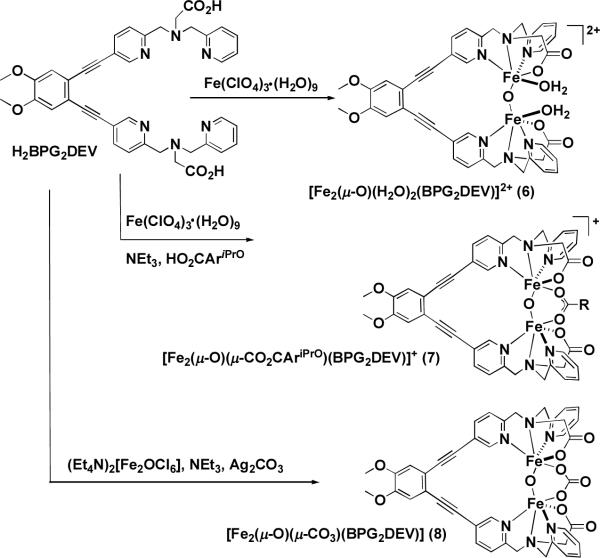
Abstract
In order to model the syn disposition of histidine residues in carboxylate-bridged non-heme diiron enzymes, we prepared a new dinucleating ligand, H2BPG2DEV, that provides this geometric feature. The ligand incorporates biologically relevant carboxylate functionalities, which have not been explored as extensively as nitrogen-only analogs. Three novel oxo-bridged diiron(III) complexes [Fe2(μ-O)(H2O)2-(BPG2DEV)](ClO4)2 (6), [Fe2(μ-O)(μ-O CAriPrO)(BPG2DEV)](ClO4) (7), and [Fe2(μ-O)(μ-CO3)(BPG2DEV)] (8) were prepared. Single crystal X-ray structural characterization confirms that two pyridines are bound syn with respect to the Fe–Fe vector in these compounds. The carbonato-bridged complex 8 forms quantitatively from 6 in a rapid reaction with gaseous CO2 in organic solvents. A common maroon-colored intermediate (λmax = 490 nm; ε = 1500 M−1 cm−1) forms in reactions of 6, 7, or 8 with H2O2 and NEt3 in CH3CN/H2O solutions. Mass spectrometric analyses of this species, formed using 18O-labeled H2O2, indicate the presence of a peroxide ligand bound to the oxo-bridged diiron(III) center. The Mössbauer spectrum at 90 K of the EPR-silent intermediate exhibits a quadrupole doublet with = 0.58 mm/s and ΔEQ = 0.58 mm/s. The isomer shift is typical for a peroxodiiron(III) species, but the quadrupole splitting parameter is unusually small compared to related complexes. These Mössbauer parameters are comparable to those observed for a peroxo intermediate formed in the reaction of reduced toluene/o-xylene monooxygenase hydroxylase (ToMOH) with dioxygen. Resonance Raman studies reveal an unusually low-energy O–O stretching mode in the peroxo intermediate that is consistent with a short diiron distance. Although peroxodiiron(III) intermediates generated from 6, 7, and 8 are poor O-atom transfer catalysts, they display highly efficient catalase activity, with turnover numbers up to 10,000. In contrast to hydrogen peroxide reactions of diiron(III) complexes that lack a dinucleating ligand, the intermediates generated here could be reformed in significant quantities after a second addition of H2O2, as observed spectroscopically and by mass spectrometry.
Introduction
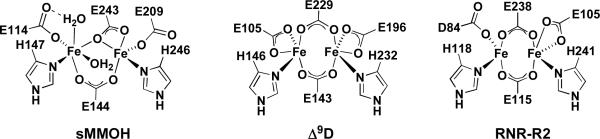
Carboxylate-bridged non-heme diiron centers occur in a variety of enzymes that activate dioxygen to catalyze key reactions in nature.1–5 Members of this class include ribonucleotide reductase (RNR-R2),6–8 Δ9-desaturase (Δ9D),9,10 and the hydroxylase components of bacterial multicomponent monooxygenases (BMMs).11,12 Soluble methane monooxygenase (sMMOH),4 toluene/o-xylene monooxygenase (ToMOH),13,14 and phenol hydroxylase (PHH)15 belong to the family of BMMs and function as catalysts for the selective oxidation of hydrocarbons. Remarkably, despite their diverse roles in biology, the active sites in these enzymes all share common structural features, a carboxylate-rich environment with two histidine donors that are bound in a syn fashion with respect to the diiron vector.16 Representations of the active sites of some of these enzymes in their reduced diiron(II) forms are provided in Chart 1.
Chart 1.
In these enzymes, dioxygen activation occurs following 2-electron reduction of their diiron(III) resting states to generate an O2-reactive diiron(II) species. Following reaction of this reduced form with dioxygen, peroxodiiron(III) intermediate species are generated that share common spectroscopic features.4 Resonance Raman spectral studies of peroxo intermediates in RNR-R217 and Δ9D10 suggest a μ-1,2-peroxo binding mode. Theoretical analysis of the peroxo intermediate in sMMOH, for which no vibrational spectroscopic data are yet available, find a μ-η2:η2-peroxo butterfly structure to be more stable,18 and pH-dependent studies of the dioxygen activation chemistry indicate that proton transfer reactions,19 most likely involving some kind of hydroperoxo intermediate, are involved. Recently, novel peroxodiiron(III) transient intermediates were observed in the ToMOH and PHH systems, having no visible or near-IR optical bands, notably different Mössbauer parameters, and possibly a different coordination mode.12,20 Peroxodiiron(III) intermediates in these enzymes can in some cases undergo O–O bond cleavage to form high-valent species, such as the mixed-valent (μ-oxo)diiron(III,IV) intermediate X in RNR-R26 and the methane-oxidizing di(μ-oxo)diiron(IV) intermediate Q in sMMO.21,22 Elucidating the structures of these oxygenated intermediates remains an important challenge.
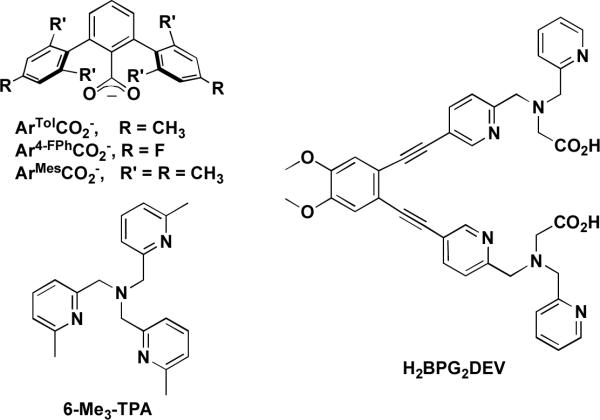
Significant effort has been expended to construct model complexes that mimic the active site structures and functions of non-heme diiron enzymes.23,24 These studies have predominantly been conducted to probe the mechanism of O2 activation, the influence of structure on reactivity, and the requirements of iron-based catalysts for hydrocarbon oxidation. Two general ligand motifs have been extensively employed in the construction of these model complexes, namely, tris(2-pyridylmethyl)amine (TPA)-based and sterically encumbering 2,6-diarylbenzoate ligands (Chart 2).23–26 Whereas the latter group of ligands facilitates formation of diiron(II) complexes with the same composition as the enzyme active sites, TPA-based constructs afford complexes that react with dioxygen to generate species more closely resembling intermediates observed spectroscopically in the reactions of BMMs with dioxygen. A structural feature that neither of these ligand motifs can rigidly enforce, however, is the syn orientation of nitrogen donors with respect to the diiron vector. This feature may be important and it is likely that nature did not choose such a stereochemistry arbitrarily. The significance of the syn N-donor disposition of imidazoles from histidine residues with respect to the diiron vector is still unclear, although preliminary DFT calculations on intermediate Q of sMMOH suggest that a stereoelectronic effect derived from this configuration tunes its reactivity.27 Apart from their inability to enforce syn N-donor character, terphenylcarboxylate- and TPA-based ligands are not pre-organized to stabilize a diiron core, which can lead to dissociation or aggregation to form monomeric or oligomeric species, respectively. To address these issues, we have been exploring N-donor ligands that are covalently tethered by a diethynylbenzene unit.16,28–31 Selection of this moiety as the appropriate linker fixes the nitrogen donor atoms at a distance and orientation similar to that in the enzymes. Of particular interest is the 1,2-bis(pyridin-3-ylethynyl)benzene scaffold, because of convenience in functionalizing the pyridine moiety, as demonstrated in the synthesis of several ligands based on this platform.28
Chart 2.
In the present article we report the synthesis of the syn N-donor ligand H2BPG2DEV (Chart 2) and three of its derivatives containing oxo-bridged diiron(III) cores. Two tripodal N,N'-bis(2-pyridylmethyl)-3-aminoacetate (BPG−) centers bridged by diethynylveratrole converge to incorporate diiron units and provide carboxylate groups that more accurately represent the aspartate and glutamate residues of non-heme diiron enzymes than earlier constructs. We describe how this dinucleating ligand stabilizes the oxo-bridged diiron(III) cores and considerably influences the chemical and physical properties of a peroxodiiron(III) intermediate generated by addition of hydrogen peroxide.
Experimental Section
Materials and Methods
Reagents were purchased from commercial sources and used as received. Acetonitrile (CH3CN), dichloromethane (CH2Cl2), and tetrahydrofuran (THF) were saturated with nitrogen and purified by passing though activated alumina columns under argon. Triethylamine (Et3N) was distilled from CaH2. The compounds (5-bromo-pyridin-2-yl)methanol (2b),32 [(pyridin-2-ylmethyl)amino]acetic acid ethyl ester (3),33 4,5-diethynylveratrole (DEV),31 and (Et4N)2[Fe2(μ-O)Cl6]34,35 were prepared using methods described in the literature. The compound 2,6-diisopropoxybenzoic acid (HO2CAriPrO) was synthesized by using a modified literature procedure.36 Caution! The perchlorate salts used in this study are potentially explosive and should be handled with care!
NMR spectra were recorded on a Varian 300 spectrometer in the Massachusetts Institute of Technology Department of Chemistry Instrument Facility (MIT DCIF). All spectra were recorded at ambient probe temperature, 293 K. IR spectra were taken on a Thermo Nicolet Avatar 360 spectrometer with OMNIC software. Mass spectra were recorded in electrospray ionization mode. ESI-MS data were obtained with an Agilent 1100 series LC/MSD mass spectrometer. UV-vis experiments were performed on a Cary 50 spectrophotometer.
[(5-Bromo-pyridin-2-ylmethyl)-pyridin-2-ylmethyl-amino]acetic Acid Ethyl Ester (4)
A solution of (5-bromo-pyridin-2-yl)methanol (2b) (4.69 g, 24.9 mmol) and Et3N (4.53 mL, 32.4 mmol) in THF (95 mL) was cooled to 0 °C and treated dropwise with methanesulfonyl chloride (MsCl; 2.32 mL, 29.9 mmol). The mixture was warmed to room temperature, stirred for 2 h, and then combined with aqueous NH4Cl (200 mL). The aqueous phase was extracted with CH2Cl2 (3 × 200 mL) and the organic layers were dried (Na2SO4), filtered, and concentrated to afford a solid (6.78 g, quant) that was used without further purification. This purple solid, K2CO3 (4.12 mg, 29.9 mmol), and [(pyridin-2-ylmethyl)-amino]-acetic acid ethyl ester (3) (5.80 g, 29.9 mmol) were stirred overnight in CH3CN (100 mL). During this period the color changed from purple-red to orange. The reaction mixture was combined with CH2Cl2 (300 mL) and the organics were washed with aq Na2CO3 (3 × 100 mL). The organic layer was dried (Na2SO4), filtered, and evaporated to dryness. The crude product was purified by column chromatography (alumina; EtOAc/hexanes, 1:3) to give 4 as a yellow oil, which was identical to the compound synthesized previously as judged by 1H NMR spectroscopy and ESI-MS.28 Yield: 7.92 g (87%). 1H NMR (300 MHz, CDCl3): δ = 8.56–8.50 (m, 2H), 7.67–7.61 (dt, J = 1.8, 7.5 Hz, 1H), 7.29 (d, J = 8.1 Hz, 1H), 7.17–7.12 (m, 2H), 4.19–4.11 (q, J = 7.2 Hz, 2H), 3.96 (s, 2H), 3.94 (s, 2H), 3.44 (s, 2H), 1.27–1.22 (t, J = 7.2 Hz, 3H). LRMS (ESI) calcd for C16H18BrN3O2Na [M+Na]+: 386, found: 386.
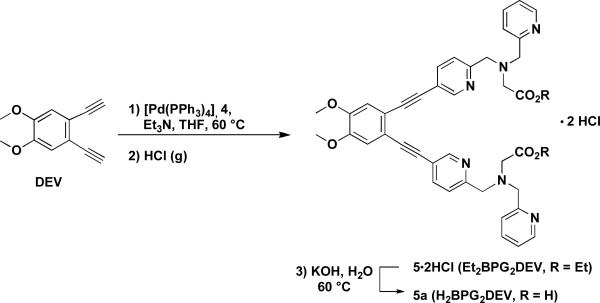
Et2BPG2DEV·2HCl (5·2HCl) (Diethyl 2,2'-(5,5'-(4,5-dimethoxy-1,2-phenylene) bis(ethyne-2,1-diyl)bis(pyridine-5,2-diyl))bis(methylene)bis((pyridin-2-ylmethyl)-azane-diyl)diacetate) Hydrochloride
4,5-Diethynylveratrole (0.474 g, 2.55 mmol), 4 (1.82 g, 5.03 mmol), [Pd(PPh3)4] (0.250 g, 0.216 mmol), Et3N (3.6 mL, 26 mmol), and THF (24 mL) were combined in a sealed tube under an inert atmosphere and stirred for 2.5 d at 60 °C. After the reaction mixture was cooled to room temperature, it was combined with EtOAc (50 mL) and washed three times with a solution of Na2CO3 (aq.), dried over Na2SO4, filtered, and evaporated to dryness. The crude material was purified by column chromatography (alumina; EtOAc/hexanes, 1:1–1:0) to give 5 as thick yellow oil. 1H NMR spectroscopy confirmed complete conversion to the desired product, but a phosphine oxide impurity was observed. To purify the material, the hydrochloride salt of the amine, 5·2HCl, was synthesized. The oil was dissolved in ca. 100 mL of EtOAc and ca. 4 mL of a solution of HCl in Et2O (2 M) was added dropwise with stirring until no further precipitation occurred. The yellow solid was filtered off and dried in vacuum. Yield: 1.20 g (57%). 1H NMR (300 MHz, CD3OD): δ = 8.92 (m, 2H); 8.89 (m, 2H); 8.54 (dt, J = 1.5; 7.8 Hz, 2H); 8.37 (dd, J = 2.1, 9.0 Hz, 2H); 8.07 (d, J = 7.8 Hz, 2H); 8.00–7.96 (m, 2H); 7.90 (d, J = 8.4 Hz, 2H); 7.25 (s, 2H); 4.58 (s, 4H); 4.56 (s, 4H); 4.18 (q, J = 7.2 Hz, 4H); 3.92 (s, 6H); 3.74 (s, 4H); 1.25 (t, J = 7.2 Hz, 6H). 13C NMR (125 MHz, CD3OD): δ = 171.27, 154.21, 153.90, 151.40, 147.44, 146.96, 145.77, 142.35, 127.61, 127.31, 126.77, 122.78, 117.88, 115.47, 94.90, 86.30, 61.68, 56.97, 56.48, 56.24, 54.90, 13.87. HRMS (ESI) calcd for [M+H]+: 753.3397, found: 753.3397. IR (KBr, cm−1): 2979 (m), 2914 (m), 2611 (m), 2214 (m, νC≡C), 1731 (s), 1614 (m), 1593 (m), 1511 (s), 1464 (m), 1371 (m), 1251 (s), 1215 (s), 1155 (m), 1086 (m), 1024 (m), 987 (m), 858 (w), 772 (w). Mp: 103–105 °C.
H2BPG2DEV (5a), 2,2'-(5,5'-(4,5-Dimethoxy-1,2-phenylene)bis(ethyne-2,1-diyl)bis(pyridine-5,2-diyl))bis(methylene)bis((pyridin-2-ylmethyl)azanediyl)diacetic Acid
An aqueous solution of 5·2HCl (0.55 g, 0.67 mmol) and KOH (1.4 g, 25 mmol) were combined to yield a total volume of 100 mL. The resulting suspension was heated to 60 °C under a nitrogen atmosphere for ca. 4 h. After cooling down to room temperature, the reaction mixture was acidified with dilute HCl to pH 5 and the product was extracted with CH2Cl2. The organic phase was dried over Na2SO4, filtered, and reduced to dryness to yield a yellow-brown solid. Yield: 0.42 g (91%). 1H NMR (300 MHz, CDCl3): δ = 13.11 (s, 2H), 8.68 (m, 2H), 8.58 (m, 2H), 7.73 (m, 4H), 7.35 (m, 4H), 7.24 (m, 2H), 7.03 (s, 2H), 4.13 (s, 4H), 4.07 (s, 4H), 3.94 (s, 6H), 3.57 (s, 4H). 13C NMR (125 MHz, d6-DMSO): δ = 173.44, 160.29, 160.05, 151.76, 150.64, 149.93, 139.86, 137.83, 123.93, 123.65, 123.40, 118.94, 118.40, 115.49, 92.16, 90.19, 60.32, 60.22, 57.00, 55.46. HRMS (ESI) calcd for [M+H]+: 697.2770, found: 697.2770. IR (KBr, cm−1): 3054 (w), 3002 (w), 2912 (w), 2832 (w), 2206 (w, νC≡C), 1714 (m), 1637 (w), 1593 (m), 1551 (w), 1511 (s), 1437 (m), 1401 (m), 1359 (m), 1248 (s), 1215 (s), 1149 (m), 1119 (m), 1085 (m), 1024 (m), 993 (m), 858 (w), 761 (m), 722 (m), 695 (m), 649 (w), 621 (w), 541 (m). Mp: 60–62 °C.
[Fe2(μ-O)(H2O)2BPG2DEV](ClO4)2 (6)
To a solution (2 mL; CH3CN/H2O, 10:1) of Fe(ClO4)3·9H2O (77 mg, 150 μmol) was added a suspension of 5a (50 mg, 72 μmol) in the same solvent mixture (2 mL). The color instantly changed to deep-red and the resulting solution was stirred for ca. 5 min. After filtration, the solution was subjected to vapor diffusion of Et2O to yield crystalline red plates that were analyzed by X-ray crystallography. The yield of crystalline material was highly dependent on the H2O content of the solution. Yield: 60 mg (78%). X-ray diffraction quality crystals were grown from vapor diffusion of Et2O into a solution of 6 in CH3OH and H2O. LRMS (ESI) calcd for [M-H]+: 857.1, found: 875.2. IR (KBr, cm−1): 3430 (m), 3073(w), 2921 (w), 2854 (w), 2210 (w, νC≡C), 1608 (s), 1590 (s), 1550 (m), 1512 (s), 1492 (m), 1462 (w), 1446 (m), 1402 (w), 1367 (m), 1348 (m), 1301 (w), 1286 (w), 1250 (s), 1212 (m), 1099 (vs), 1086 (vs), 1024 (m), 992 (w), 927 (w), 902 (w), 807 (m, νFe–O–Fe), 768 (m), 731 (w), 667 (w), 651 (w), 622 (m), 570 (w), 476 (w), 423 (w). Anal. Calcd. for 6·2CH3OH, C42H46N6O19Cl2Fe2: C, 44.98; H, 4.13; N, 7.49. Found: C, 45.18; H, 4.19; N, 7.12. UV–vis (CH3CN:H2O, 10:1) (λmax, nm (ε, M−1 cm−1)): 480 (440). Mp: 175–180 °C (dec).
[Fe2(μ-O)(μ-O2CAriPrO)BPG2DEV](ClO4) (7)
Method A. A red CH3OH/CH2Cl2 (1:1) solution of 6 (63 mg, 60 μmol) was allowed to react with HO2CAriPrO (21 mg, 90 μmol) in the presence of NEt3 (40 μL) to form an intense green solution. Clusters of green needles were isolated by vapor diffusion of Et2O into this reaction mixture. Yield: 40 mg (57%). Method B. A CH3OH/CH2Cl2 (1:1) solution of Fe(ClO4)3·9H2O (77 mg, 150 μmol) was combined with HO2CAriPrO (19 mg, 79 μmol) and NEt3 (60 μL). To this reaction mixture, a solution of 5a (50 mg, 72 μmol) was instantly added and the resulting deep green solution was stirred for 10 min, filtered, and subjected to vapor diffusion of Et2O. Deep green needle-shaped crystals, suitable for X-ray crystallographic analysis, were isolated. Yield: 38 mg (46%). LRMS (ESI) calcd for [M–ClO4]+: 1059.2, found: 1059.5. IR (KBr, cm−1): 3076 (w), 2975 (w), 2930 (w), 2211 (w, νC≡C), 1645 (s), 1608 (s), 1593 (s), 1530 (s), 1513 (s), 1459 (s), 1422 (s), 1372 (m), 1356 (m), 1332 (m), 1289 (m), 1249 (s), 1087 (vs), 1024 (m), 996 (w), 923 (w), 906 (w), 848 (w), 834 (w), 771 (m, νFe–O–Fe), 729 (w), 668 (w), 623 (m), 551 (w), 512 (w). Anal. Calcd for 7·0.5CH2Cl2, C53.5H53N6O15Cl2Fe2: C, 53.43; H, 4.44; N, 6.99. Found: C, 53.84; H, 4.87; N, 7.08. UV–vis (CHCl3:CH3OH, 1:1) (λmax, nm (ε, M−1 cm−1)): 642 (130); 522 (sh, 218); 490 (730); 475 (sh, 569); 436 (sh, 1171); 413 (1577). Mp: 195–200 °C (dec).
[Fe2(μ-O)(μ-CO3)BPG2DEV] (8)
A solution (6 mL, CH2Cl2/CH3OH, 1:1) of 5a (100 mg, 144 μmol) and Et3N (40 mL, 290 μmol) was added dropwise to a solution (7 mL, CH2Cl2/CH3OH, 1:1) of (Et4N)2[Fe2(μ-O)Cl6] (87 mg, 144 μmol). Solid Ag2CO3 (159 mg, 576 μmol) was then added to the brown solution. The resulting suspension turned green and was allowed to react for ca. 45 min. The mixture was filtered and subjected to vapor diffusion of Et2O. After 2–3 days, emerald-green needles of 8 were harvested that were suitable for X-ray crystallography. Yield: 93 mg (73%). LRMS (ESMS) calcd for (M+H)+: 883.1, found: 883.0; calcd for (M–CO3+OH)+: 839.1, found: 839.2. IR (KBr, cm−1): 3065 (w), 2956 (w), 2920 (w), 2846 (w), 2210 (w, νC≡C), 1617 (s), 1610 (s), 1571 (w), 1533 (m), 1513 (s), 1495 (m), 1444 (m), 1403 (w), 1383 (w), 1358 (m), 1321 (m), 1304 (m), 1289 (m), 1272 (w), 1248 (m), 1214 (m), 1182 (m), 1158 (w), 1142 (w), 1123 (w), 1102 (w), 1087 (w), 1055 (w), 1040 (w), 1024 (w), 997 (w), 927 (w), 908 (w), 860 (w), 847 (w), 838 (w), 770 (m, νFe–O–Fe), 742 (w), 695 (w), 663 (w), 564 (w), 552 (w), 528 (w), 460 (w), 437 (w), 423 (w). UV–vis (CHCl3:CH3OH, 1:1) (λmax, nm (ε, M−1 cm−1)): 638 (124); 517 (193); 487 (567); 473 (sh, 342); 411 (1576). Anal. Calcd for 8·0.75CH2Cl2, C41.75HH35.5N6O10Cl1.5Fe2: C, 53.00; H, 3.78; N, 8.88. Found: C, 52.99; H, 4.27; N, 9.10. Mp: 150–155 °C (dec).
X-ray Crystallographic Studies
Intensity data were collected on a Bruker SMART APEX CCD diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å), controlled by a Pentium-based PC running the SMART software package.37 Single crystals were mounted on the tips of glass fibers, coated with Paratone-N oil, and cooled to 110 K under a stream of N2 maintained by a KRYO-FLEX low-temperature apparatus. A total of 2800 frames were collected for each compound. The structures were solved by direct methods and refined on F2 by using the SHELXTL-97 software included in the SHELXTL software package.38,39 Empirical absorption corrections were applied by using the SADABS program,40 and the structures were checked for higher symmetry with the PLATON software.41 All non-hydrogen atoms were located and their positions refined with anisotropic thermal parameters by least-squares cycles. All hydrogen atoms were assigned to idealized positions and given thermal parameters equivalent to either 1.5 (methyl hydrogen atoms) or 1.2 (all other hydrogen atoms) times the thermal parameter of the carbon atoms to which they were attached. The hydrogen atoms of O8 and O9 of the H2O ligands in the diiron(III) complex 6 and of O1W were located from a difference map and their bond distances to the corresponding oxygen atoms restrained using a DFIX command. Refinement details, data collection parameters, and crystal data for 6, 7, and 8 are reported in the Supporting Information.
Mössbauer Spectroscopy
Mössbauer spectra were recorded on an MSI spectrometer (WEB Research Company) with a 57Co source in a Rh matrix maintained at room temperature. Solid samples of 6, 7, and 8 were prepared by suspension of 13 to 20 mg of the powdered solids in Apiezon M grease and placed in a nylon sample holder. Solution samples of reaction intermediates 8a and 7a were prepared by respectively treating suspensions (ca. 0.8 mL) of the oxo-bridged diiron complexes 8 (ca. 20 μmol, CH3CN/H2O, 1:1) and 7 (ca. 10 μmol, CH3OH/H2O, 4:1) with Et3N (15 μL) followed by addition of aqueous solution of H2O2 (50 μL, 30%). After ca. 1 min, the red-brown reaction mixture was transferred to a sample holder and instantly frozen in liquid N2. A sample of 6a was prepared similarly, but using a more dilute solution (ca. 2 μmol, CH3OH/H2O, 20:1) of 57Fe-enriched complex 6, 57Fe-6, which was allowed to react with Et3N (5 μL) and excess H2O2 (10 μL, 30%) for ca. 40–50 s and then frozen instantly. The synthesis of 57Fe-6 is described in Supporting Information. Data were acquired at 4.2 K and 90 K, and isomer shift (δ) values are reported relative to the room temperature Mössbauer spectrum of a metallic iron foil, which was used for calibration of the velocity scale. The spectra were fit to Lorentzian lines with the WMOSS plot and fit program.42
Resonance Raman Spectroscopy
Resonance Raman (RR) spectra were obtained on a custom built McPherson 2061/207 spectrometer equipped with a Princeton Instrument liquid-N2 cooled CCD detector (LN-1100PB). Excitation at 647, 568, and 413 nm was provided by a Kr laser (Innova 302, Coherent) and 514- and 488- nm excitations by an Ar laser (Innova 90, Coherent). The laser beam was kept at low power, between 40 and 10 mW, and was focused with a cylindrical lens onto frozen samples in NMR tubes. Long wave pass filters (RazorEdge filters, Semrock) or super-notch filters (Kaiser Inc.) were used to attenuate Rayleigh scattering. Sets of ~4 min accumulations were acquired at 4-cm−1 resolution. Frequency calibrations were performed using aspirin and are accurate to ±1 cm−1. The samples were subjected to continuous spinning at 110 K to prevent adverse effects from laser illumination. Regular visual inspection of the sample colors during the course of the RR experiments and comparison of consecutive RR spectra provided a reliable means to evaluate photobleaching of species of interest.
Electrospray Ionization Mass Spectrometry of the Peroxo Intermediate
Products 6a, 7a, and 8a, which result from reaction of H2O2 and NEt3 with 6, 7, or 8, respectively, were identified by ESI-MS using a Finnigan LTQ ion trap mass spectrometer maintained at the Department of Chemistry, Tufts University. The samples were prepared by addition of a 50–100 fold excess of a 2% aqueous H2O2 solution containing 5 equiv of NEt3 to solutions of the diiron(III) complexes (ca. 1–2 mM) in solvent mixtures of CH3CN/H2O or CH3OH/CH2Cl2. The peaks of interest were confirmed by comparison of the observed and calculated m/z values including isotope patterns.
Reaction of 6, 7, and 8 with H2O2 and Quantification of O2 Evolution
In order to quantify the amount of dioxygen formed during hydrogen peroxide decomposition, 2 mL of a 0.4 mM solution of the diiron complex were placed in a 25-mL round-bottom flask equipped with a stir bar. The flask was then tightly sealed with a rubber septum and electrical tape. The solution was treated with ca. 5 μL of NEt3 and cooled to 0 °C before H2O2 was added through the septum with a syringe. The concentration of diiron complex was kept constant by adjusting the final volume of the reaction mixture. The reaction flask was connected by a cannula to an inverted graduated cylinder, filled with H2O, and the amount of O2 generated was determined applying the ideal gas law. In the case of 6, the solution was thoroughly purged with nitrogen to remove any traces of CO2 that can potentially react with 6 under basic conditions to form the carbonato-bridged complex 8.
Substrate Oxidation Studies
Oxidation reactions were carried out under an inert atmosphere of N2 (g). In each experiment, cis-cyclooctene (0.26 mL, 2.0 mmol) and NEt3 (5 μL) were added to 2.0 mL of a 1.0 M solution of 6 or 7 in CH3CN/H2O (95:5) at 25 °C. To this mixture, H2O2 (97 μL, 10.3 M; diluted with CH3CN to a total volume of 400 μL) was added by syringe pump over a period of one h. After stirring for an additional 2h, the products were determined by GC analysis. The structures of the products were confirmed by GC-MS spectrometry, and by comparison with authentic samples.
Results and Discussion
Synthesis of H2BPG2DEV (5a)
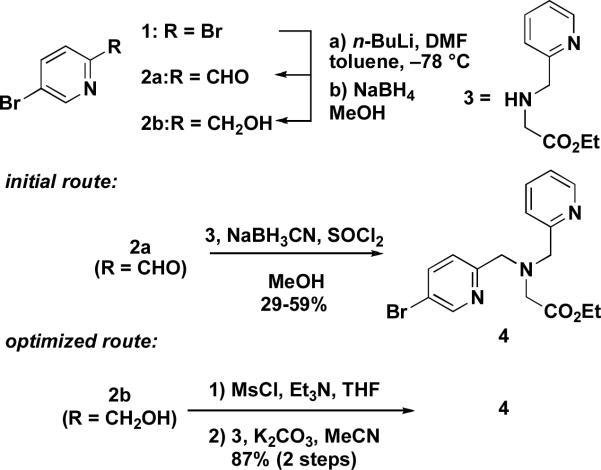
Previously we reported an efficient route to a new family of diethynyltriptycene-linked dipyridyl ligands, including Et2BPG2DET (DET = 2,3-diethynyltriptycene), an analog of Et2BPG2DEV (5).28 Our initial route started from commercially available 2,5-dibromopyridine (1). Following a known reaction, we synthesized 2-formyl-5-bromopyridine (2a) by treatment of 1 with n-BuLi, followed by quenching with DMF.32 With aldehyde 2a in hand, we prepared the pyridylbromide coupling partner 4 by reductive amination of 2a with the N-pyridylmethylglycine ethyl ester 3, which is available in one step from commercially available materials and can be isolated easily by distillation on a multi-gram scale.33 Although the reaction sequence used to prepare 4 proceeded with moderate yields of less than a gram, we observed that isolated yields of 2a and 4 diminished by roughly 30% for each step upon scale up. We therefore devised an optimized route to the pyridylbromide 4 from 1, which is compared to the previous method in Scheme 1. In this procedure we prepared alcohol 2b by reduction of 2a with NaBH4 in situ.32 Isolated yields for 2b ranged between 54–60% starting with 10 g of 1. In addition to the higher yield, 2b could be readily purified by crystallization, in contrast to the aldehyde 2a, which was difficult to separate from undesired byproducts. Mesylation of 2b with MsCl and Et3N afforded a mesylate that was allowed to react with 3 in the presence of K2CO3 to furnish 4 in 87% yield over two steps. Using this optimized route, we were able to prepare multi-gram quantities of 4 from 1 in a three step synthetic sequence that required only a single alumina column chromatography purification procedure.
Scheme 1.
Preparation of pyridylbromide 4.
With sufficient amounts of the pyridylbromide 4 in hand, we were able to synthesize large quantities of Et2BPG2DEV (5) via a cross-coupling reaction between 4 and 4,5-diethynylveratrole (DEV)31 using 10 mol% of [Pd(PPh3)4] catalyst in a mixture of Et3N and THF at 60°C (Scheme 2). We chose the DEV over the DET backbone due to its greater solubility in organic solvents and more facile synthesis. The Sonogashira cross-coupling reaction worked remarkably well upon scale up and the conversion to the reaction product was greater than 90% as determined by 1H NMR spectroscopy. In order to remove residual phosphine impurities that were present after purification of the crude product by alumina column chromatography, the HCl salt of the amine was isolated by treatment of an EtOAc solution of the purified material with an etheral hydrogen chloride solution. This purification sequence lowered the isolated yield of the corresponding dihydrochloride salt to 57%. Saponification of the esters with KOH in H2O proceeded cleanly, affording H2BPG2DEV in nearly quantitative yield. Although the salt of the ester is stable for months under ambient atmospheric conditions, the dicarboxylic acid decays to form a complex mixture of products over time as evidenced by 1H NMR spectroscopy. Therefore, we stored the ligand as the hydrochloride salt of the diester and prepared the acid freshly for the following metal complexation studies.
Scheme 2.
Synthesis of the Oxo-Bridged Diiron(III) Compounds [Fe2(μ-O)(H2O)BPG2DEV](ClO4)2 (6), [Fe2(μ-O)(μ-O2CAr iPrO)BPG2DEV](ClO4) (7), [Fe2(μ-O)(μ-CO3)BPG2DEV] (8)
Compound 6 crystallizes in good yield as deep red-colored blocks by vapor diffusion of Et2O into a reaction mixture containing H2BPG2DEV and two equiv of Fe(ClO4)3·9H2O in CH3CN/H2O (10:1). The yields and the crystal quality depended strongly on the ratio of CH3CN and H2O. Only a few examples of oxo-bridged diiron(III) compounds with aqua ligands have been reported because H2O is prone to substitution and tends to form oxo-bridged polyiron(III) complexes.43 When excess base, such as NEt3, was added under aerobic conditions in the synthesis of 6, the color changed from deep red to intense green. The resulting complex 8 contained a carbonato- and oxo-bridged diiron(III) center that forms upon reaction with CO2 from the air. This reaction was complete in less than a few seconds when CO2 gas was introduced directly into the reaction mixture. With the aforementioned procedure, isolated yields of 8 varied widely. Therefore, an optimized protocol for the synthesis of 8 was developed. This procedure involved treating H2BPG2DEV with one equiv of the pre-formed (μ-oxo)diiron(III) complex (Et4N)2[Fe2(μ-O)Cl6] dissolved in CH3OH/CH2Cl2, producing a yellow-brown solution. Addition of excess Ag2CO3 resulted in nearly instantaneous formation of a green suspension that was stirred for 45 min, filtered, and subjected to vapor diffusion of Et2O. Emerald-green needles were isolated in 73% yield after several days and analyzed by X-ray crystallography. Compound 8 belongs to a rather rare group of carbonato-bridged diiron complexes.44–50
As with the synthesis of the carbonate complex 8, the carboxylato-bridged complex 7 could be prepared in a variety of ways in moderate yields, either in a reaction between the ligand H2BPG2DEV and two equivalents of Fe(ClO4)3·9H2O in the presence of base and excess HO2CAriPrO, or by addition of the carboxylic acid to a CH3CN solution of 6 in the presence of base. Scheme 3 summarizes the pathways for the synthesis of compounds 6, 7, and 8.
Scheme 3.
Structural Characterization
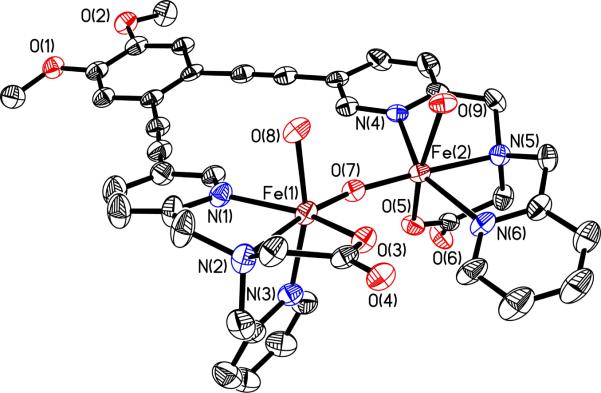
An ORTEP diagram of 6 is displayed in Fig. 1 and selected bond lengths and angles are reported in Table S2. Compound 6 is a dication and crystallizes with two perchlorate anions in the asymmetric unit and numerous water molecules in the lattice, one of which bridges the two methoxy groups of the veratrole backbone via hydrogen bonds. A packing diagram (Fig. S1 and Table S3, Supporting Information) reveals strong hydrogen-bonding interactions between the H2O ligands and carboxylate groups of neighboring molecules as well as stacking of the diethynyl-veratrole units. The two iron atoms are separated by 3.4837(9) Å with an Fe-O-Fe angle of 155.37(16)°. The two H2O molecules bind in a syn fashion with respect to the diiron vector, similar to stoichiometrically analogous complexes that contain either two unlinked BPG- or two BPP- (bis(2-pyridylmethyl)aminopropionate) ligands.51,52 In contrast to these two compounds, the pyridine nitrogen atoms of the BPG- moieties in 6 are bound syn instead of anti to each other, which is a consequence of the linker. Furthermore, the values for the Fe-Fe distance and the Fe-O-Fe angle in 6 are significantly smaller in comparison to these two complexes (Table 1). In a similar complex with the ligand 6-HPA, where the two neutral TPA units are linked to one another by an ethylene bridge, the distances between the two iron atoms are even longer than 3.56 Å and the Fe-O-Fe angles close to linear.51–53 The shorter distance and angle in 6 are most likely a consequence of more rigid DEV linker, which draws the iron atoms closer together.
Figure 1.
ORTEP diagram of 6 displaying thermal ellipsoids (40%). For clarity, the ClO4− anions are not shown.
Table 1.
Comparison of Fe-Fe Distances and Fe-O-Fe Angles (deg) of Diiron Compounds with a [Fe2(μ-O)(H2O)2] Core.
| Compound | Fe-Fe (Å) | Fe-O-Fe (deg) | Ref. |
|---|---|---|---|
| 6 | 3.4837(9) | 155.37(16) | a |
| [Fe2(μ-O)(H2O)2BPG2]2+ | 3.56 | 169.8(2) | 51 |
| [Fe2(μ-O)(H2O)2BPP2]2+ | 3.564(2) | 168.8(3) | 52 |
| [Fe2(μ-O)(H2O)2(6-HPA)2]4+ | 3.607(3) | 179.2 | 53 |
This work.
Abbreviations: BPP = bis(2-pyridylmethyl)aminopropionate; 6-HPA = 1,2-bis[2-{bis(2-pyridylmethyl)aminomethyl}-6-pyridyl]ethane.
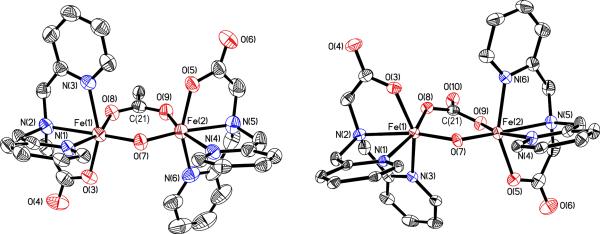
Compounds 7 and 8 have very similar structural parameters. These complexes differ in the chemical nature of the bridging ligand, a carboxylate in the case of 7 and a carbonate ion in 8. Accordingly, compound 7 is positively charged and 8 is neutral. Both diiron cores are displayed in Fig. 2, and crystallographic information and selected bond distances and angles are summarized in Table S2. The BPG2DEV2- ligand again stabilizes the dinuclear structures by bridging the diiron centers. The carbonato and carboxylato bridges in 7 and 8 lie approximately in the plane defined by the Fe-O-Fe unit, a feature generally observed in these types of compounds.47,54
Figure 2.
ORTEP diagram of 7 (left) and 8 (right) displaying thermal ellipsoids (50%). For clarity, the diethynylveratrole backbones, the AriPrO group, and the ClO4− anion are not shown.
For compound 7 there are two crystallographically independent molecules in the asymmetric unit and geometric comparisons are reported for averaged values. In 7, the Fe-Fe distance is 3.218 Å and the Fe-O-Fe angle 128.5°. Compound 8 contains a (μ-oxo)(μ-carbonato)diiron(III) core in which the two iron atoms are separated by 3.1142(18) Å and related by a pseudo-C2 axis. The Fe-O-Fe angle is 125.97(15)° and the distance to the bridging oxygen atom is 1.740(3) Å, which is consistent with values for other oxo-bridged diiron(III) complexes.55,56
Despite an extensive literature on diiron complexes having tripodal amine ligands, especially TPA derivatives, little has been reported for more carboxylate-rich systems.24,25 In a CSD search, only three crystal structures with the simple BPG− ligand bound to iron are listed.57 The first has a structure similar to that of 7, but with a bridging benzoate.54 No reactivity studies of this compound were reported. The second is a hydroxo-bridged diiron compound58 and the third structure contains two H2O ligands bound to an oxo-bridged diiron core (vide infra).51 Although we obtained evidence for a hydroxo-bridged BPG2DEV2– diiron(III) complex by mass spectrometry (vide infra), we were not able to isolate such a species.
Spectroscopic Characterization of 6, 7, and 8
The UV-vis spectrum of 6, which contains only oxo as a bridging ligand, differs dramatically from those of the oxo/carboxylato- and oxo/carbonato-bridged compounds 7 and 8. Fig. 3 reveals that 6 has a maximum in its optical absorption spectrum around 490 nm, whereas more bands appear in the spectra of 7 and 8, which are similar to one another. Despite this spectral similarity, the solubilities of the two latter complexes differ significantly. Compound 7 has excellent solubility in CH3CN and other organic solvents, presumably owing to the lipophilic carboxylate ligand, but 8 is only appreciably soluble in mixtures of CH3OH/CH2Cl2 or CH3CN/H2O.
Figure 3.
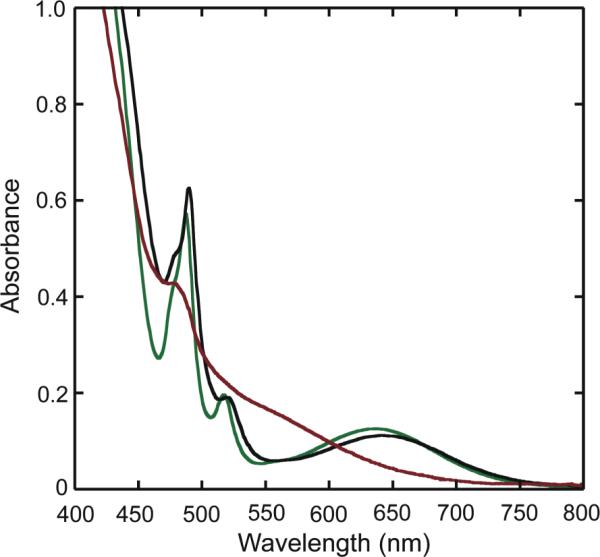
UV-vis absorption spectra of 1 mM solutions of 7 (black) and 8 (green) in CH3OH/CHCl3 (1:1) and of 6 (red) in CH3CN/H2O (10:1).
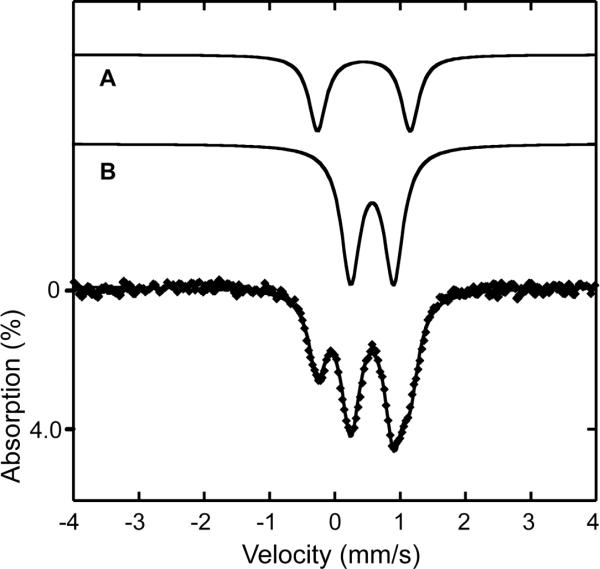
Zero-field Mössbauer spectra of solid samples of 6, 7, and 8 were acquired at 4.2 K and/or 90 K. Table 2 summarizes the derived Mössbauer parameters from these experiments and the spectra are displayed in Fig. 4. The isomer shifts acquired at 90 K lie between δ = 0.45 and 0.47 mm/s and quadrupole splitting parameters between ΔEQ = 1.35 and 1.59 mm/s for the three compounds. These values are characteristic of 6-coordinate high-spin (μ-oxo)diiron(III) complexes, which generally have quadrupole splitting values of > 1 mm/s.55
Table 2.
Zero-Field Mössbauer Parameters of Solid 6, 7, and 8, Acquired at 90 K.
| Compound | δ (mm/s) | ΔEQ (mm/s) | Γ (mm/s) |
|---|---|---|---|
| 6 | 0.47(2) | 1.59(2) | 0.29 |
| 7 | 0.46(2) | 1.47(2) | 0.31 |
| 8 | 0.47(2)a | 1.35(2)a | 0.28a |
| 0.45(2) | 1.36(2) | 0.31 |
Acquired at 4.2 K.
Figure 4.
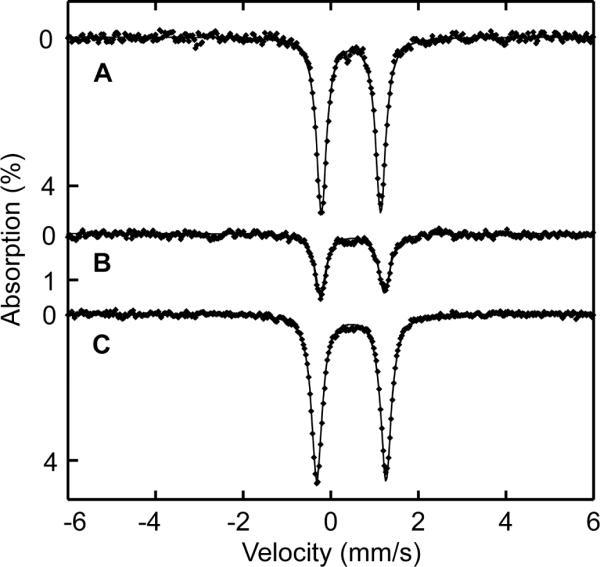
Zero-field Mössbauer spectrum of 8 (A), 7 (B), and 6 (C) acquired at 90 K [experimental data (❶) and calculated fits (—)].
Fixation of CO2 to Form the Carbonato-Bridged Complex 8
Complex 6 reacts with atmospheric CO2 in organic solvents containing triethylamine to form the carbonato-bridged diiron(III) complex 8. When this reaction was monitored by UV-vis spectroscopy, rapid and quantitative formation of a species having the spectral properties of 8 was apparent (Fig. 5). Even at low temperatures (−78 °C), the reaction occurred in a few seconds following introduction of excess CO2 (g). Fixation of CO2 by hydroxo-bridged diiron(II) complexes has been observed previously,59 but only three examples exist in which oxo-bridged diiron(III) complexes react with CO2 to form carbonato-bridged species.46,47,60
Figure 5.
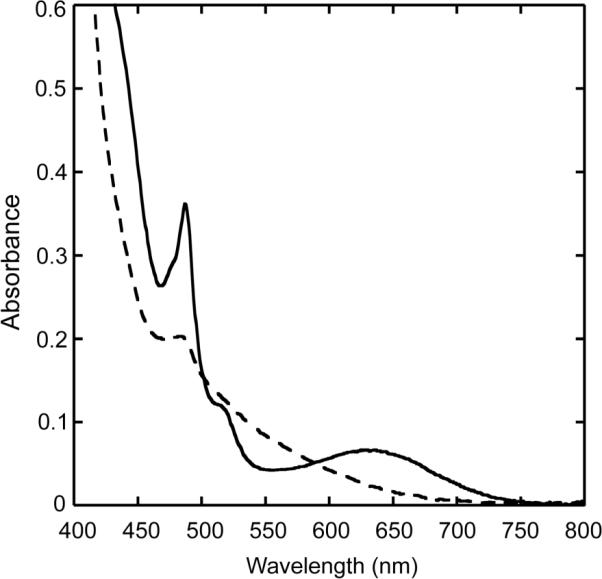
UV-vis spectra of 6 before (- - -) and after reaction with CO2 (—) in a basic CH3OH/CH2Cl2 (1:1) solution.
We propose that 8 forms in stepwise manner via formation of a metal hydroxo species. An analogous mechanism was established for the enzyme carbonic anhydrase, which catalyzes the physiologically essential hydration of CO2 to form bicarbonate.61,62 Deprotonation of a water ligand in 6 could produce a bridging hydroxo ion, which is a reasonable supposition because a diiron species with this composition is observed in the electrospray mass spectrum of 6 under basic conditions (vide infra) and because base is required to promote the reaction in organic solvents. In a second step, nucleophilic attack of the hydroxo ligand on the carbon atom of CO2 would form bicarbonate. The latter is readily deprotonated to produce the observed carbonato-bridged diiron(III) complex.
Mass Spectrometry – Stability of the Dinuclear Core
The coordination chemistry of complexes 6, 7, and 8 is also reflected in ESI mass spectrometry experiments. Mass spectra of the dinuclear complexes dissolved in neutral solution revealed the parent compounds, but in basic aqueous solutions, several new ion clusters are detected. Chart 3 displays the core structures of the resulting diiron species. Two new prominent peaks at m/z = 839 and 883 correspond to positively charged hydroxo- and carbonato-bridged complexes having the formula [Fe2(μ-O)(μ-OH)(BPG2DEV)]+ and {[Fe2(μ-O)(μ-CO )(BPG2DEV)]+H}+ (8), respectively. The hydroxo-bridged complex can form either by substitution of a bridging ligand by OH− or by deprotonation of a ligated H2O molecule in 6. Although no crystal structure was obtained that revealed this particular unit, it is reasonable to propose that the core can readily form, by analogy to related diiron(III) complexes with a BPG− ligand and derivatives thereof.51,58 The carbonato-bridged species most likely derives from a reaction in basic solution of the diiron complex with atmospheric CO2, as occurs during the synthesis of 8. Since complex 6 contains labile H2O ligands, it is not surprising that a peak corresponding to the parent ion is not observed in the mass spectrum. When excess carboxylic or carbamic acid was added to solutions of 6, 7, and 8, a dominant peak for the corresponding carboxylate- or carbamate-bridged species was observed. Interestingly, very bulky carboxylates could also be introduced this manner, suggesting that even sterically hindered bridging ligands are accommodated by the platform (data not shown). In contrast to the described systems, analogous Fe–O–Fe complexes containing non-covalently linked BPG− units and derivatives thereof readily dissociate or oligomerize to give mononuclear or trinuclear complexes, respectively.51,58
Chart 3.

Reaction of 6, 7, and 8 with H2O2 – Characterization of a Common Peroxo Intermediate
(a) UV-vis Spectroscopic Studies
When 6, 7, and 8 were treated with excess H2O2 in the presence of a small amount of NEt3, the solutions turned to a more intense, maroon color, concomitant with evolution of a gaseous product. A test with an alkaline pyrogallol-solution confirmed the formation of dioxygen.63,64 The intermediate products from reactions with H2O2, 6a, 7a, and 8a, in solutions of CH3CN/H2O were monitored by UV-vis spectroscopy. Buildup of a broad visible absorption band at 490 nm (ε = 1500 M−1 cm−1) was observed in all three cases. The corresponding spectra are presented in Fig. 6 and in Figs. S5 and S6. When the reactions were repeated without the addition of NEt3, the solutions turned yellow and no spectra analogous to those for 6a, 7a, or 8a were observed. The formation rate of 6a, 7a, and 8a was strongly dependent on the amount of H2O2 used. An excess of ca. 1000 equiv of H2O2 was required to achieve the maximum absorption intensity at 490 nm for the carbonato-bridged complex 8 over a period of more than an hour. With compound 7, which has a bridging carboxylate ligand, the band maximized more rapidly (ca. 10–15 min) in the presence of a smaller amount of H2O2 (ca. 50–100 equiv, Fig. S5). In contrast, only 5–10 equiv of H2O2 and less than a minute of reaction time were required to form 6a (Fig. S6). These observations are consistent with loss of a H2O ligand occurring more readily than the less labile monoanionic carboxylate or dianionic carbonate ligands. Intermediates 6a, 7a, and 8a were also observed in other solvent mixtures, including CH3OH/H2O and CH2Cl2/CH3OH. When 6a was generated in a solution of aqueous methanol, an absorption with essentially the same features as found for 6a in CH3CN/H2O was observed, but the peak maximum shifted from 490 nm to 470 nm (Fig. S7).
Figure 6.
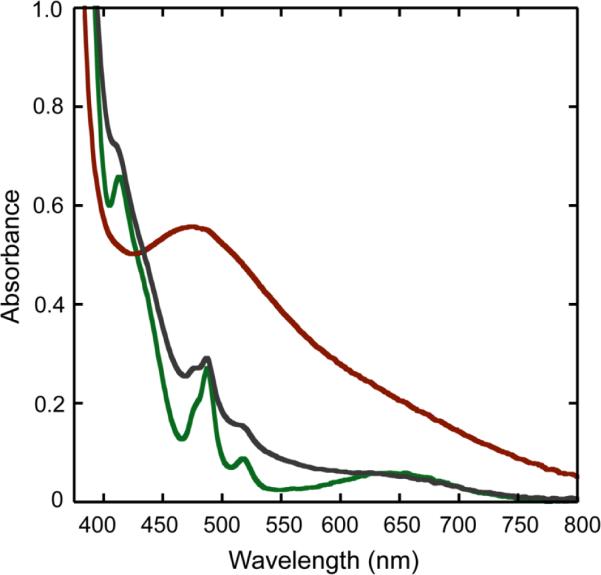
UV-vis spectra, recorded at 0 °C, of a reaction mixture of 8 (0.37 mM, green trace) in CH3CN/H2O (2:1), NEt3, and H2O2 to form a peroxo intermediate 8a (red trace). The grey trace corresponds to the product from the reaction.
Reaction of 8 with 1000-fold excess H2O2 and a catalytic amount of base was monitored by UV-vis spectroscopy, as displayed in Fig. 6. Early in the time course of the reaction (ca. 90 min), the intermediate builds up but it subsequently decays to a species having a similar absorption spectrum as the starting material. After a second addition of a 1000-fold excess of H2O2, the band at 490 nm reappears. These experiments, together with mass spectral evidence, reveal that the oxo-bridged diiron core remains largely intact and is most likely bridged by a carbonate ligand because of the similarity of the final spectrum to that of the starting material.
(b) Mössbauer Spectroscopic Studies
Zero-field Mössbauer spectra of frozen solution samples of 6a, 7a, and 8a were acquired at 90 K. The spectrum of 6a is shown in Fig. 7, and those for 7a and 8a are displayed in Figs. S8 and S9. Mössbauer parameters for the three compounds are listed in Table 3. A sample of 8a (in CH3CN/H2O) was measured at 4.2 K and fit to two quadrupole doublets with an area ratio of 78:22. Because of limited solubility, the sample contains nearly 80% of starting material 8, which can be assigned to one of the doublets. The second doublet, however, belongs to the reaction intermediate 8a with an isomer shift of δ = 0.63(6) mm/s and quadrupole splitting parameter of ΔEQ = 0.64(6) mm/s. Similarly, in the 90 K spectrum of 7a (in CH3OH/H2O), there is evidence for a population of the starting material 7 in the spectrum, together with another quadrupole doublet assigned to a species having δ = 0.58(2) mm/s and ΔEQ = 0.56(2) mm/s. The best conversion to the intermediate was achieved in a reaction of a dilute (ca. 2 mM) solution of 57Fe-enriched 6 with H2O2 and base. Here, 66% of 6a was generated with Mössbauer parameters of δ = 0.58(2) mm/s and ΔEQ = 0.58(2) mm/s, identical within error limits to those for 7a. Taking into account that the spectrum of 8a was acquired at temperatures and from solvents different than those of 6a and 7a, the data strongly suggest that 6a, 7a, and 8a are the same species. The presence of a single quadrupole doublet is most consistent with two high-spin iron sites symmetrically bridged by a peroxo-ligand.
Figure 7.
Zero-field Mössbauer spectrum [experimental data (❶) and calculated fits (—)] recorded at 90 K for a frozen solution sample of the product of the reaction of 6 with excess H2O2 and a trace of NEt3. The sample contained 66% of 6a (B) and 34% of a diiron(III) species (A; δ = 0.45 mm/s and ΔEQ = 1.41 mm/s) with Mössbauer parameters similar to those of the starting material.
Table 3.
Zero-Field Mössbauer Parameters of Intermediates 6a, 7a, and 8a, Acquired at 90 K.
| δ (mm/s) | ΔEQ (mm/s) | Γ (mm/s) | |
|---|---|---|---|
| 6a | 0.58(2) | 0.58(2) | 0.35 |
| 7a | 0.58(2) | 0.56(2) | 0.29 |
| 8a | 0.63(6) | 0.64(6) | 0.32 |
We were interested to learn whether the starting diiron complex reforms upon completion of reactions with H2O2, as implied by the UV-vis spectroscopic experiments. We therefore measured a frozen solution sample [CH3OH/H2O (20:1)] of the decomposition product from a reaction of 6 with ca. 50 equiv of H2O2 and a trace of NEt3. The Mössbauer parameters of δ = 0.46(3) mm/s and ΔEQ = 1.39(3) mm/s were very similar to those for the starting material 6 (δ = 0.45(3) mm/s; ΔEQ = 1.51(3) mm/s) in the same basic solution mixture. We conclude that this species is most likely a (μ-oxo)(μ-hydroxo)diiron(III) complex and that no decomposition occurred (vide supra and Chart 3).
The spectroscopic and mass spectrometric (vide infra) data determined for the common intermediate formed in the H2O2 reactions of 6, 7, and 8 are characteristic of a (μ-oxo)(μ-peroxo)diiron(III) complex. Table 4 lists some examples of transient peroxo species observed in pre-steady-state studies of non-heme diiron enzymes and of oxo-bridged diiron(III) model systems. Well-defined peroxodiiron(III) intermediates are observed in sMMOH, RNR-R2, and Δ9D4 and in the T201S variant of ToMOH20 that display characteristic peroxo ligand-to-metal charge transfer (LMCT) bands between 650 to 750 nm and Mössbauer parameters of δ = 0.62–0.68 mm/s and ΔEQ > 1.0 mm/s.4 In contrast, the recently characterized, putative peroxodiiron(III) intermediate of ToMOH has Mössbauer isomer shifts smaller than δ = 0.6 mm/s, quadrupole splitting parameters significantly less than 1 mm/s (ΔEQ = 0.66 mm/s), and lack an optical band in their absorption spectra.65 These significant differences can be rationalized by a different peroxo coordination mode and/or protonation state. Synthetically prepared peroxodiiron(III) complexes have the peroxo ligand generally bound in a μ-1,2-fashion with spectroscopic properties very similar to those of peroxo intermediates in the former group of enzymes.5 The UV-vis and Mössbauer spectroscopic properties of the peroxodiiron(III) intermediate reported here, however, differ significantly from these complexes. To our knowledge, of all the synthetic examples of (μ-oxo)(μ-peroxo)diiron(III) species, only the intermediates reported here, 6a, 7a, and 8a, have a Mössbauer quadrupole splitting parameter that is nearly identical to the values for colorless peroxo intermediates in ToMOH and PHH. In addition to their unique Mössbauer spectroscopic properties, intermediates 6a, 7a, and 8a display a considerably blue-shifted LMCT band, assigned by RR spectroscopy (see below), in their UV-vis spectra compared to other peroxodiiron(III) species (Table 4). A comparison with two (μ-oxo)(μ-peroxo)diiron(III) complexes providing an N4 (6-Me3-TPA)66 and a closely related N3O (6-Me2-BPP) coordination environment on each iron atom,67 similar to that supplied by the BPG− unit, display LMCT bands at 648 nm and 577 nm, respectively, consistent with the carboxylate being a stronger donor. This observation might explain the hypsochromic shift in intermediates containing the BPG2DEV2– ligand.
Table 4.
Spectroscopic Parameters for Peroxo Intermediates in Non-Heme Diiron Enzymes and Synthetic Oxo-Bridged Diiron(III) Compounds.
| Optical | Mössbauer | ||||
|---|---|---|---|---|---|
|
| |||||
| γmax (nm) | ε (M−1cm−1) | δ (mm/s) | ΔEQ (mm/s) | Ref. | |
| sMMOH (M. caps. (Bath)) | 700 | 1800 | 0.66 | 1.51 | a |
| ToMOH (Pseudomonas sp. OX1) | - | - | 0.54 | 0.66 | 65 |
| ToMOH T201S (Pseudomonas sp. OX1) | 675 | 1500 | 0.67 | 1.51 | 20 |
| RNR-R2 D84E | 700 | 1500 | 0.63 | 1.58 | a |
| Δ9-desaturase | 700 | 1200 | 0.68 | 1.90 | a |
| [Fe2(μ-O)(μ-1,2-O2)(6-Me3-TPA)2]2+ | 494; 648c | 1100; 1200 | 0.54 | 1.68 | 66 |
| [Fe2(μ-O)(μ-1.2-O2)(μ-O2CR)(hexpy)2]+ | 510; 605c | 1300; 1310 | 0.53 | 1.67 | 68 |
| [Fe2(μ-O)(μ-1,2-O2)(6-Me2-BPP)2] | 577 | 1500 | 0.50 | 1.46 | 67 |
| [Fe2(μ-O)(μ-O2)BPG2DEV] | 490 | 1500 | 0.58 | 0.58 | b |
Parameters were taken from ref. 1.
This work.
peroxo-Fe(III) charge transfer band.
(c) EPR Spectroscopy
EPR spectroscopic analysis of 8a reveals an axial signal with g values of 2.062 and 2.004, which we attribute to a mixed-valent diiron(II,III) (S = ½) species. Spin quantitation indicated only 2.6% abundance, however. The bulk of the sample is EPR-silent, which we assign to two iron(III) centers antiferromagnetically coupled to one another. This result is consistent with a peroxo-bridged diiron(III) complex.5 The absence of an EPR signal centered at g = 4.3, characteristic for mononuclear high-spin iron(III) complexes, is also in agreement with these findings. The EPR spectrum is displayed as Fig. S12.
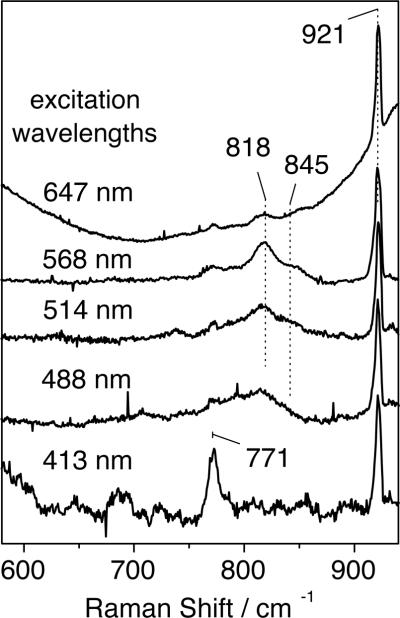
(d) Resonance Raman Spectroscopy
RR spectra of intermediate 6a, prepared with either H2 16O2 (or H2 18O2) and obtained with 568-nm excitation, revealed two features at 819 and 845 (772 and 797) cm−1, respectively (Fig. 8). These RR signals are not observed in samples that were allowed to proceed in time beyond decay of intermediate 6a (Fig. 8B). The intensities of the RR doublets also decrease after prolonged laser exposure and photobleaching of the samples from dark brown to light yellow. Relative to solvent bands, these intensities also vary with excitation wavelength, revealing a significant decrease at 647 nm and at 413 nm, where intra-pyridine vibrations enter into resonance (Fig. 9). Because of the complexity of these RR signals and the photosensitivity of intermediate 6a, extracting excitation profiles would be an overinterpretation of the data. Nevertheless, the excitation wavelength dependence of the isotope-sensitive signals qualitatively matches expectation based on the absorption spectra of intermediate 6a (Fig. S6).
Fig. 8.
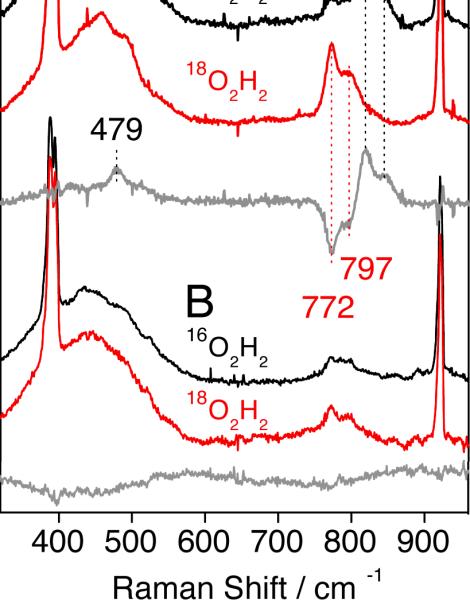
RR spectra of intermediate 6a (A) and its decomposition product (B) obtained with 568-nm excitation at 110 K. Sharp Raman bands below 400 and above 900 cm−1 correspond to solvent vibrations.
Fig. 9.
RR spectra of intermediate 6a obtained with different laser excitations at 110 K. All traces are normalized to the 921-cm−1 solvent band.
The observed RR frequencies and 18O-shifts (−47/−48 cm−1) are consistent with metal-peroxo vibrations. The ν(O–O) frequencies in symmetrically-bridged (μ-1,2- peroxo)diiron(III) complexes range from 830 to 908 cm−1 and low frequency O–O stretching bands are generally correlated with short Fe-Fe distances.69–71 The RR bands associated with ν(O–O) in intermediate 6a are at the lower end of this range and a comparison to these systems indicates that the Fe-Fe distance in intermediate 6a may be as short as 3.1–3.2 Å. Short Fe-Fe distances require stabilization from bridging ligands in addition to the peroxo group, with a μ-oxo group providing the shortest such distance. Although Fe-O-Fe modes were not identified in the RR spectra of intermediate 6a, it is reasonable to assume that the oxo bridge, present in the starting material, remains in intermediate 6a. This assignment is strongly supported by mass spectrometric analysis (vide infra). The covalent linkage between the two iron sites supplied by the syn N-donor ligand BPG2DEV2- may also play an important role in promoting a short Fe-Fe distance. In a similar peroxodiiron(III) species, with a dinucleating ligand having two covalently linked tris(2-pyridyl)methane moieties, the O–O mode of the bound peroxide appears at very low value of 830 cm–1, which is consistent with this hypothesis.71,72
From spectroscopic studies we can conclude that the peroxo ligand bridges the two iron centers in 6a, but we cannot definitively assign its binding mode. Detailed experimental and theoretical studies on (μ-oxo)(μ-1,2-peroxo)diiron(III) intermediates having unlinked tetradentate capping ligands, related to those in BPG2DEV2-, confirmed the 1,2-peroxo bridging mode. Furthermore, a crystal structure of [Fe2(μ-O)(μ-1,2-O2)6-Me2-BPP] (see Table 4), which has an identical ligand set to that of 6a, reveals a 1,2-bridging peroxo ligand lying approximately in the plane defined by the Fe-O-Fe unit.67 The spectroscopic properties and the O–O stretching frequencies of the peroxodiiron(III) compound described here, however, differ significantly from those reported for these species and we cannot rule out an alternative peroxo binding mode. Because the mass spectra of 6a–8a reveal the presence of an added proton as well as the peroxo ligand (vide infra), the structure might contain a μ-1,1-hydroperoxodiiron(III) unit in which the dangling O-atom is protonated, a unit that is consistent with all of the data.
The presence of a doublet rather than a single peak for the isotope-sensitive RR bands may result either from two different conformations of the peroxodiiron complex or Fermi splitting of a fundamental O–O stretch corresponding to the doublet average of 832 cm−1. Fermi coupling of the ν(O–O) with underlying non-resonant vibrations has been invoked for several peroxodiiron complexes.10,17,66,70 On the other hand, the possible occurrence of two distinct peroxodiiron species must also be taken into consideration, perhaps reflecting different protonation states. Optical spectroscopic73 and kinetic studies indicate the presence of two peroxo intermediates in the oxygenation chemistry of sMMOH and ToMOH.20 In the low-frequency region, the [16O-intermediate – 18O-intermediate] RR difference spectrum isolates a positive feature at 479 cm−1 that may correspond to the expected ν(Fe-16O2) mode, even though its negative 18O-counterpart does not emerge from the broad and nearly featureless background signal that occurs in this region (Fig. 8).
(e) Mass Spectrometry
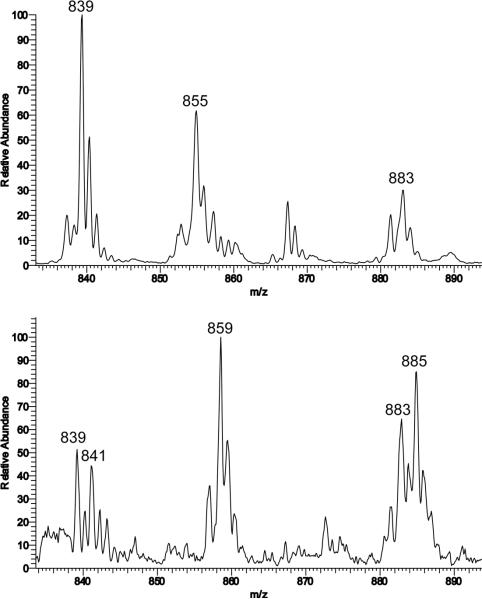
ESI-MS of 6a, 7a, and 8a were acquired using solutions of CH3CN/H2O (3:1) or CH2Cl2/CH3OH (1:1) in low- and high-resolution modes. In each high-resolution mass spectrum of 6a, 7a, and 8a there was an ion cluster having a mass and isotope pattern identical to that calculated for the M+H (peroxo)diiron(III) species {[Fe2(μ-O)(μ-O2)BPG2DEV]+H}+ with a theoretical m/z = 855.1195. The recorded values were m/z = 855.1205, 855.1189, and 855.1161 for 6a, 7a, and 8a, respectively. A comparison between the theoretical and observed spectra is presented in Fig. S13. Isotope-labeling experiments carried out with H2182O2 (2% in H2O) revealed a peak shift of four units from m/z = 855 to 859, demonstrating that the peroxide is bound to the diiron center. A comparison of the two mass spectra is shown in Fig. 10.
Figure 10.
ESI mass spectrum of 7a at 295 K in CH3CN displaying the isotope patterns for ions at m/z = 855 for {[Fe2(μ-O)(μ-16O2)BPG2 DEV]+H+} (top) and m/z = 859 for for {[Fe2(μ-O)(μ-18O2)BPG22 DEV]+H+} (bottom). The peak m/z = 867 in the upper spectrum can be assigned to a formate-bridged diiron complex [Fe2(μ-O)(μ-O CH)BPG2DEV]+, which is due to the presence of residual formic acid impurities.
Singly-charged ion clusters of [Fe2(μ-O)(μ-OH)(BPG2DEV)]+ (m/z = 839) and {[Fe2(μ-O)(μ-CO3)(BPG2DEV)]+H}+ (m/z = 883) were also observed in these mass spectra, which derive from the reaction of the diiron complex with hydroxide and CO2 from the air (vide supra). Interestingly, two additional peaks at m/z = 841 and 885 were detected in the 18O-labeled sample. These values are shifted by two mass units from the peaks for these two diiron complexes, indicating that an 18O atom is incorporated into the hydroxo- and carbonato-bridged compounds, respectively. This result suggests that the O–O bond of the bound peroxide is indeed cleaved and, presumably, that H2O is formed. An O–O bond scission is reasonable, because this reaction has been investigated extensively in related (μ-oxo)(μ-1,2-peroxo)diiron(III) complexes, which form high-valent diiron centers, and has been proposed for a similar compound in a dinucleating ligand system.25,53,74 The intermediate could react with another equivalent of H2O2 to form H2O and O2. We propose that the newly formed H2O molecule stays bound to the diiron center and can readily be deprotonated under basic conditions to form a bridging hydroxide ion. Such an intermediate could additionally react with CO2 from air to form the carbonato-bridged species. By mass spectrometry, we were able to establish that the peroxo species is regenerated upon additional treatment with H2O2 (data not shown).
Catalase Activity
When H2O2 was added to a basic solution of the diiron compounds 6, 7, or 8, O2 formation was observed, as confirmed by a test with an alkaline pyrogallol solution.63,64 The catalase-like activity of the carbonato complex 8 was investigated in more detail and the O2 formed in the reactions was quantified. To a fixed concentration of diiron(III) complex 8 and NEt3 in a solution of CH3CN/H2O (2:1) at 0 °C were added various amounts of H2O2. The quantity of O2 produced was measured volumetrically. The yield of O2 depends linearly on the amount of H2O2, with a slope of 0.53 (R2 = 0.99), which corresponds to catalase-like reaction stoichiometry (Fig. 11 and eq. 1). The diiron catalyst is very efficient and achieves ~10,000 turnovers. Experiments
| (1) |
with compounds 6 and 7 under the same conditions revealed them to be as efficient as 8 with respect to catalase function.
Figure 11.
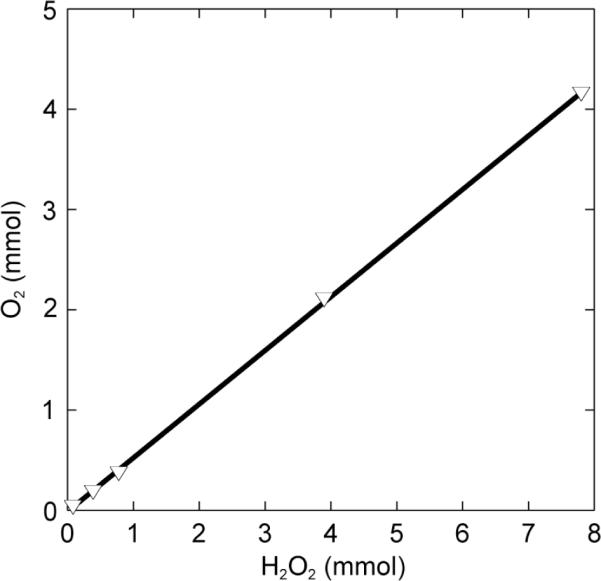
O2 formation from 0.078 to 7.8 mmol H2O2 at 0 °C as catalyzed by 8 (2 mL of 0.4 mM solution in CH3CN/H2O, 2:1) and 10 μL of NEt3.
Catalase activity has been reported in reactions of a relatively small number of oxo-bridged diiron(III) compounds with H O 75–80 In all cases, intermediates with spectroscopic properties characteristic for peroxo-bridged diiron(III) complexes were observed. Some common features of these H2O2-disproportionation catalysts include complex decomposition, TON (turn-over number) dependence on the exchangeability of the bridging ligand, and increasing O2 formation rates at higher pH values. In contrast, to these previously reported systems, decomposition of the complexes 6, 7, and 8 upon treatment with H2O2 does not occur. The increased stability of the complexes is attributed to the BPG2DEV2- ligand. Remarkably, quantitative O2 production is observed for all three complexes 6–8, with TONs significantly higher than those reported for other systems (the ratio of [Fe2]:H2O2 never exceeded 1:500) and only when base was added to the reaction mixture. In samples that were lacking additional base, quantitative O2 production was not observed.
Catalase activity is important for protecting the cell from oxidative damage by excess H2O2. A ubiquitous family of metalloenzymes disproportionate H2O2 in an exothermic reaction to form H2O and dioxygen. One of the two most abundant classes of catalases contains an iron protoporphyrin IX cofactor with an axial tyrosinate ligand.81 The second most common catalase enzyme contains a dimanganese active site.82 For non-heme diiron enzymes, catalase activity has been reported for ToMOH83 and toluene-2-monooxygenase from Burkholderia cepacia G4,84. By comparison with previous studies of diiron model compounds, the present findings are important for our general understanding of catalase activity in BMMs and the decomposition pathway of H2O2 catalyzed by diiron centers.
Substrate Oxidation
We also investigated whether the peroxo intermediates 6a, 7a, and 8a might function as oxidases. The common intermediate quantitatively oxidizes PPh3 to Ph3PO, a very easy conversion, but is a poor catalyst for the epoxidation of cis-cyclooctene, unlike some related systems.53,85,86 Quantitation of the epoxide revealed yields < 1% and TONs of ca. 8 and 3, for 6a and 7a, respectively (Table S4). The production of O2 was strongly diminished by addition of phosphine to the reaction, but was not significantly affected when cyclooctene was present, which is consistent with competitive catalase and substrate oxidation reactions.
Summary and Perspective
This study describes the successful application of the dinucleating ligand H2BPG2DEV to specifically model the syn coordination of histidine residues in non-heme diiron enzymes. This scaffold is more biologically relevant than previous versions due to its greater carboxylate content. Three (μ-oxo)diiron(III) derivatives were characterized, one with a terminal water molecule on each iron atom and two with additional bridging ligands, either carbonate or carboxylate. Reactivity studies with H2O2 show that the dinucleating ligand conveys an inherent stability to all three oxo-bridged diiron complexes, which serve as catalases in multi-turnover reactions with large excesses of H2O2. These characteristics distinguish these complexes from the broader class of diiron(III) complexes that do not have covalently linked tripodal ligands. The present system belongs to a small group of non-heme diiron model complexes having catalase activity that dominates over its oxygenating properties. Similar catalase activity has been observed in ToMOH and PHH, suggesting additional relevance of the present model complexes to biological counterparts. Spectroscopic analysis of a transient (μ-oxo)(μ-peroxo)diiron(III) species generated in this chemistry reveals properties that deviate from the characteristic parameters for peroxo-bridged intermediates in most model complexes and non-heme diiron enzymes. The Mössbauer spectroscopic properties closely resemble those for a peroxo intermediate in ToMOH, with ΔEQ values less than 1 mm/s. Raman studies reveal an O–O stretching frequency that is atypically low, suggesting a short iron-iron distance in the peroxo intermediate, presumably facilitated by the dinucleating ligand and possibly reflecting a previously unknown μ-1,1 bridging mode. These physical properties of these complexes differentiate them from compounds with mononucleating ligands. Further studies of related complexes are warranted in pursuit of information to understand the origin of the spectroscopic properties and reactivity of non-heme diiron sites in biology.
Supplementary Material
Acknowledgements
This work was supported by grants GM032134 (SJL) and GM74785 (PM-L) from the National Institute of General Medical Sciences. We thank Ms. Wanhua Ye at the Chemistry Department at Tufts University and Ms. Li Li at the DCIF at MIT for help with mass spectrometric measurements, Dr. Sebastian Stoian for assistance in acquiring the EPR spectra, and Drs. Peter Müller and Daniela Buccella for assistance with X-ray crystallography.
Footnotes
Supporting Information Available: Experimental details for the synthesis of [57Fe2(μ-O)(H2O)2BPG2DEV](ClO4)2 (57Fe-6), crystallographic refinement details, crystallographic tables (Tables S1–S3), a packing diagram of 6 (Fig. S1), ORTEP diagrams of the complete molecules 7 and 8 (Fig. S2 and S3), space-filling diagrams of 6–8 (Fig. S4), and CIF files for 6–8, UV-vis spectra of 6a and 7a (Fig. S5–S7), solution Mössbauer spectra of 7a, 8a, 6 and the decomposition product of 6a (Fig. S8–S11); the EPR spectrum of 8a (Fig. S12), high-resolution mass spectra of 6a–8a (Fig.S13 and S14), and a table for substrate oxidation studies (Table S4) are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Feig AL, Lippard SJ. Chem. Rev. 1994;94:759–805. [Google Scholar]
- (2).Wallar BJ, Lipscomb JD. Chem. Rev. 1996;96:2625–2657. doi: 10.1021/cr9500489. [DOI] [PubMed] [Google Scholar]
- (3).Kurtz DM., Jr. J. Biol. Inorg. Chem. 1997;2:159–167. [Google Scholar]
- (4).Merkx M, Kopp DA, Sazinsky MH, Blazyk JL, Müller J, Lippard SJ. Angew. Chem., Int. Ed. 2001;40:2782–2807. doi: 10.1002/1521-3773(20010803)40:15<2782::AID-ANIE2782>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- (5).Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee S-K, Lehnert N, Neese F, Skulan AJ, Yang Y-S, Zhou J. Chem. Rev. 2000;100:235–349. doi: 10.1021/cr9900275. [DOI] [PubMed] [Google Scholar]
- (6).Sturgeon BE, Burdi D, Chen S, Huynh B-H, Edmondson DE, Stubbe J, Hoffman BM. J. Am. Chem. Soc. 1996;118:7551–7557. [Google Scholar]
- (7).Logan DT, Su XD, Åberg A, Regnström K, Hajdu J, Eklund H, Nordlund P. Structure. 1996;4:1053–1064. doi: 10.1016/s0969-2126(96)00112-8. [DOI] [PubMed] [Google Scholar]
- (8).Bollinger JM, Jr., Edmondson DE, Huynh BH, Filley J, Norton JR, Stubbe J. Science. 1991;253:292–298. doi: 10.1126/science.1650033. [DOI] [PubMed] [Google Scholar]
- (9).Lindqvist Y, Huang W, Schneider G, Shanklin J. EMBO J. 1996;15:4081–4092. [PMC free article] [PubMed] [Google Scholar]
- (10).Broadwater JA, Ai J, Loehr TM, Sanders-Loehr J, Fox BG. Biochemistry. 1998;37:14664–14671. doi: 10.1021/bi981839i. [DOI] [PubMed] [Google Scholar]
- (11).Sazinsky MH, Lippard SJ. Acc. Chem. Res. 2006;39:558–566. doi: 10.1021/ar030204v. [DOI] [PubMed] [Google Scholar]
- (12).Murray LJ, Lippard SJ. Acc. Chem. Res. 2007;40:466–474. doi: 10.1021/ar600040e. [DOI] [PubMed] [Google Scholar]
- (13).Cafaro V, Izzo V, Scognamiglio R, Notomista E, Capasso P, Casbarra A, Pucci P, Di Donato A. Appl. Environ. Microbiol. 2004;70:2211–2219. doi: 10.1128/AEM.70.4.2211-2219.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Sazinsky MH, Bard J, Di Donato A, Lippard SJ. J. Biol. Chem. 2004;279:30600–30610. doi: 10.1074/jbc.M400710200. [DOI] [PubMed] [Google Scholar]
- (15).Sazinsky MH, Dunten PW, McCormick MS, DiDonato A, Lippard SJ. Biochemistry. 2006;45:15392–15404. doi: 10.1021/bi0618969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Kuzelka J, Farrell JR, Lippard SJ. Inorg. Chem. 2003;42:8652–8662. doi: 10.1021/ic034928e. [DOI] [PubMed] [Google Scholar]
- (17).Moënne-Loccoz P, Baldwin J, Ley BA, Loehr TM, Bollinger JM. Biochemistry. 1998;37:14659–14663. doi: 10.1021/bi981838q. [DOI] [PubMed] [Google Scholar]
- (18).Rinaldo D, Philipp DM, Lippard SJ, Friesner RA. J. Am. Chem. Soc. 2007;129:3135–3147. doi: 10.1021/ja0654074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Lee S-K, Lipscomb JD. Biochemistry. 1999;38:4423–4432. doi: 10.1021/bi982712w. [DOI] [PubMed] [Google Scholar]
- (20).Song WJ, Behan RK, Naik SG, Huynh BH, Lippard SJ. J. Am. Chem. Soc. 2009;131:6074–6075. doi: 10.1021/ja9011782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Lee S-K, Fox BG, Froland WA, Lipscomb JD, Münck E. J. Am. Chem. Soc. 1993;115:6450–6451. [Google Scholar]
- (22).Liu KE, Valentine AM, Wang D, Huynh BH, Edmondson DE, Salifoglou A, Lippard SJ. J. Am Chem. Soc. 1995;117:10174–10185. [Google Scholar]
- (23).Du Bois J, Mizoguchi TJ, Lippard SJ. Coord. Chem. Rev. 2000;200-202:443–485. [Google Scholar]
- (24).Tshuva EY, Lippard SJ. Chem. Rev. 2004;104:987–1012. doi: 10.1021/cr020622y. [DOI] [PubMed] [Google Scholar]
- (25).Que L, Jr., Tolman WB. Angew. Chem., Int. Ed. 2002;41:1114–1137. doi: 10.1002/1521-3773(20020402)41:7<1114::aid-anie1114>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- (26).Tolman WB, Que L., Jr. J. Chem. Soc., Dalton Trans. 2002;5:653–660. [Google Scholar]
- (27).Baik M-H, Gherman BF, Friesner RA, Lippard SJ. J. Am Chem. Soc. 2002;124:14608–14615. doi: 10.1021/ja026794u. [DOI] [PubMed] [Google Scholar]
- (28).Kodanko Jeremy J, Morys Anna J, Lippard Stephen J. Org. Lett. 2005;7:4585–4588. doi: 10.1021/ol051700h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kodanko JJ, Xu D, Song DT, Lippard SJ. J. Am. Chem. Soc. 2005;127:16004–16005. doi: 10.1021/ja054943d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kodanko JJ, Lippard SJ. Inorg. Chim. Acta. 2008;361:894–900. doi: 10.1016/j.ica.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Reisner E, Lippard SJ. Eur. J. Org. Chem. 2008;2008:156–163. [Google Scholar]
- (32).Wang X, Rabbat P, O'Shea P, Tillyer R, Grabowski EJJ, Reider PJ. Tetrahedron Lett. 2000;41:4335–4338. [Google Scholar]
- (33).Policar C, Lambert F, Cesario M, Morgenstern-Badarau I. Eur. J. Inorg. Chem. 1999:2201–2207. [Google Scholar]
- (34).Armstrong WH, Lippard SJ. Inorg. Chem. 1985;24:981–982. [Google Scholar]
- (35).Dunbar KR, Longridge JJ, Rawson JM, Sun J-S, Hagen KS, Do B. Inorg. Synth. 2002;33:103–107. [Google Scholar]
- (36).Gumanov LL, Shteinman AA, Nordlander E, Koldobskii GI. Russ. J. Org. Chem. 2002;38:606–608. [Google Scholar]
- (37).SMART, Software for the CCD Detector System. version 5.6 Bruker AXS; Madison, WI: 2000. [Google Scholar]
- (38).Sheldrick GM. SHELXTL-97. University of Göttingen; Göttingen, Germany: 2000. [Google Scholar]
- (39).Sheldrick GM. Acta Crystallogr., Sect. A. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- (40).Sheldrick GM. SADABS: Area-Detector Absorption Correction. University of Göttingen; Göttingen, Germany: 2001. [Google Scholar]
- (41).Spek AL. PLATON, A Multipurpose Crystallographic Tool. Utrecht University; Utrecht, The Netherlands: 2000. [Google Scholar]
- (42).Kent TA. WMOSS. Minneapolis: 1998. [Google Scholar]
- (43).Lippard SJ. Angew. Chem., Int. Ed. 1988;27:344–361. [Google Scholar]
- (44).Schmitt W, Hill JP, Malik S, Volkert CA, Ichinose I, Anson CE, Powell AK. Angew. Chem., Int. Ed. 2005;44:7048–7053. doi: 10.1002/anie.200501748. [DOI] [PubMed] [Google Scholar]
- (45).Schmitt W, Hill JP, Juanico MP, Caneschi A, Costantino F, Anson CE, Powell AK. Angew. Chem., Int. Ed. 2005;44:4187–4192. doi: 10.1002/anie.200500516. [DOI] [PubMed] [Google Scholar]
- (46).Glaser T, Lügger T, Hoffmann R-D. Eur. J. Inorg. Chem. 2004:2356–2362. [Google Scholar]
- (47).Hazell R, Jensen KB, McKenzie CJ, Toftlund H. J. Chem. Soc., Dalton Trans. 1995:707–717. [Google Scholar]
- (48).Fujita T, Ohba S, Nishida Y, Goto A, Kokii T. Acta Crystallogr., Sect. C. 1994;C50:544–546. [Google Scholar]
- (49).Arulsamy N, Hodgson DJ, Glerup J. Inorg. Chim. Acta. 1993;209:61–69. [Google Scholar]
- (50).Norman RE, Holz RC, Menage S, Que L, Jr., Zhang JH, O'Connor CJ. Inorg. Chem. 1990;29:4629–4637. [Google Scholar]
- (51).Mortensen MN, Jensen B, Hazell A, Bond AD, McKenzie CJ. J. Chem. Soc., Dalton Trans. 2004:3396–3402. doi: 10.1039/B409559F. [DOI] [PubMed] [Google Scholar]
- (52).Hazell A, Jensen KB, McKenzie CJ, Toftlund H. J. Chem. Soc., Dalton Trans. 1993:3249–3257. [Google Scholar]
- (53).Kodera M, Itoh M, Kano K, Funabiki T, Reglier M. Angew. Chem., Int. Ed. 2005;44:7104–7106. doi: 10.1002/anie.200501825. [DOI] [PubMed] [Google Scholar]
- (54).Menage S, Que L., Jr. New J. Chem. 1991;15:431–438. [Google Scholar]
- (55).Kurtz DM., Jr. Chem. Rev. 1990;90:585–606. [Google Scholar]
- (56).Drüeke S, Wieghardt K, Nuber B, Weiss J. Inorg. Chem. 1989;28:1414–1417. [Google Scholar]
- (57).ConQuest. Cambridge Crystallographic Data Center; 2007. [Google Scholar]
- (58).Furutachi H, Ohyama Y, Tsuchiya Y, Hashimoto K, Fujinami S, Uehara A, Suzuki M, Maeda Y. Chem. Lett. 2000:1132–1133. [Google Scholar]
- (59).Kitajima N, Hikichi S, Tanaka M, Morooka Y. J. Am. Chem. Soc. 1993;115:5496–5508. [Google Scholar]
- (60).Schmitt W, Anson CE, Sessoli R, van Veen M, Powell AK. J. Inorg. Biochem. 2002;91:173–189. doi: 10.1016/s0162-0134(02)00404-x. [DOI] [PubMed] [Google Scholar]
- (61).Silverman DN, Lindskog S. Acc. Chem. Res. 1988;21:30–36. [Google Scholar]
- (62).Vahrenkamp H. Acc. Chem. Res. 1999;32:589–596. [Google Scholar]
- (63).Duncan IA, Harriman A, Porter G. Anal. Chem. 1979;51:2206–2208. [Google Scholar]
- (64).Williams DD, Blachly CH, Miller RR. Anal. Chem. 1952;24:1819–21. [Google Scholar]
- (65).Murray LJ, García-Serres R, Naik S, Huynh BH, Lippard SJ. J. Am. Chem. Soc. 2006;128:7458–7459. doi: 10.1021/ja062762l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Dong Y, Zang Y, Shu L, Wilkinson EC, Que L, Jr., Kauffmann K, Münck E. J. Am. Chem. Soc. 1997;119:12683–12684. [Google Scholar]
- (67).Zhang X, Furutachi H, Fujinami S, Nagatomo S, Maeda Y, Watanabe Y, Kitagawa T, Suzuki M. J. Am. Chem. Soc. 2005;127:826–827. doi: 10.1021/ja045594a. [DOI] [PubMed] [Google Scholar]
- (68).Kodera M, Taniike Y, Itoh M, Tanahashi Y, Shimakoshi H, Kano K, Hirota S, Iijima S, Ohba M, Okawa H. Inorg. Chem. 2001;40:4821–4822. doi: 10.1021/ic0155434. [DOI] [PubMed] [Google Scholar]
- (69).Brunold TC, Tamura N, Kitajima N, Moro-oka Y, Solomon EI. J. Am. Chem. Soc. 1998;120:5674–5690. [Google Scholar]
- (70).Moënne-Loccoz P, Krebs C, Herlihy K, Edmondson DE, Theil EC, Huynh BH, Loehr TM. Biochemistry. 1999;38:5290–5295. doi: 10.1021/bi990095l. [DOI] [PubMed] [Google Scholar]
- (71).Fiedler AT, Shan X, Mehn MP, Kaizer J, Torelli S, Frisch JR, Kodera M, Que L., Jr. J. Phys. Chem. A. 2008;112:13037–13044. doi: 10.1021/jp8038225. and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Kodera M, Itoh M, Kano K, Funabiki T. Bull. Chem. Soc. Jpn. 2006;79:252–261. [Google Scholar]
- (73).Brazeau BJ, Lipscomb JD. Biochemistry. 2000;39:13503–13515. doi: 10.1021/bi001473l. [DOI] [PubMed] [Google Scholar]
- (74).Xue G, Fiedler AT, Martinho M, Münck E, Que L., Jr. Proc. Natl. Acad. Sci. U.S.A. 2008;105:20615–20620. [Google Scholar]
- (75).Mauerer B, Crane J, Schuler J, Wieghardt K, Nuber B. Angew. Chem., Int. Ed. 1993;32:289–291. [Google Scholar]
- (76).Balogh-Hergovich E, Speier G, Reglier M, Giorgi M, Kuzmann E, Vertes A. Eur. J. Inorg. Chem. 2003:1735–1740. [Google Scholar]
- (77).Than R, Schrodt A, Westerheide L, Van Eldik R, Krebs B. Eur. J. Inorg. Chem. 1999:1537–1543. [Google Scholar]
- (78).Ito S, Okuno T, Matsushima H, Tokii T, Nishida Y. J. Chem. Soc., Dalton Trans. 1996:4479–4484. [Google Scholar]
- (79).Okuno T, Ito S, Ohba S, Nishida Y. J. Chem. Soc., Dalton Trans. 1997:3547–3551. [Google Scholar]
- (80).Menage S, Vincent JM, Lambeaux C, Fontecave M. J. Chem. Soc., Dalton Trans. 1994:2081–2084. [Google Scholar]
- (81).Maté MJ, Murshudov G, Bravo J, Melik-Adamyan W, Loewen PC, Fita I. Handb. Metalloproteins. 2001;1:486–502. [Google Scholar]
- (82).Wu AJ, Penner-Hahn JE, Pecoraro VL. Chem. Rev. 2004;104:903–938. doi: 10.1021/cr020627v. [DOI] [PubMed] [Google Scholar]
- (83).Murray LJ, Naik SG, Ortillo DO, GarcíaSerres R, Lee JK, Huynh BH, Lippard SJ. J. Am. Chem. Soc. 2007;129:14500–14510. doi: 10.1021/ja076121h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Newman LM, Wackett LP. Biochemistry. 1995;34:14066–76. doi: 10.1021/bi00043a012. [DOI] [PubMed] [Google Scholar]
- (85).White MC, Doyle AG, Jacobsen EN. J. Am. Chem. Soc. 2001;123:7194–7195. doi: 10.1021/ja015884g. [DOI] [PubMed] [Google Scholar]
- (86).Taktak S, Kryatov SV, Haas TE, Rybak-Akimova EV. J. Mol. Catal. A: Chem. 2006;259:24–34. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.