel AL, Pierre J. Induction of the IL-13 receptor alpha2-chain by IL-4 and IL-13 in human keratinocytes: involvement of STAT6, ERK and p38 MAPK pathways. Oncogene. 2001;20:6660–8. doi: 10.1038/sj.onc.1204629. [DOI] [PubMed] [Google Scholar]
- Elloumi HZ, Holland SM. Complex regulation of human cathelicidin gene expression: novel splice variants and 5′UTR negative regulatory element. Mol Immunol. 2008;45:204–17. doi: 10.1016/j.molimm.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G, Bours V, Park S, Tomita-Yamaguchi M, Kelly K, Siebenlist U. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-kappa B-mediated inhibition. Nature. 1992;359:339–42. doi: 10.1038/359339a0. [DOI] [PubMed] [Google Scholar]
- Fujita T, Nolan GP, Liou HC, Scott ML, Baltimore D. The candidate proto-oncogene bcl-3 encodes a transcriptional coactivator that activates through NF-kappa B p50 homodimers. Genes Dev. 1993;7:1354–63. doi: 10.1101/gad.7.7b.1354. [DOI] [PubMed] [Google Scholar]
- Hata TR, Gallo RL. Antimicrobial peptides, skin infections, and atopic dermatitis. Semin Cutan Med Surg. 2008;27:144–50. doi: 10.1016/j.sder.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Howell MD, Boguniewicz M, Pastore S, Girolomoni G, Leung DY. Mechanism of HBD-3 deficiency in atopic dermatitis. Clin Immunol. 2006a;121:332–8. doi: 10.1016/j.clim.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006b;24:341–8. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Howell MD, Novak N, Bieber T, Pastore S, Girolomoni G, Boguniewicz M, et al. Interleukin-10 downregulates anti-microbial peptide expression in atopic dermatitis. J Invest Dermatol. 2005;125:738–45. doi: 10.1111/j.0022-202X.2005.23776.x. [DOI] [PubMed] [Google Scholar]
- Kolls JK, McCray PB, Jr, Chan YR. Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol. 2008;8:829–35. doi: 10.1038/nri2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwata H, Watanabe Y, Miyoshi H, Yamamoto M, Kaisho T, Takeda K, et al. IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-alpha production in macrophages. Blood. 2003;102:4123–9. doi: 10.1182/blood-2003-04-1228. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125:665–77. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- McKeithan TW, Ohno H, Diaz MO. Identification of a transcriptional unit adjacent to the breakpoint in the 14;19 translocation of chronic lymphocytic leukemia. Genes Chromosomes Cancer. 1990;1:247–55. doi: 10.1002/gcc.2870010310. [DOI] [PubMed] [Google Scholar]
- Nishikori M, Maesako Y, Ueda C, Kurata M, Uchiyama T, Ohno H. High-level expression of BCL3 differentiates t(2;5)(p23;q35)-positive anaplastic large cell lymphoma from Hodgkin disease. Blood. 2003;101:2789–96. doi: 10.1182/blood-2002-08-2464. [DOI] [PubMed] [Google Scholar]
- Ong PY, Hamid QA, Travers JB, Strickland I, Al Kerithy M, Boguniewicz M, et al. Decreased IL-15 may contribute to elevated IgE and acute inflammation in atopic dermatitis. J Immunol. 2002;168:505–10. doi: 10.4049/jimmunol.168.1.505. [DOI] [PubMed] [Google Scholar]
- Rebollo A, Dumoutier L, Renauld JC, Zaballos A, Ayllon V, Martinez AC. Bcl-3 expression promotes cell survival following interleukin-4 deprivation and is controlled by AP1 and AP1-like transcription factors. Mol Cell Biol. 2000;20:3407–16. doi: 10.1128/mcb.20.10.3407-3416.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M, Louahed J, Demoulin JB, Renauld JC. Interleukin-9 regulates NF-kappaB activity through BCL3 gene induction. Blood. 1999;93:4318–27. [PubMed] [Google Scholar]
- Rocha S, Martin AM, Meek DW, Perkins ND. p53 represses cyclin D1 transcription through down regulation of Bcl-3 and inducing increased association of the p52 NF-kappaB subunit with histone deacetylase 1. Mol Cell Biol. 2003;23:4713–27. doi: 10.1128/MCB.23.13.4713-4727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of innate antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunol. 2006;118:509–19. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Iffland K, Frisch S, Kudlich T, Schmausser B, Eck M, et al. Histone-deacetylase inhibitors induce the expression of the cathelicidin LL-37 in human gastrointestinal cells. Mol Immunol. 2004;41:847–54. doi: 10.1016/j.molimm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Schauber J, Svanholm C, Termen S, Iffland K, Menzel T, Scheppach W, et al. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:735–41. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita N, Lefort S, Laurent P, Caput D, Ferrara P. Characterization and comparison of the interleukin 13 receptor with the interleukin 4 receptor on several cell types. J Biol Chem. 1995;270:3512–7. doi: 10.1074/jbc.270.8.3512. [DOI] [PubMed] [Google Scholar]
- Wang T-T, Nestel F, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Mayo MW, Anest V, Hanson JL, Baldwin AS., Jr The putative oncoprotein Bcl-3 induces cyclin D1 to stimulate G(1) transition. Mol Cell Biol. 2001;21:8428–36. doi: 10.1128/MCB.21.24.8428-8436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing N, Maldonado ML, Bachman LA, McKean DJ, Kumar R, Griffin MD. Distinctive dendritic cell modulation by vitamin D(3) and glucocorticoid pathways. Biochem Biophys Res Commun. 2002;297:645–52. doi: 10.1016/s0006-291x(02)02262-3. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- Yu XP, Bellido T, Manolagas SC. Down-regulation of NF-kappa B protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc Natl Acad Sci USA. 1995;92:10990–4. doi: 10.1073/pnas.92.24.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MY, Harhaj EW, Bell L, Sun SC, Miller BA. Bcl-3 expression and nuclear translocation are induced by granulocyte–macrophage colony-stimulating factor and erythropoietin in proliferating human erythroid precursors. Blood. 1998;92:1225–1234. [PubMed] [Google Scholar]
- Zhang Q, Didonato JA, Karin M, McKeithan TW. BCL3 encodes a nuclear protein which can alter the subcellular location of NF-kappa B proteins. Mol Cell Biol. 1994;14:3915–26. doi: 10.1128/mcb.14.6.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]