Abstract
Fully synthetic, carbohydrate-based antitumor vaccine candidates have been synthesized in highly clustered modes. Multiple copies of tumor-associated carbohydrate antigens, Tn and STn, were assembled on a single cyclic peptide scaffold in a highly convergent manner. Ring-closing metathesis-mediated incorporation of an internal cross-linker was also demonstrated. In particular, this rigidified cross-linked construct would enhance a cluster-recognizing antibody response by retaining an appropriate distance between glycans attached to the peptide platform. Details of the design and synthesis of highly clustered antigens are described herein.
Introduction
Our laboratory is seeking the development of novel, fully synthetic carbohydrate-based vaccines for the treatment of cancer. This research program is based on the finding that malignantly transformed cells often exhibit significant alteration in the nature and quantity of carbohydrates presented on their cell surfaces, either as glycosphingolipids or as glycoproteins.1 Presumably, when properly introduced to the immune system, a tumor-associated carbohydrate-based antigen could set into motion an exploitable immune response, leading to the generation of antibodies which would selectively bind to and hopefully eliminate those tumor cells which over-express the carbohydrates in question. We are certainly not alone in this area. In particular, impressive advances of carbohydrate based anticancer vaccines have been reported by Boons,2 Kunz,3 and Schmidt.4 The viability of this carbohydrate vaccine concept has been confirmed experimentally in our laboratory. Thus, when these tumor-associated antigens are presented to the immune system as glycoconjugates appended to immunogenic carrier molecules (such as KLH carrier protein)5 and co-administered with an immunological adjuvant (such as QS21),6 a carbohydrate-specific antibody response may be induced. A number of complex, fully synthetic carbohydrate-based constructs, synthesized in our laboratories, have shown significant promise in preclinical, and even clinical settings.7 In designing a carbohydrate-based vaccine construct, it may be of considerable value to consider the way in which the antigen is displayed in its natural environment, i.e. on the surface of the transformed cell, to more directly mimic this natural setting in the context of the vaccine.
Along these lines, we have taken note of the mucin-bound carbohydrate antigens, Tn and STn, which are over-expressed on the surfaces of a variety of epithelial cancers, such as prostate, breast, colon, and ovarian.8 On the tumor cell surface, Tn and STn are presented in broadly conserved “clusters” of 2–4 carbohydrate units, O-linked to the mucin peptide through serine or threonine residues.
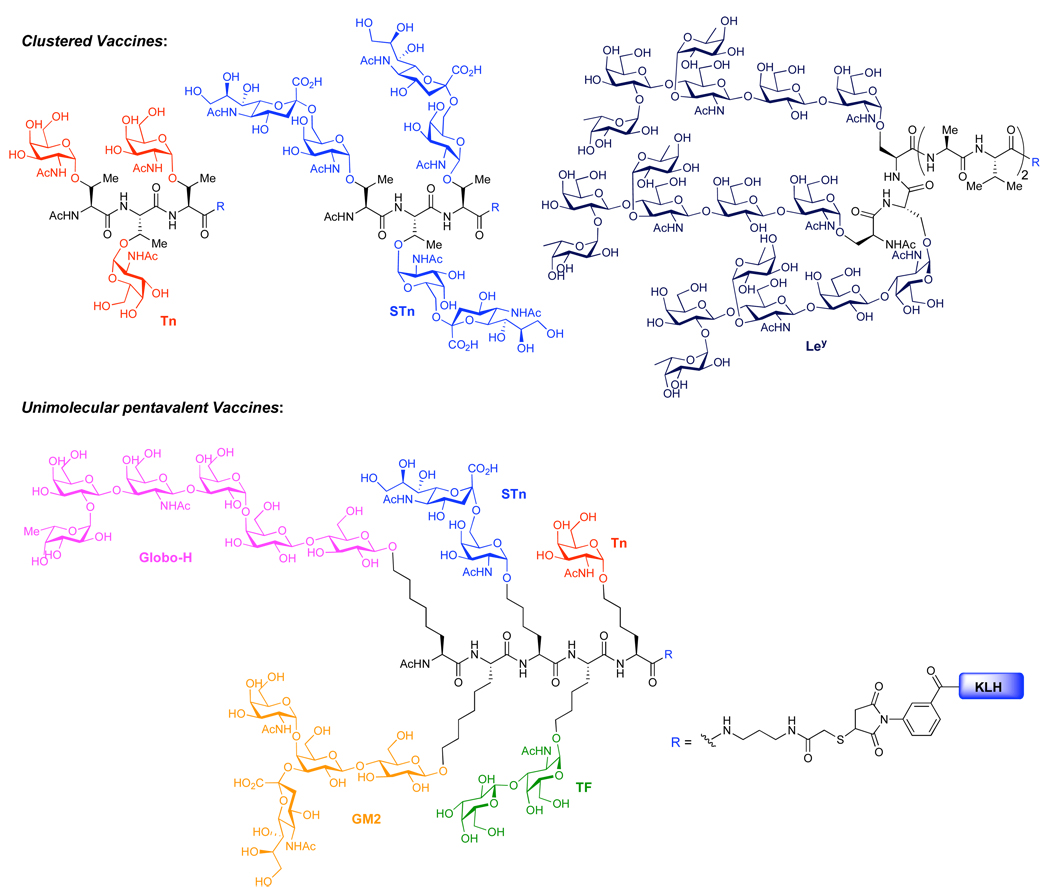
Approaches using monomeric Tn or STn antigen, in which one glycan unit is covalently appended to an immunogenic carrier protein, have proven beneficial.9 However, it has been shown that clustered vaccines – wherein multiple copies of the carbohydrate are incorporated on a peptide backbone (Figure 1) –induce higher titers against some carbohydrate epitopes.10a Indeed, recent studies with the antitumor monoclonal antibody (MAb) B72.3 revealed that it preferentially recognized clustered STn in preference to monovalent STn.9a,10b–c
Figure 1.
Representative anticancer vaccine constructs.
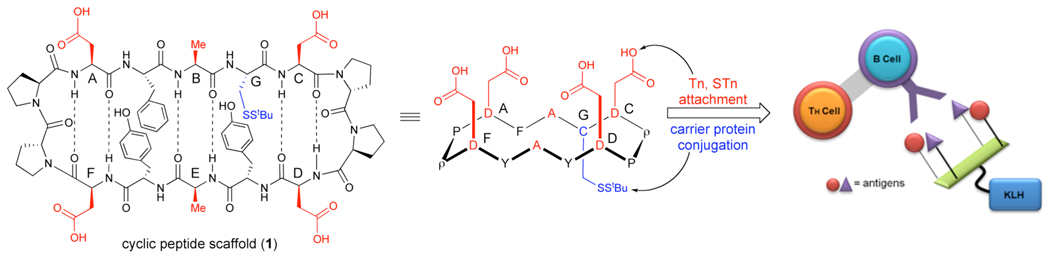
To some extent, the lowered entropic penalty associated with the increase in valency seems to enhance carbohydrate-protein interactions. However, the choice of template for multivalent carbohydrate display may be crucial. Excessively flexible scaffolds will permit attached glycans to remain far apart in most conformations thereby perhaps decreasing effective clustering of the antigen. In considering a template for the presentation of the clustered carbohydrates, we have been attracted to the model, depicted in Figure 2, upon which the clustered glycans would be displayed in a well-defined orientation. Our design, clearly inspired by Dumy11 and Robinson12 is amenable to variations in the number and type of carbohydrates displayed, as well as the spacing of the carbohydrate domains. Moreover, the promise of such templates is evidenced by recent studies demonstrating their use for clustered antigen syntheses in our lab and elsewhere.13,14 In fact, we have recently employed this scaffold in the context of a separate program, directed toward the development of an HIV vaccine.15
Figure 2.
Cyclic peptide scaffold 1 and antibody response to multivalent-KLH conjugate. TH Cell = T helper cell.
The essential element of our cyclic peptide design is a pair of β-turn-inducing D-Pro-L-Pro sequences16 at both ends of the macrocycle. Positions A–F (red, with side chain projecting “above” the plane of the scaffold) may contain handles for glycan attachment, whereas position G is a cysteine residue (blue, with side chain projecting from the “bottom” face of the scaffold), suitable for linkage to a carrier protein or biological marker. This scaffold is tunable in that differential placement of aspartate residues in positions A–F permits variation in the number and spacing of the glycan attachments (1, Figure 2).
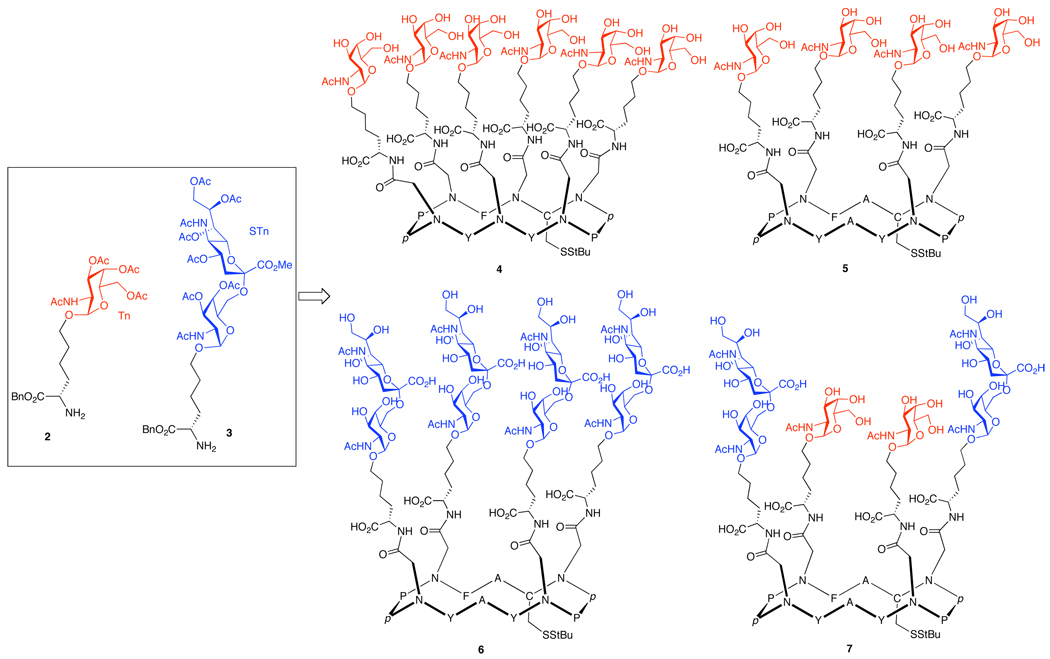
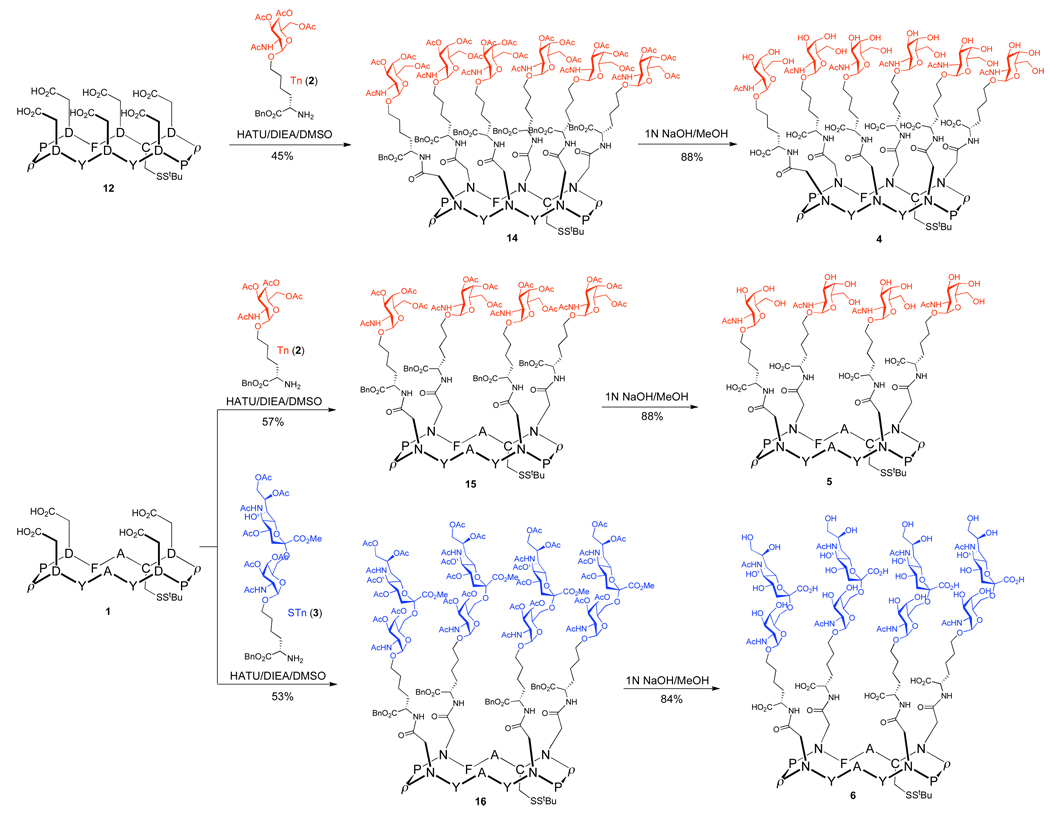
In an effort to identify an optimal presentation of the carbohydrates on the cyclic peptide scaffold, we chose as our targets the four constructs shown in Figure 3. Structures 4 and 5 incorporate six and four replicate copies of the Tn antigen, respectively, and construct 6 presents four copies of the STn disaccharide. We also sought to prepare a multiantigenic construct, 7, incorporating both the Tn and STn antigens.
Figure 3.
Protected glycosylamino acids and clustered antigens.
Protected O-linked glycosylamino acids 2 and 3 (Figure 3) were prepared from the L-hydroxynorleucine benzyl ester, according to our previously established protocol.17 These Tn and STn “cassettes,” which we originally employed in earlier approaches to the synthesis of clustered antigens, serve as useful building blocks for glycal assembly. In this system, the N-termini of both the Tn and STn cassettes serve as handles for coupling to the peptide scaffold, and the remaining carboxyl function may provide a handle for further elaboration (i.e., as T-helper or additional B-epitope attachments).
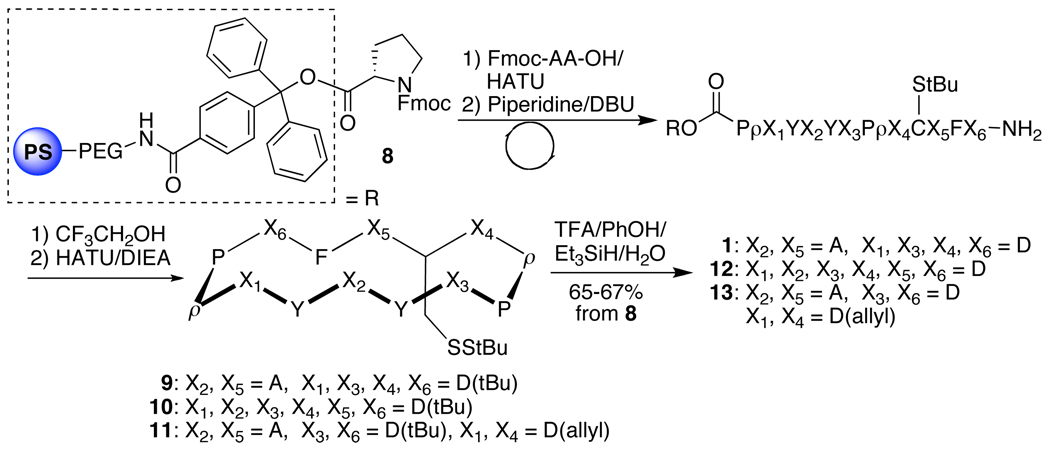
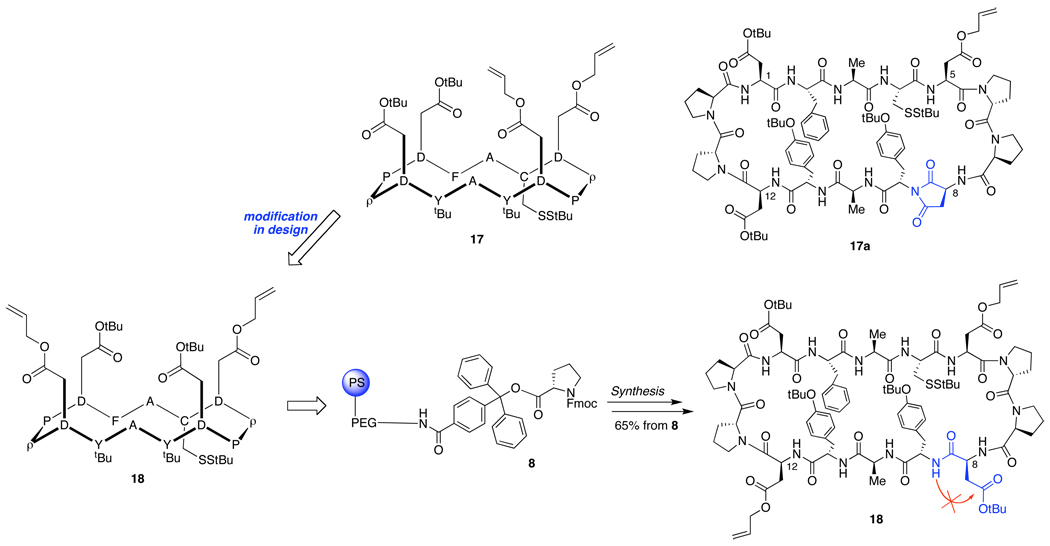
Cyclic peptides 9, 10, and 11 (containing 4 or 6 aspartate residues) were prepared in parallel through automated solid-phase synthesis from the prolinated trityl resin 8 (Scheme 1). Cleavage from the resin, macrocyclization,18 and deprotection of the tert butyl esters of the aspartate and tyrosine residues furnished 1, 12, and 13 in good overall yields.
Scheme 1.
Synthesis of cyclic peptide scaffolds.
With the components of the target structures in hand, we directed our efforts toward the covalent attachment of the carbohydrate antigens to the scaffolds. The Lansbury aspartylation reaction19 is often employed in glycopeptide synthesis. However, the standard Lansbury aspartylation protocol employs a glycosidic amine as the coupling partner of activated aspartic acid, rather than the primary amino acid nitrogen which would be required by our strategy. In considering the application of this protocol to our own system, there was concern that altered nucleophilicity of the amine might favor in the emergence of nonproductive pathways. For example, the competing, relatively facile cyclization of the peptide itself might lead to the formation of aspartimide.20 Indeed, this side reaction was found to be a problem in the coupling of peptide 12 with glycosylamino acid 2. Under standard conditions (HOAt, HATU, DIEA/DMSO), formation of the undesired aspartimide was predominant, with little indication of the requisite hexavalent product, 14. Upon close investigation, it was found that the HOAt, which is generally used as an activation additive, along with HATU, plays a significant role in promoting undesired aspartimide formation. Fortunately, activation of the aspartic acids with HATU alone in the presence of DIEA in DMSO effectively minimized aspartimide formation, and consequently allowed high yielding conversion to the desired hexavalent product, 14 (Scheme 2).
Scheme 2.
Syntheses of clustered glycopeptides.
Having identified workable coupling conditions, we were able to assemble the tetravalent constructs 15 and 16 in a similar fashion. These less congested glycopeptides exhibit different spatial arrangements, yet they still express the epitopes in highly clustered fashions. Following global deprotection, the highly clustered antigens 4, 5, and 6 were in hand.
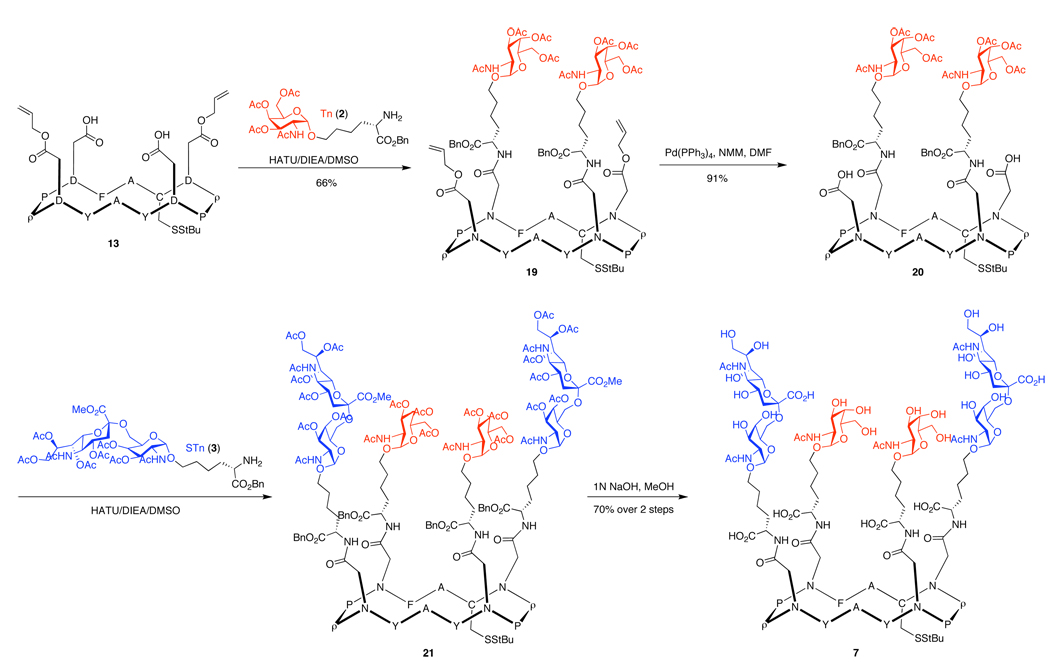
We next turned to the synthesis of the unimolecular multiantigenic construct, 7,21 wherein both the Tn and STn carbohydrate antigens are displayed on the peptidic backbone. This type of multivalent construct is intended to reflect the degree of carbohydrate heterogeneity associated with most cancers.22 There is significant variation in the types of carbohydrates which are over-expressed on the tumor cell surface, even within a particular cancer type. By combining clusters of both the Tn and STn antigens within a single cyclic peptide scaffold, we would hope to induce a more effective immune response, in which the antibodies raised would target a greater proportion of transformed tumor cells. It is not unlikely that careful strategic considerations in the peptide scaffold design could well influence the effectiveness of a multiantigenic construct.
Initial efforts to reach cyclic peptide 17 were unsuccessful, due to complications arising from aspartimide formation at position 8, leading to the undesired product, 17a (Scheme 3). Unlike the bulky Asp-β-tert-butyl esters, the less hindered Asp-β-allyl ester is susceptible to undesired aspartimide formation, arising from intramolecular nucleophilic attack by the amide nitrogen at the aspartyl C-terminus.23 However, aspartimide formation of the Asp-β-allyl ester at position 5 was impeded by the presence of proline in position 6. Thus, we initiated a design decision to transpose the -β-allyl and -β-tert-butyl protecting groups of positions 8 and 12. Gratifyingly, no evidence of aspartimide formation was found in the synthesis of cyclic peptide 18.
Scheme 3.
Cyclic peptide scaffold design for multi-antigen attachments.
Following selective tert-butyl deprotection of 18 under the conditions described in Scheme 1, intermediate 13 was in hand. We then undertook the sequential attachment of the Tn and STn carbohydrate antigens. In the event, coupling of Tn 2 with peptide 13 proceeded efficiently to produce the Tn glycopeptide construct, as shown in Scheme 4. The liberation of additional reaction sites by palladium-mediated allyl deprotection24 resulted in a high yield of divalent product 20. Addition of STn 3 to activated 20 and subsequent global deacetylation completed the synthesis of tetravalent antigen 7.
Scheme 4.
Synthesis of unimolecular multiantigenic glycopeptide 7.
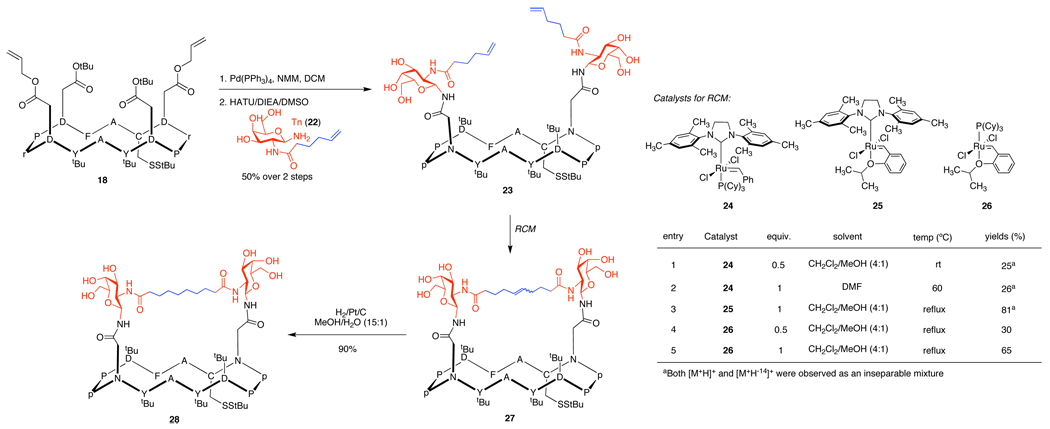
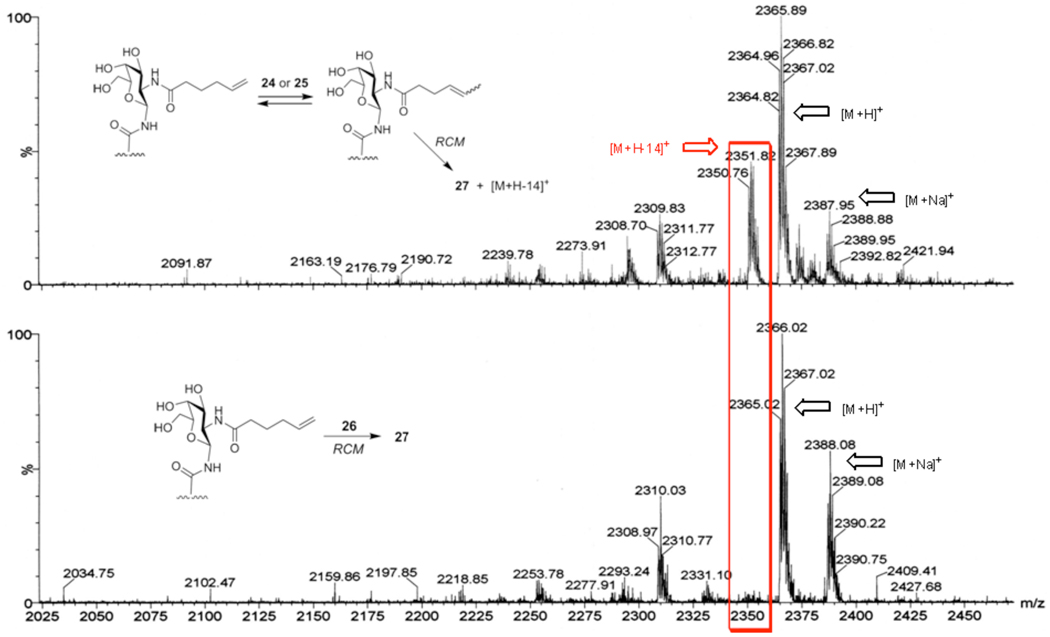
An intriguing question concerning the clustered antigen design could be the potential flexibility of linkers which attach the glycans to the cyclic peptide scaffold. Cross-linking elements between carbohydrates would rigidify the construct and prevent undesired spreading of glycan units. This should better mimic a tightly-clustered antigen and enhance the cluster-recognizing antibody response. Ring closing metathesis (RCM) has proven to be a powerful tool for the formation of peptide macrocycles in numerous settings.25 In particular, fascinating works for the synthesis of multivalent glycoconjugates by Kiessling26 have further encouraged us to explore the ruthenium-catalyzed metathesis for the incorporation of cross-linker in our system, which would lead to highly clustered cross-linked constructs. Our initial attempted RCM of di-Tn precursor 23 employing Grubbs catalyst (24) proceeded to generate spacer-linked construct 27 in modest yield (Scheme 5). Variation of the reaction conditions, including higher catalyst loading, was unable to induce complete conversion of 23. Additionally, mass spectra of isolated RCM products indicated the existence of inseparable contaminants in which a methylene group has been deleted,27(a) presumably due to the ruthenium complex-mediated isomerization27 of the terminal olefin of 23 (Figure 4, top) prior to ring closure. Although a significant increase in yield was observed when the Hoveyda-Grubbs II catalyst (25) 28 was employed, terminal olefin isomerization could not be suppressed. Gratifyingly, no isomerization of 23 occurred with the use of Hoveyda-Grubbs I catalyst (26) 29, which afforded the desired RCM product 27 in good yield (Scheme 5 and Figure 4 (bottom)). The product olefins, a mixture of E- and Z- isomers, were then hydrogenated to furnish 28. The protected aspartyl residues of 28 may serve as additional handles for further modification of the construct; i.e. attachment of multiple different antigens, to appropriately reflect the heterogeneity of target cancers.
Scheme 5.
Synthesis of cross-linked glycopeptide 28.
Figure 4.
Low-resolution mass spectrums of RCM products.
Conclusion
In summary, we have described the design and synthesis of multivalent anticancer vaccine constructs. The individual carbohydrate-based antigens were prepared in well-defined and highly clustered modes by convergent synthesis. In addition, the successful introduction of a cross-linker provides a wider scope of future modifications in epitope design either by spacer length variations or additional glycan attachments on the scaffolds. Upon conjugation to KLH carrier protein, the anticipated higher molecular ratio of Tn or STn versus KLH could offer an additional benefit in terms of immunogenicity. We expect that these highly clustered anticancer constructs will help in gaining further insight into the way in which carbohydrate clusters are recognized by the immune system. Conjugation to KLH carrier protein and immunological investigations will be reported in due course.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institutes of Health (CA28824 to SJD). DL is grateful for support from the Terri Brodeur Breast Cancer Foundation.
Footnotes
Supporting Information. Experimental procedures and characterization data (PDF). This material is available free of charge via the Internet at http://pubs.acs.org
Reference
- 1.(a) Danishefsky SJ, Allen JR. Angew. Chem., Int. Ed. 2000;39:836–863. doi: 10.1002/(sici)1521-3773(20000303)39:5<836::aid-anie836>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]; (b) Livingston PO, Zhang S. Cancer Immunol. Immunother. 1997;45:1–9. doi: 10.1007/s002620050394. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Livingston PO, Ragupathi G. Cancer Immunol. Immunother. 1997;45:10–19. doi: 10.1007/s002620050395. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Toyokuni T, Singhal AK. Chem. Soc. Rev. 1995:231–242. [Google Scholar]; (e) Hakomori SI. Advances in Exp. Med. Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 2.(a) Ingale S, Wolfert MA, Gaekwad J, Buskas T, Boons G-J. Nat. Chem. Biol. 2007;3:663–667. doi: 10.1038/nchembio.2007.25. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Buskas T, Ingale S, Boons G-J. Angew. Chem., Int. Ed. 2005;44:5985–5988. doi: 10.1002/anie.200501818. [DOI] [PubMed] [Google Scholar]
- 3.(a) Kunz H, Dziadek S, Wittrock S, Becker T. ACS Symp. Ser. 2008;989:293–310. [Google Scholar]; (b) Westerlind U, Hobel A, Gaidzik N, Schmitt E, Kunz H. Angew. Chem., Int. Ed. 2008;47:7551–7556. doi: 10.1002/anie.200802102. [DOI] [PubMed] [Google Scholar]; (c) Wittrock S, Becker T, Kunz H. Angew. Chem., Int. Ed. 2007;46:5226–5230. doi: 10.1002/anie.200700964. [DOI] [PubMed] [Google Scholar]; (d) Dziadek S, Brocke C, Kunz H. Chem. Eur. J. 2004;10:4150–4162. doi: 10.1002/chem.200400228. [DOI] [PubMed] [Google Scholar]; (e) Sprengard U, Schudok M, Schmidt W, Kretzschmar G, Kunz H. Angew. Chem., Int. Ed. 1996;35:321–324. [Google Scholar]
- 4.(a) Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris Adrian L, Old L, Cerundolo V. J. Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]; (b) Schmidt RR, Castro-Palomino JC, Retz O. Pure Appl. Chem. 1999;71:729–744. [Google Scholar]
- 5.(a) Helling F, Shang A, Calves M, Zhang SL, Ren SL, Yu RK, Oettgen HF, Livingston PO. Cancer Res. 1994;54:197–203. [PubMed] [Google Scholar]; (b) Helling F, Zhang S, Shang A, Adluri S, Calves M, Koganty R, Longenecker BM, Yao TJ, Oettgen HF, Livingston PO. Cancer Res. 1995;55:2783–2788. [PubMed] [Google Scholar]
- 6.(a) Kensil CR, Patel U, Lennick M, Marciani D. J. Immunol. 1991;146:431–437. [PubMed] [Google Scholar]; (b) Marciani DJ, Press JB, Reynolds RC, Pathak AK, Pathak VL, Gundy E, Farmer JT, Koratich MS, May RD. Vaccine. 2000;18:3141–3151. doi: 10.1016/s0264-410x(00)00118-3. [DOI] [PubMed] [Google Scholar]; (c) Kim S-K, Ragupathi G, Musselli C, Choi SJ, Park YS, Livingston PO. Vaccine. 1999;18:597–603. doi: 10.1016/s0264-410x(99)00316-3. [DOI] [PubMed] [Google Scholar]; (d) Kim S-K, Ragupathi G, Cappello S, Kagan EP, Livingston O. Vaccine. 2000;19:530–537. doi: 10.1016/s0264-410x(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 7.(a) Ragupathi G, Park TK, Zhang SL, Kim IJ, Graber L, Adluri S, Lloyd KO, Danishefsky SJ, Livingston PO. Angew. Chem., Int. Ed. 1997;36:125–128. [Google Scholar]; (b) Slovin SF, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann WG, Fazzari M, Dantis L, Olkiewicz K, Lloyd KO, Livingston PO, Danishefsky SJ, Scher HI. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gilewski T, Ragupathi G, Bhuta S, Williams LJ, Musselli C, Zhang XF, Bencsath KP, Panageas KS, Chin J, Hudis CA, Norton L, Houghton AN, Livingston PO, Danishefsky S. J. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3270–3275. doi: 10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Krug LM, Ragupathi G, Hood C, Kris MG, Miller VA, Allen JR, Keding SJ, Danishefsky SJ, Gomez J, Tyson L, Pizzo B, Baez V, Livingston PO. Clin. Cancer Res. 2004;10:6094–6100. doi: 10.1158/1078-0432.CCR-04-0482. [DOI] [PubMed] [Google Scholar]; (e) Sabbatini PJ, Kudryashov V, Ragupathi G, Danishefsky SJ, Livingston PO, Bornmann W, Spassova M, Zatorski A, Spriggs D, Aghajanian C, Soignet S, Peyton M, O’Flaherty C, Curtin J, Lloyd KO. Int. J. Cancer. 2000;87:79–85. [PubMed] [Google Scholar]
- 8.(a) Springer GF. Science. 1984;224:1198–1206. doi: 10.1126/science.6729450. [DOI] [PubMed] [Google Scholar]; (b) Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, Takahashi HK, Bigbee WL, Kim YS. Cancer Res. 1989;49:197–204. [PubMed] [Google Scholar]; (c) Kim YS, Gum J, Brockhausen I. Glyconjugate J. 1996;13:693–707. doi: 10.1007/BF00702333. [DOI] [PubMed] [Google Scholar]; (d) Springer GF. J. Mol. Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]; (e) Springer GF. Crit. Rev. Oncogenesis. 1995;6:57–85. doi: 10.1615/critrevoncog.v6.i1.50. [DOI] [PubMed] [Google Scholar]
- 9.(a) Adluri S, Helling F, Ogata S, Zhang S, Itzkowitz SH, Lloyd KO, Livingston PO. Cancer Immunol. Immunother. 1995;41:185–192. doi: 10.1007/BF01521345. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) MacLean GD, Reddish M, Koganty RR, Wong T, Gandhi S, Smolenski M, Samuel J, Nabholtz JM, Longenecker BM. Cancer Immunol. Immunother. 1993;36:215–222. doi: 10.1007/BF01740902. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) MacLean GD, Reddish MA, Bowen-Yacyshyn MB, Poppema S, Longenecker BM. Cancer Invest. 1994;12:46–56. doi: 10.3109/07357909409021392. [DOI] [PubMed] [Google Scholar]; (d) MacLean GD, Miles DW, Rubens RD, Reddish MA, Longenecker BM. J. Immunother. 1996;19:309–316. doi: 10.1097/00002371-199607000-00006. [DOI] [PubMed] [Google Scholar]; (e) MacLean GD, Reddish MA, Koganty RR, Longenecker BM. J. Immunother. 1996;19:59–68. doi: 10.1097/00002371-199601000-00007. [DOI] [PubMed] [Google Scholar]; (f) O'Boyle KP, Markowitz AL, Khorshidi M, Lalezari P, Longenecker BM, Lloyd KO, Welt S, Wright KE. Hybridoma. 1996;15:401–408. doi: 10.1089/hyb.1996.15.401. [DOI] [PubMed] [Google Scholar]
- 10.(a) Kudryashov V, Glunz PW, Williams LJ, Hintermann S, Danishefsky SJ, Lloyd KO. Proc. Natl. Acad. Sci. U.S.A. 2001;98:3264–3269. doi: 10.1073/pnas.051623598. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Slovin SF, Ragupathi G, Musselli C, Olkiewicz K, Verbel D, Kuduk SD, Schwarz JB, Sames D, Danishefsky SJ, Livingston PO, Scher HI. J. Clin. Oncol. 2003;21:4292–4298. doi: 10.1200/JCO.2003.04.112. [DOI] [PubMed] [Google Scholar]; (c) Zhang S, Walberg LA, Ogata S, Itzkowitz SH, Koganty RR, Reddish M, Gandhi SS, Longenecker BM, Lloyd KO, Livingston PO. Cancer Res. 1995;55:3364–3368. [PubMed] [Google Scholar]
- 11.(a) Dumy P, Eggleston IM, Cervigni S, Sila U, Sun X, Mutter M. Tetrahedron Lett. 1995;36:1225–1258. [Google Scholar]; (b) Dumy P, Renaudet O. Org. Lett. 2003;5:243–246. doi: 10.1021/ol0270935. [DOI] [PubMed] [Google Scholar]; (c) Singh Y, Dolphin GT, Razkin J, Dumy P. ChemBioChem. 2006;7:1298–1314. doi: 10.1002/cbic.200600078. [DOI] [PubMed] [Google Scholar]
- 12.Jiang L, Moehle K, Dhanapal B, Obrecht D, Robinson JA. Helv. Chim. Acta. 2000;83:3097–3112. Favre M, Moehle K, Jiang J, Pfeiffer B, Robinson JA. J. Am. Chem. Soc. 1999;121:2679–2685. For a review, see Robinson JA. Synlett. 2000:429–441.
- 13.Grigalevicius S, Chierici S, Renaudet O, Lo-Man R, Deriaud E, Leclerc C, Dumy P. Bioconjugate Chem. 2005;16:1149–1159. doi: 10.1021/bc050010v. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Li H, Zou G, Wang LX. Org. Biomol. Chem. 2007;5:1529–1540. doi: 10.1039/b702961f. [DOI] [PubMed] [Google Scholar]
- 15.(a) Krauss IJ, Joyce JG, Finnefrock AC, Song HC, Dudkin VY, Geng XJ, Warren D, Chastain M, Shiver JW, Danishefsky SJ. J. Am. Chem. Soc. 2007;129:11042–11044. doi: 10.1021/ja074804r. [DOI] [PubMed] [Google Scholar]; (b) Joyce JG, Krauss IJ, Song HC, Opalka DW, Grimm KM, Nahas DD, Esser MT, Hrin R, Feng M, Dudkin VY, Chastain M, Shiver JW, Danishefsky SJ. Proc. Natl. Acad. Sci. USA. 2008;105:15684–15689. doi: 10.1073/pnas.0807837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bean JW, Kopple KD, Peishoff CE. J. Am. Chem. Soc. 1992;114:5328–5334. [Google Scholar]
- 17.Keding SJ, Endo A, Danishefsky SJ. Tetrahedron. 2003;59:7023–7031. [Google Scholar]
- 18.The macrocyclization was essentially instantaneous at room temperature, indicating that cross-strand hydrogen bonding may preorganize the acyclic peptide for cyclization. Moreover, downfield 1H NMR chemical shifts of selected NH resonances, slow D2O exchange times, and the temperature profile of these chemical shifts provided preliminary evidence of the desired β-sheet character of the peptide scaffold.
- 19.Cohen-Anisfeld ST, Lansbury PT. J. Am. Chem. Soc. 1993;115:10531–10537. [Google Scholar]
- 20.(a) Bodanszky M, Natarajan S. J. Org. Chem. 1975;40:2495–2499. doi: 10.1021/jo00905a016. [DOI] [PubMed] [Google Scholar]; (b) Bodanszky M, Kwei JZ. Int. J. Pept. Protein Res. 1978;12:69–74. [PubMed] [Google Scholar]; (c) Tam JP, Riemen MW, Merrifield RB. Pept. Res. 1988;1:6–18. [PubMed] [Google Scholar]; (d) Mergler M, Dick F, Sax B, Weiler P, Vorherr T. J. Peptide Sci. 2003;9:36–46. doi: 10.1002/psc.430. [DOI] [PubMed] [Google Scholar]; (e) Lauer JL, Fields CG, Fields GB. Lett. Peptide Sci. 1994;1:197–205. [Google Scholar]
- 21.(a) Ragupathi G, Koide F, Livingston PO, Cho YS, Endo A, Wan Q, Spassova MK, Keding SJ, Allen J, Ouerfelli O, Wilson RM, Danishefsky SJ. J. Am. Chem. Soc. 2006;128:2715–2725. doi: 10.1021/ja057244+. [DOI] [PubMed] [Google Scholar]; (b) Allen JR, Harris CR, Danishefsky SJ. J. Am. Chem. Soc. 2001;123:1890–1897. doi: 10.1021/ja002779i. [DOI] [PubMed] [Google Scholar]; (c) Ragupathi G, Coltart DM, Williams LJ, Koide F, Kagan E, Allen J, Harris C, Glunz PW, Livingston PO, Danishefsky SJ. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13699–13704. doi: 10.1073/pnas.202427599. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Keding SJ, Danishefsky SJ. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11937–11942. doi: 10.1073/pnas.0401894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.(a) Zhang SL, Cordon-Cardo C, Zhang HS, Reuter VE, Adluri S, Hamilton WB, Lloyd KO, Livingston PO. Int. J. Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]; (b) Zhang SL, Zhang HS, Cordon-Cardo C, Reuter VE, Singhal AK, Lloyd KO, Livingston PO. Int. J. Cancer. 1997;73:50–56. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Mergler M, Dick F, Sax B, Weiler P, Vorherr T. Peptide Sci. 2003;9:36–46. doi: 10.1002/psc.430. [DOI] [PubMed] [Google Scholar]
- 24.Kunz H, Waldmann H. Angew. Chem., Int. Ed. 1984;23:71–72. [Google Scholar]
- 25.(a) Blackwell HE, Sadowsky Jack D, Howard RJ, Sampson JN, Chao JA, Steinmetz WE, O’Leary DJ, Grubbs RH. J. Org. Chem. 2001;66:5291–5302. doi: 10.1021/jo015533k. [DOI] [PubMed] [Google Scholar]; (b) Miller SJ, Blackwell HE, Grubbs RH. J. Am. Chem. Soc. 1996;118:9606–9614. [Google Scholar]; (c) Blackwell HE, Grubbs RH. Angew. Chem. Int. Ed. 1998;37:3281–3284. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]; (d) Schmiedeberg N, Kessler H. Org. Lett. 2002;4:59–62. doi: 10.1021/ol016891c. [DOI] [PubMed] [Google Scholar]; (e) Kazmaier U, Maier S. Org. Lett. 1999;1:1763–1766. [Google Scholar]; (f) Jarvo ER, Copeland GT, Papaioannou N, Bonitatebus PJ, Jr, Miller SJ. J. Am. Chem. Soc. 1999;121:11638–11643. [Google Scholar]
- 26.(a) Yang Z-Q, Puffer EB, Pontrello JK, Kiessling LL. Carbohydr. Res. 2002;337:1605–1613. doi: 10.1016/s0008-6215(02)00270-7. [DOI] [PubMed] [Google Scholar]; (b) Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. J. Am. Chem. Soc. 2002;124:14922–14933. doi: 10.1021/ja027184x. [DOI] [PubMed] [Google Scholar]; (c) Strong LE, Kiessling LL. J. Am. Chem. Soc. 1999;121:6193–6196. [Google Scholar]; (d) Kanai M, Mortell KH, Kiessling LL. J. Am. Chem. Soc. 1997;119:9931–9932. [Google Scholar]; (e) Schuster MC, Mortell KH, Hegeman AD, Kiessling LL. J. Mol. Catal. A: Chem. 1997;116:209–216. [Google Scholar]; (f) Mortell KH, Weatherman RV, Kiessling LL. J. Am. Chem. Soc. 1996;118:2297–2298. [Google Scholar]; (g) Mortell KH, Gingras M, Kiessling LL. J. Am. Chem. SOC. 1994;116:12053–12054. [Google Scholar]
- 27.(a) Wan Q, Cho YS, Lambert TH, Danishefsky SJ. J. Carbohydr. Chem. 2005;24:425–440. [Google Scholar]; (b) Hu Y-J, Dominique R, Das SK, Roy R. Can. J. Chem. 2000;78:838–845. [Google Scholar]; (c) Wipf P, Rector SR, Takahashi H. J. Am. Chem. Soc. 2002;124:14848–14849. doi: 10.1021/ja028603t. [DOI] [PubMed] [Google Scholar]; (d) Cadot C, Dalko PI, Cossy J. Tetrahedron Lett. 2002;43:1839–1841. [Google Scholar]
- 28.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. J. Am. Chem. Soc. 2000;122:8168–8179. [Google Scholar]
- 29.Kingsbury JS, Harrity JPA, Bonitatebus PJ, Jr, Hoveyda AH. J. Am. Chem. Soc. 1999;121:791–799. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.