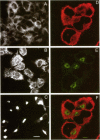
Abstract
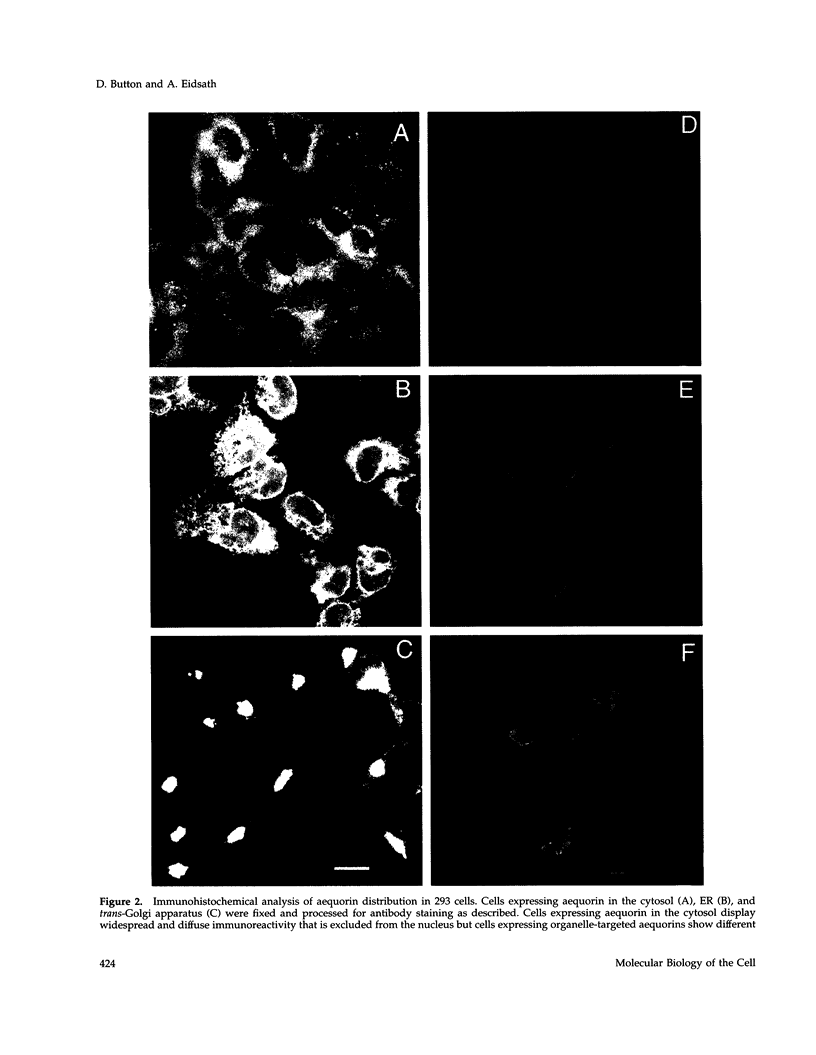
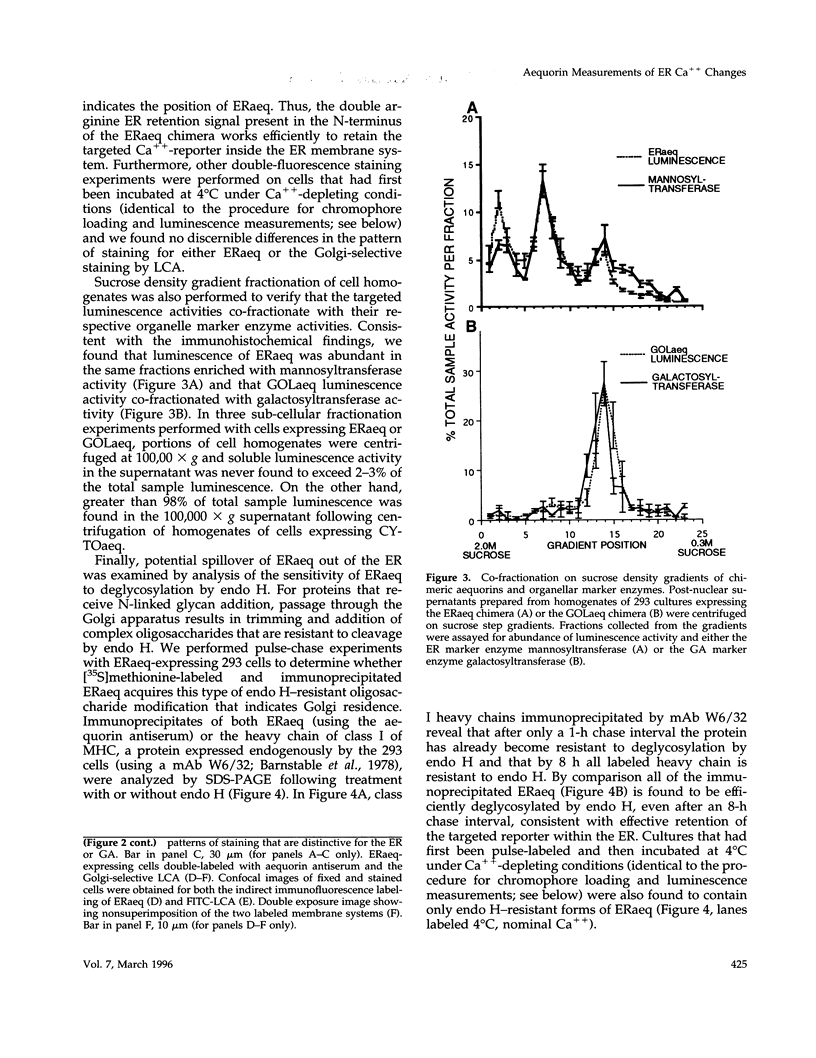
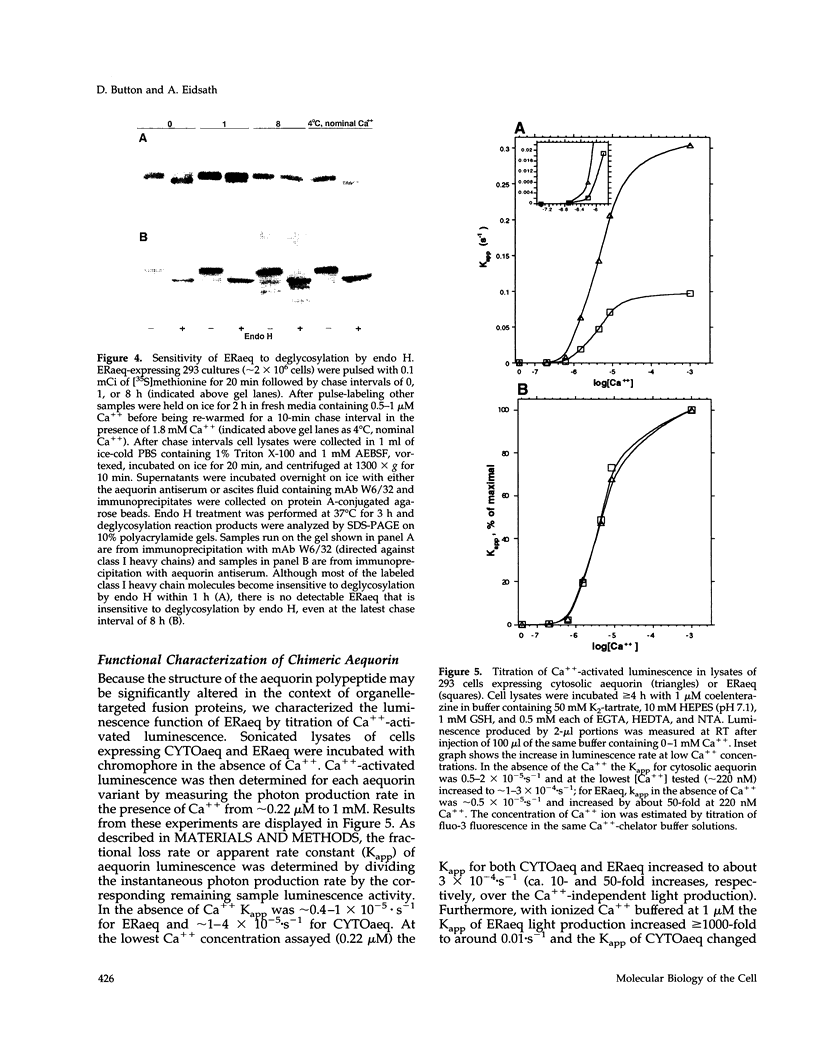
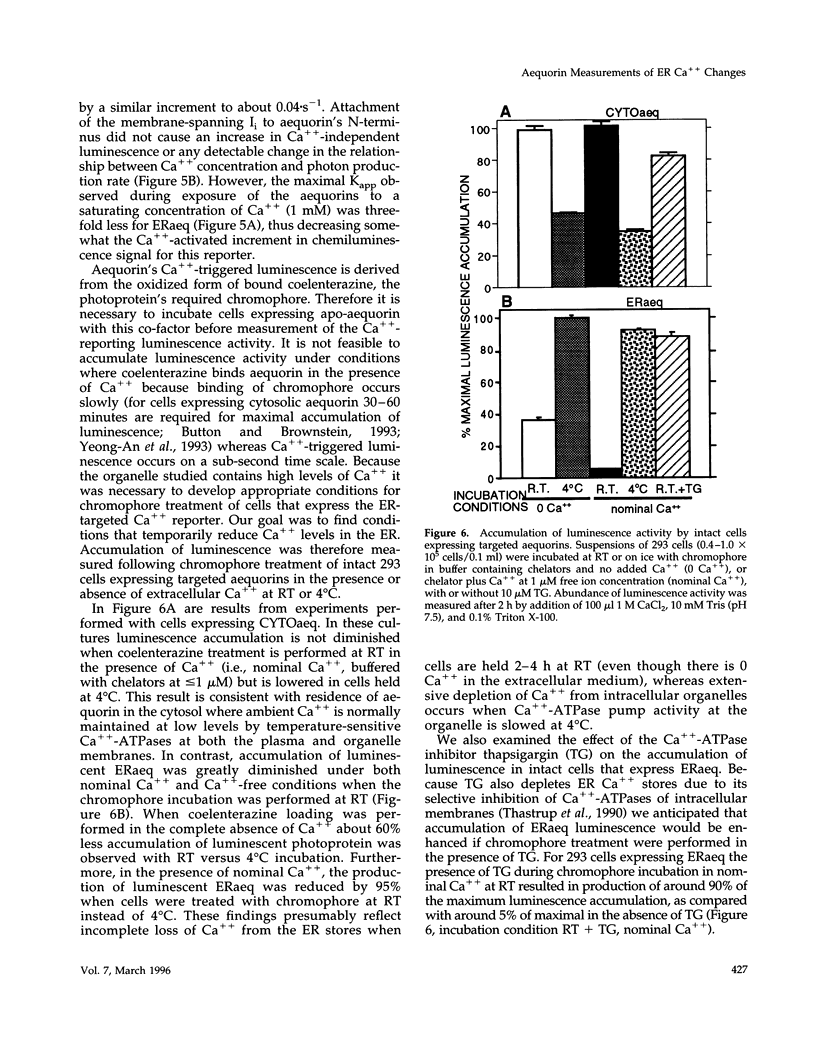
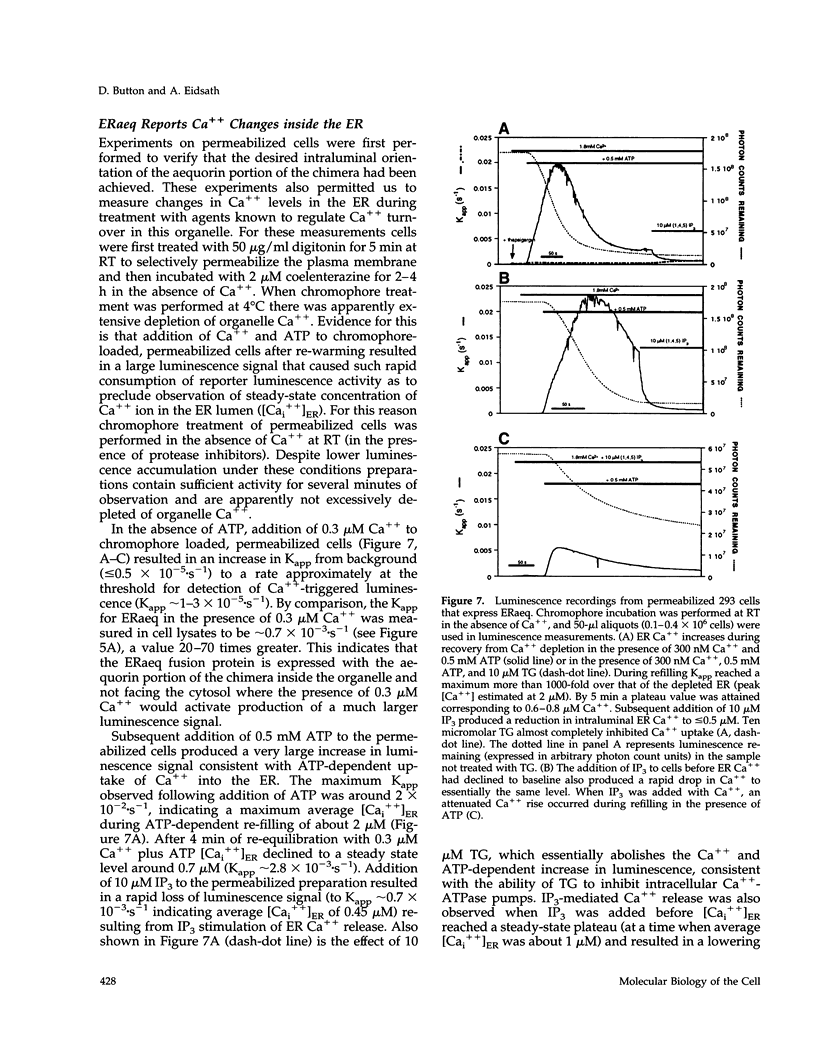
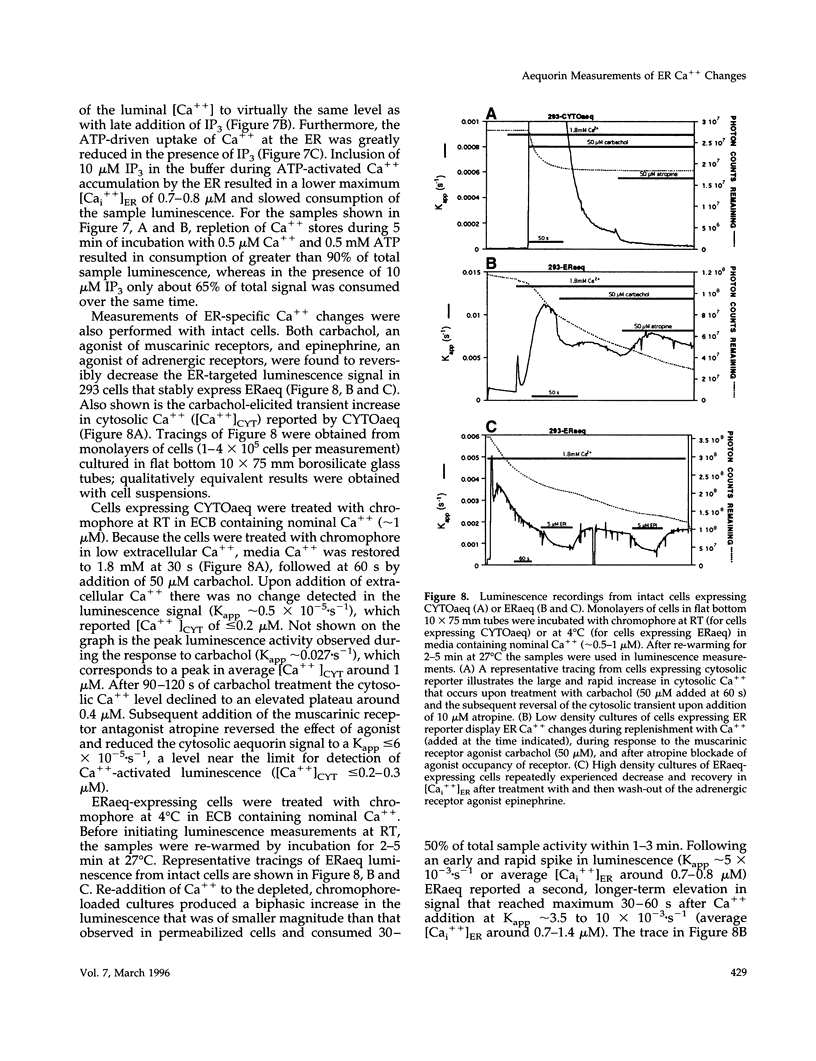
A chimeric protein (ERaeq) comprised of the invariant chain (Ii) of class II major histocompatability complex (MHC-II) and aequorin was localized in the endoplasmic reticulum (ER) of transfected human embryonal kidney 293 cells. The targeted aequorin resided in the lumen of the ER membrane system, including the nuclear cistern, and following addition of the chromophore coelenterazine underwent Ca(++)-activated chemiluminescence. The majority of chemiluminescence produced by coelenterazine treatment of ERaeq-expressing 293 cells was consumed rapidly (within 2-4 min) upon re-addition of Ca++ to coelenterazine-loaded cells, a finding consistent with very high Ca++ concentrations (approximately 10(-5)-10(-3) M Ca++ ion) inside the ER. However, following the initial rapid consumption of ERaeq chemiluminescence, the activity that remained (10-30% of total sample luminescence of permeabilized cells or 50-70% of total sample luminescence of intact cells) was found to produce a stable baseline corresponding to a Ca++ ion concentration < or = 1-2 microM. The stable baseline of luminescence observed following rapid consumption of the majority of the sample's activity was not derived from re-binding of fresh chromophore to spent photoprotein, suggesting that a minority fraction of the ER membrane system within which the ERaeq chimera was distributed contained a relatively low Ca++ concentration. Addition of IP3 to digitonin-permeabilized cells, or agonist treatment of intact cells decreased this residual signal. Luminescence recordings from cells expressing an ER-targeted aequorin with relatively high affinity for Ca++ thus reveal heterogeneity in luminal ER Ca++ concentration and permit observation of receptor- and IP3-activated release of Ca++ from the ER membrane system.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Oancea E., Kuhn M. A., Meyer T. Source of nuclear calcium signals. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12458–12462. doi: 10.1073/pnas.91.26.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki D., Lee N., Yamaguchi N., Dubois C., Fukuda M. N. Golgi retention of a trans-Golgi membrane protein, galactosyltransferase, requires cysteine and histidine residues within the membrane-anchoring domain. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4319–4323. doi: 10.1073/pnas.89.10.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badminton M. N., Kendall J. M., Sala-Newby G., Campbell A. K. Nucleoplasmin-targeted aequorin provides evidence for a nuclear calcium barrier. Exp Cell Res. 1995 Jan;216(1):236–243. doi: 10.1006/excr.1995.1030. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Blinks J. R., Prendergast F. G., Allen D. G. Photoproteins as biological calcium indicators. Pharmacol Rev. 1976 Mar;28(1):1–93. [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M., Murgia M., Pasti L., Picard D., Pozzan T., Rizzuto R. Nuclear Ca2+ concentration measured with specifically targeted recombinant aequorin. EMBO J. 1993 Dec;12(12):4813–4819. doi: 10.1002/j.1460-2075.1993.tb06170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button D., Brownstein M. Aequorin-expressing mammalian cell lines used to report Ca2+ mobilization. Cell Calcium. 1993 Oct;14(9):663–671. doi: 10.1016/0143-4160(93)90091-j. [DOI] [PubMed] [Google Scholar]
- Campbell A. K., Daw R. A., Hallett M. B., Luzio J. P. Direct measurement of the increase in intracellular free calcium ion concentration in response to the action of complement. Biochem J. 1981 Feb 15;194(2):551–560. doi: 10.1042/bj1940551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. A., Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988 Jul-Aug;6(7):632–638. [PubMed] [Google Scholar]
- Clapham D. E. Calcium signaling. Cell. 1995 Jan 27;80(2):259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Rink T. J. Fluorescence and bioluminescence measurement of cytoplasmic free calcium. Biochem J. 1987 Dec 1;248(2):313–328. doi: 10.1042/bj2480313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Intracellular calcium mobilization by inositol 1,4,5-trisphosphate: intracellular movements and compartmentalization. Cell Calcium. 1993 Mar;14(3):185–200. doi: 10.1016/0143-4160(93)90066-f. [DOI] [PubMed] [Google Scholar]
- Fewtrell C. Ca2+ oscillations in non-excitable cells. Annu Rev Physiol. 1993;55:427–454. doi: 10.1146/annurev.ph.55.030193.002235. [DOI] [PubMed] [Google Scholar]
- Galione A. Cyclic ADP-ribose: a new way to control calcium. Science. 1993 Jan 15;259(5093):325–326. doi: 10.1126/science.8380506. [DOI] [PubMed] [Google Scholar]
- Gerasimenko O. V., Gerasimenko J. V., Tepikin A. V., Petersen O. H. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995 Feb 10;80(3):439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- Hofer A. M., Machen T. E. Technique for in situ measurement of calcium in intracellular inositol 1,4,5-trisphosphate-sensitive stores using the fluorescent indicator mag-fura-2. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2598–2602. doi: 10.1073/pnas.90.7.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu V. W., Shah N., Klausner R. D. A brefeldin A-like phenotype is induced by the overexpression of a human ERD-2-like protein, ELP-1. Cell. 1992 May 15;69(4):625–635. doi: 10.1016/0092-8674(92)90226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S. Y., Tokimasa T., Takasawa S., Furuya Y., Nohmi M., Okamoto H., Kuba K. Cyclic ADP-ribose modulates Ca2+ release channels for activation by physiological Ca2+ entry in bullfrog sympathetic neurons. Neuron. 1994 May;12(5):1073–1079. doi: 10.1016/0896-6273(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Kendall J. M., Badminton M. N., Dormer R. L., Campbell A. K. Changes in free calcium in the endoplasmic reticulum of living cells detected using targeted aequorin. Anal Biochem. 1994 Aug 15;221(1):173–181. doi: 10.1006/abio.1994.1394. [DOI] [PubMed] [Google Scholar]
- Kendall J. M., Dormer R. L., Campbell A. K. Targeting aequorin to the endoplasmic reticulum of living cells. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1008–1016. doi: 10.1016/0006-291x(92)92304-g. [DOI] [PubMed] [Google Scholar]
- Malviya A. N., Rogue P., Vincendon G. Stereospecific inositol 1,4,5-[32P]trisphosphate binding to isolated rat liver nuclei: evidence for inositol trisphosphate receptor-mediated calcium release from the nucleus. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9270–9274. doi: 10.1073/pnas.87.23.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M., Brini M., Marsault R., Alvarez J., Sitia R., Pozzan T., Rizzuto R. Monitoring dynamic changes in free Ca2+ concentration in the endoplasmic reticulum of intact cells. EMBO J. 1995 Nov 15;14(22):5467–5475. doi: 10.1002/j.1460-2075.1995.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Orrenius S., Nilsson T., Berggren P. O. An inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in liver nuclei. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6858–6862. doi: 10.1073/pnas.87.17.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Inouye S., Ohmiya Y., Tsuji F. I. A C-terminal proline is required for bioluminescence of the Ca(2+)-binding photoprotein, aequorin. FEBS Lett. 1991 Dec 16;295(1-3):63–66. doi: 10.1016/0014-5793(91)81385-l. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Rizzuto R., Volpe P., Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994 Jul;74(3):595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Ridgway N. D., Dawson P. A., Ho Y. K., Brown M. S., Goldstein J. L. Translocation of oxysterol binding protein to Golgi apparatus triggered by ligand binding. J Cell Biol. 1992 Jan;116(2):307–319. doi: 10.1083/jcb.116.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993 Oct 29;262(5134):744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Pozzan T. Targeting recombinant aequorin to specific intracellular organelles. Methods Cell Biol. 1994;40:339–358. doi: 10.1016/s0091-679x(08)61121-8. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Simpson A. W., Brini M., Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992 Jul 23;358(6384):325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- Rutter G. A., Theler J. M., Murgia M., Wollheim C. B., Pozzan T., Rizzuto R. Stimulated Ca2+ influx raises mitochondrial free Ca2+ to supramicromolar levels in a pancreatic beta-cell line. Possible role in glucose and agonist-induced insulin secretion. J Biol Chem. 1993 Oct 25;268(30):22385–22390. [PubMed] [Google Scholar]
- Schutze M. P., Peterson P. A., Jackson M. R. An N-terminal double-arginine motif maintains type II membrane proteins in the endoplasmic reticulum. EMBO J. 1994 Apr 1;13(7):1696–1705. doi: 10.1002/j.1460-2075.1994.tb06434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu Y. A., Kricka L. J., Pritchett D. B. Measurement of intracellular calcium using bioluminescent aequorin expressed in human cells. Anal Biochem. 1993 Mar;209(2):343–347. doi: 10.1006/abio.1993.1132. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Regeneration of the photoprotein aequorin. Nature. 1975 Jul 17;256(5514):236–238. doi: 10.1038/256236a0. [DOI] [PubMed] [Google Scholar]
- Sitia R., Meldolesi J. Endoplasmic reticulum: a dynamic patchwork of specialized subregions. Mol Biol Cell. 1992 Oct;3(10):1067–1072. doi: 10.1091/mbc.3.10.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubin M., Long E. O., Mach B. Two forms of the Ia antigen-associated invariant chain result from alternative initiations at two in-phase AUGs. Cell. 1986 Nov 21;47(4):619–625. doi: 10.1016/0092-8674(86)90626-4. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse F. W., Tse A., Hille B. Cyclic Ca2+ changes in intracellular stores of gonadotropes during gonadotropin-releasing hormone-stimulated Ca2+ oscillations. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9750–9754. doi: 10.1073/pnas.91.21.9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Tsien R., Pozzan T. Measurement of cytosolic free Ca2+ with quin2. Methods Enzymol. 1989;172:230–262. doi: 10.1016/s0076-6879(89)72017-6. [DOI] [PubMed] [Google Scholar]
- Volpe P., Simon B. J. The bulk of Ca2+ released to the myoplasm is free in the sarcoplasmic reticulum and does not unbind from calsequestrin. FEBS Lett. 1991 Jan 28;278(2):274–278. doi: 10.1016/0014-5793(91)80134-o. [DOI] [PubMed] [Google Scholar]
- Watkins N. J., Campbell A. K. Requirement of the C-terminal proline residue for stability of the Ca(2+)-activated photoprotein aequorin. Biochem J. 1993 Jul 1;293(Pt 1):181–185. doi: 10.1042/bj2930181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha X., Chandra S., Ridsdale A. J., Morrison G. H. Golgi apparatus is involved in intracellular Ca2+ regulation in epithelial LLC-PK1 cells. Am J Physiol. 1995 May;268(5 Pt 1):C1133–C1140. doi: 10.1152/ajpcell.1995.268.5.C1133. [DOI] [PubMed] [Google Scholar]