Abstract
We have demonstrated a new enzyme-induced disassembly of amphiphilic nanocontainers based on dendrimers. Disassembly and the ensuing release of non-covalently bound guest molecules are of great interest due to the implications in areas such as drug delivery and sensing. Achieving these with a protein as the stimulus is of even greater importance, because proteins are the primary indicators of biological imbalances. We have achieved the disassembly of the nanocontainers by disturbing the hydrophilic-lipophilic balance in amphiphilic dendrimer building blocks.
Stimuli-responsive drug delivery systems have gained significant attention in recent years, because their controlled release characteristics have resulted in enhanced efficacy at the site of action.1 Most of these systems respond to stimuli such as temperature, pH, light, magnetic field and ionic strength.2 An attractive class of responsive materials would involve molecular designs that respond to biological stimuli, such as proteins. This is because overexpression of proteins, especially enzymes, has been implicated frequently in the diseased state of the cells. Here, we disclose our findings with an enzyme-responsive dendrimer assembly.
Dendrimers are interesting as a macromolecular structure for a variety of applications, due to their globular shape and the high degree of control over their size.4 In the area of enzyme-responsive systems, a novel class of dendrimers called “self-immolative dendrimers” has been reported, in which all covalently appended drug/active molecules are released by a single enzymatic trigger at the dendritic core.5 A useful complement to this approach involves non-covalently sequestering the guest molecules and releasing them in response to an enzymatic trigger. This approach is attractive because: (i) hydrophobic guests are encapsulated by the water-soluble dendrimers and therefore the hydrophobicity of the molecule will not affect the fidelity of the approach. (ii) guest molecules, such as drugs, need not be converted to prodrugs and thus the molecular design is simplified and the applicability can be rather broad. We report here on the enzymatic disassembly of dendrimer-based micelles. Dendritic micellar assemblies are also appealing because: (i) these macromolecules exhibit low critical aggregation concentrations (CACs) and high stabilities, compared to their small molecule counterparts;6,7 (ii) the systematic control over the molecular weight of the dendrimer through generational variations provides an opportunity for differential release rate.
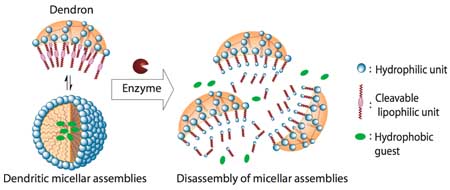
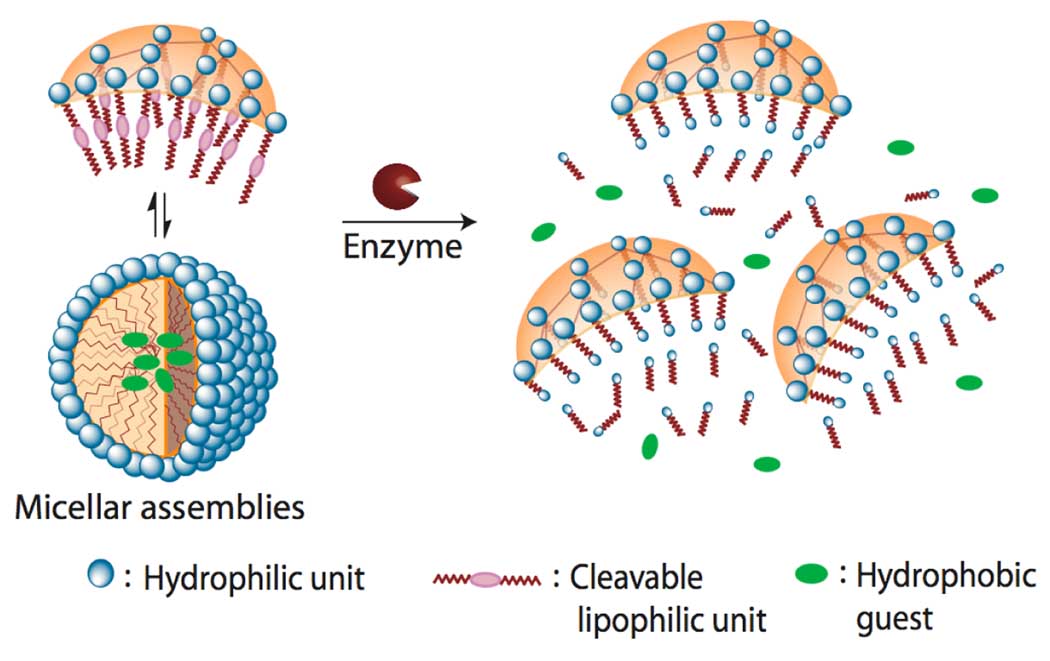
We have recently reported a distinct class of amphiphilic biaryl dendrimers that form micelle-like and inverse micelle-like structures in polar and apolar solvents, respectively.7 The fact that hydrophilic-lipophilic balance (HLB) is crucial for the formation of micellar assemblies in these dendrimer presents an opportunity for disassembly where by the HLB could be disturbed in response to an enzymatic trigger. Unlike classical amphiphilic dendrimers, our facially amphiphilic dendrimers form micellar assemblies by the aggregation of several dendritic molecules due to the orthogonal placement of hydrophilic and lipophilic units in every repeat unit of the dendrimer. To enzymatically trigger the disassembly in these assemblies, we installed enzyme cleavable ester moieties as lipophilic units. We hypothesized that the enzymatic cleavage of these lipophilic units will render the dendrimer hydrophilic and cause deaggregation. Since the hydrophobic container property of these dendrimers in water is predicated based on the micelle-like aggregation, the enzyme-triggered deaggregation event should result in a concomitant release of the sequestered hydrophobic guest molecules (Figure 1).
Figure 1.
Schematic representation of the enzyme-induced disassembly of dendritic micellar assemblies and guest release.
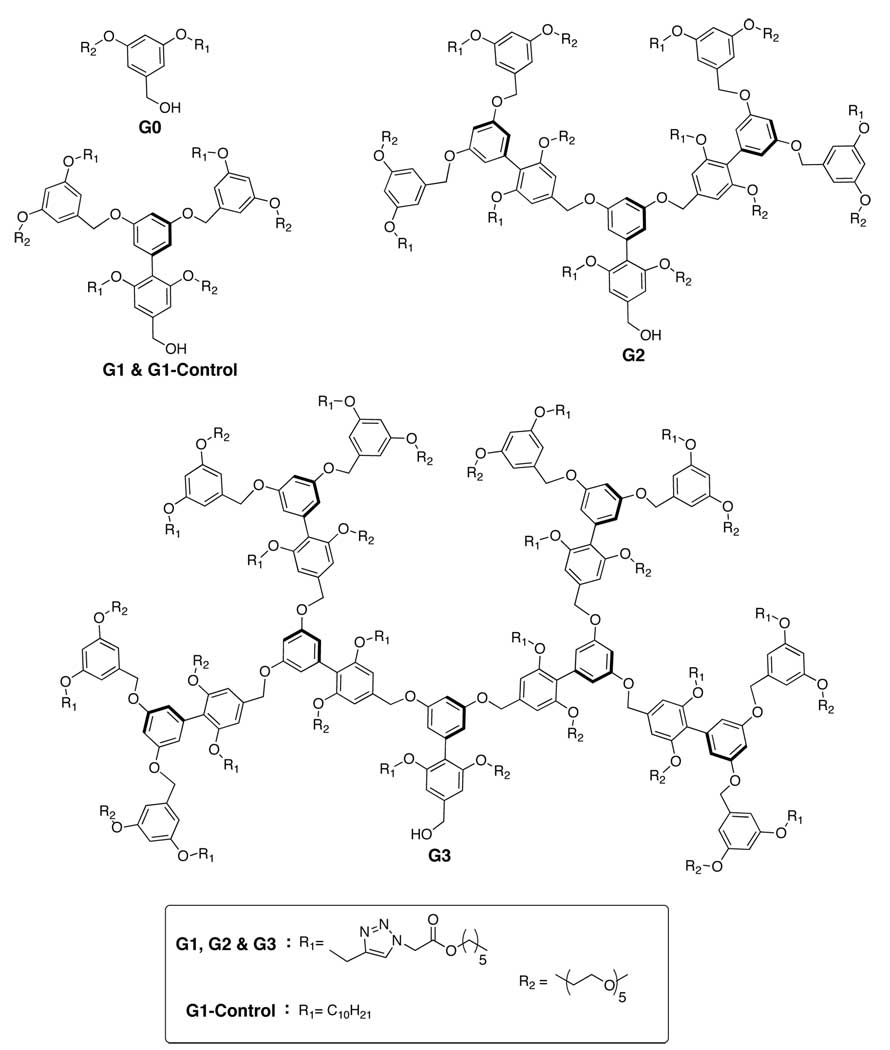
The structures of dendrons (G0-G3) with a hexyl ester functionality as the lipophilic unit and pentaethylene glycol (PEG) as the hydrophilic unit are shown in Chart 1. PEG was chosen to avoid non-specific interactions. The dendrimers were synthesized in a modular fashion, where the enzyme-sensitive functionalities were installed using the Huisgen 1,3-dipolar cycloaddition reaction.8 Prior to testing the disassembly, we investigated the micellar behavior of these dendrons in water by encapsulating pyrene as a probe. CACs of these dendrons were determined using the concentration dependence on excitation spectrum of pyrene.8 As expected, the CAC of the small molecule surfactant G0 dendron was found to be in mM range, whereas G1-G3 dendrons exhibited CACs of 4.3, 0.7 and 0.3 µM respectively.
Chart 1.
Structure of ester functionalized amphiphilic dendrons.
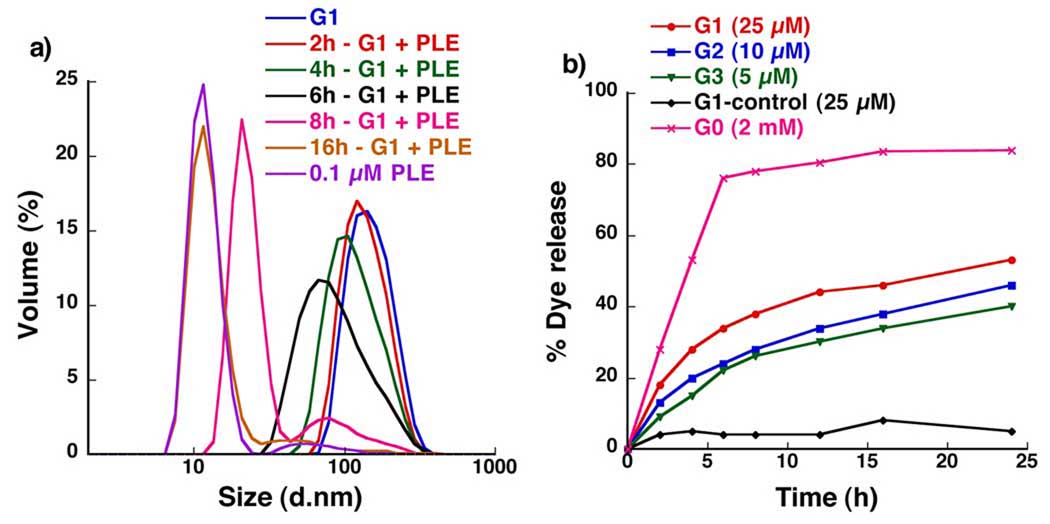
The enzyme-induced disassembly was first investigated using dynamic light scattering (DLS) studies. The assembly size in a 25 µM solution of G1 dendron was about 100 nm. Upon addition of a 0.1 µM solution of Porcine Liver Esterase (PLE), we were gratified to find a systematic decrease in size of G1 dendron assembly with time, finally reaching around 10 nm after 16 hrs (Figure 2a). The final size (10 nm) is identical to the size of enzyme. This indicates that the final unaggregated water-soluble dendrons are not discernible by DLS. To further test whether the disassembly occurs solely due to the enzymatic hydrolysis of ester functionalities, PLE was added to a solution of a structurally similar dendron G1-control (Chart 1)7b that lacks ester functionalities. The lack of disassembly in this case supports the enzyme-specific disassembly of the G1 micelle-like assembly.8
Figure 2.
Disassembly of dendritic micellar assemblies upon exposure to PLE (a) size evoluation of G1 dendritic assembly using DLS. (b) % release of pyrene using fluorescence studies.
We were also interested in the generation dependence of the disassembly with respect to the relative kinetics of disassembly. We conceived that the size of the dendrons will have an effect on this kinetics. For comparison, we maintained identical concentrations of the ester functionalities in all generations. Upon exposure to PLE, G2 assembly reduced to 10 nm in 24 hrs, while G3 took 36 hrs to reach the same size.8 This generation dependence of the size evolution is possibly because the ester functionalities are less accessible to the enzyme in the tightly packed higher generation dendrons. In other words, the higher generation dendrons sterically protect the enzymatic degradation of the ester functionalities. The rapid decrease in the size observed for G0 further supports this hypothesis.8
It is reasonable to expect that the disassembly would effect a concomitant release of any hydrophobic guest molecule sequestered within the micellar interior. To test this, PLE was added to the pyrene-encapsulated dendrons (G0-G3), where a systematic decrease in pyrene fluorescence over time was observed (Figure 2b).8 The temporal evolution of pyrene fluorescence indicates that the disassembly is indeed accompanied by the guest release. Also, no pyrene release was observed with G1-control as expected (Figure 2b).8 Three features are noteworthy: (i) rate of guest release decreases systematically with increasing dendron generation, which is consistent with the DLS data; (ii) the burst release observed in G0 is also consistent with DLS; (iii) the maximum dye release observed in the case of dendrons reached a plateau at about 50%. We attribute this to the possibility that the rather hydrophobic backbone of our biaryl dendrimers is capable of solvating a small amount of pyrene. This was supported by the significant pyrene encapsulation capability of the hydrolyzed G2 carboxylic acid-containing dendron.8
In summary, we have designed dendrimer-based amphiphilic assemblies that can non-covalently sequester hydrophobic guest molecules and release these guests in response to an enzymatic trigger. This is achieved by incorporating the enzyme-sensitive functionalities at the lipophilic face of the dendrons. This feature causes a change in the HLB upon encountering the enzyme to effect disassembly and guest molecule release. The non-covalent nature of binding and release of guest molecules is likely to further open up the repertoire of dendrimers in enzyme-responsive drug delivery systems and biosensors.
Supplementary Material
Acknowledgment
We thank NIGMS of the National Institutes of Health and DARPA for support.
Footnotes
Supporting Information Available: Experimental details, NMR, DLS and Fluorescence data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Rijcken CJF, Soga O, Hennink WE, van Nostrum CF. J. Controlled Release. 2007;120:131–148. doi: 10.1016/j.jconrel.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 2.(a) Almutairi A, Guillaudeu SJ, Berezin MY, Achilefu S, Fréchet JMJ. J. Am. Chem. Soc. 2008;130:444–445. doi: 10.1021/ja078147e. [DOI] [PubMed] [Google Scholar]; (b) Bae Y, Nishiyama N, Kataoka K. Bioconjugate Chem. 2007;18:1131–1139. doi: 10.1021/bc060401p. [DOI] [PubMed] [Google Scholar]; (c) Wang C, Tam KC, Jenkins RD, Tan CB. J. Phys. Chem. B. 2003;107:4667–4675. [Google Scholar]; (d) Lecommandoux S, Sandre O, Checot F, Rodriguez-Hernandez J, Perzynski R. Adv. Mater. 2005;17:712–718. [Google Scholar]; (e) Schmaljohann D. Adv. Drug Delivery Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 3.(a) Burke MD, Park JO, Srinivasarao M, Khan SA. J. Controlled Release. 2005;104:141–153. doi: 10.1016/j.jconrel.2005.01.017. [DOI] [PubMed] [Google Scholar]; (b) Thornton PD, McConnell G, Ulijn RV. Chem. Commun. 2005:5913–5915. doi: 10.1039/b511005j. [DOI] [PubMed] [Google Scholar]
- 4.(a) Grayson SM, Fréchet JMJ. Chem. Rev. 2001;101:3819–3867. doi: 10.1021/cr990116h. [DOI] [PubMed] [Google Scholar]; (b) Newkome GR, Moorefield CN, Vögtle F. Dendrimers. 2nd Edition. Wiley-VCH; [Google Scholar]
- 5. Amir RJ, Pessah N, Shamis M, Shabat D. Angew. Chem., Int. Ed. 2003;42:4494–4499. doi: 10.1002/anie.200351962. de Groot FMH, Albrecht C, Koekkoek R, Beusker PH, Scheeren HW. Angew. Chem., Int. Ed. 2003;42:4490–4494. doi: 10.1002/anie.200351942.. For a related strategy based on non-enzymatic trigger, see: Szalai ML, Kevwitch RM, McGrath DV. J. Am. Chem. Soc. 2003;125:15688–15689. doi: 10.1021/ja0386694. McGrath DV. Mol. Pharm. 2005;2:253–263. doi: 10.1021/mp050047x. Seebach D, Herrmann GF, Lengweiler UD, Bachmann BM, Amrein W. Angew. Chem., Int. Ed. Engl. 1996;35:2795–2797. Schmalenberg KE, Frauchiger L, Nikkhouy-Albers L, Uhrich KE. Biomacromolecules. 2001;2:851–855. doi: 10.1021/bm010042v..
- 6.(a) Hawker CJ, Wooley KL, Fréchet JMJ. J. Chem. Soc., Perkin Trans. 1. 1993:1287–1297. [Google Scholar]; (b) Newkome GR, Moorefield CN, Baker GR, Johnson AL, Behera RK. Angew. Chem., Int. Ed. 1991;30:1176–1178. [Google Scholar]
- 7.(a) Vutukuri DR, Basu S, Thayumanavan S. J. Am. Chem. Soc. 2004;126:15636–15637. doi: 10.1021/ja0449628. [DOI] [PubMed] [Google Scholar]; (b) Aathimanikandan SV, Savariar EN, Thayumanavan S. J. Am. Chem. Soc. 2005;127:14922–14929. doi: 10.1021/ja054542y. [DOI] [PubMed] [Google Scholar]
- 8.See Supporting Information for the details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.