Abstract
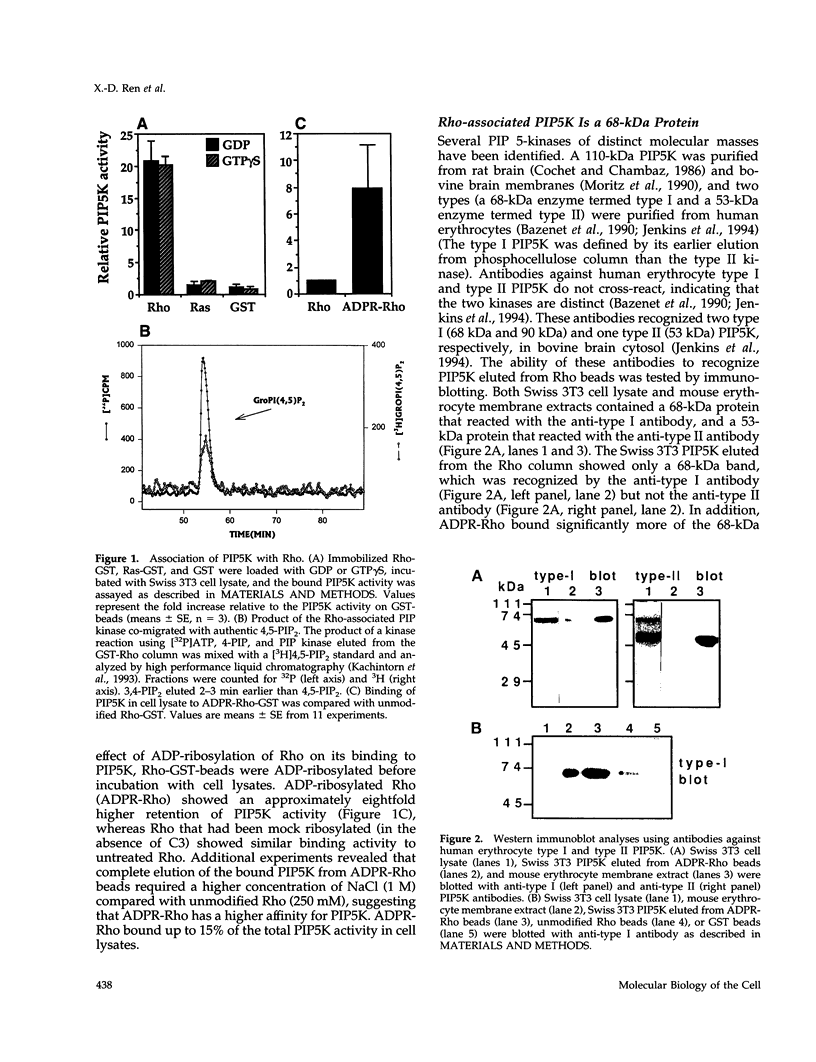
Our previous work showed that post-translationally modified Rho in its GTP-bound state stimulated phosphatidylinositol 4-phosphate 5-kinase (PIP5K) activity in mouse fibroblast lysates. To investigate whether Rho physically interacts with PIP5K, we incubated immobilized Rho-GST with Swiss 3T3 cell lysates and tested for retained PIP5K activity. Rho-GST, but not Ras-GST or GST alone, bound significant PIP5K activity. The binding of PIP5K was independent of whether Rho was in a GTP- or GDP-bound state. An antibody against a 68-kDa human erythrocyte type I PIP5K recognized a single 68-kDa protein eluted from Rho-GST column. The Rho-associated PIP5K responded to phosphatidic acid differentially from the erythrocyte type I PIP5K, suggesting that it could be a distinct isoform not reported previously. Rho co-immunoprecipitated with the 68-kDa PIP5K from Swiss 3T3 lysates, demonstrating that endogenous Rho also interacts with PIP5K. ADP-ribosylation of Rho with C3 exoenzyme enhanced PIP5K binding by approximately eightfold, consistent with the ADP-ribosylated Rho functioning as a dominant negative inhibitor. These results demonstrate that Rho physically interacts with a 68-kDa PIP5K, although whether the association is direct or indirect is unknown.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazenet C. E., Ruano A. R., Brockman J. L., Anderson R. A. The human erythrocyte contains two forms of phosphatidylinositol-4-phosphate 5-kinase which are differentially active toward membranes. J Biol Chem. 1990 Oct 15;265(29):18012–18022. [PubMed] [Google Scholar]
- Boronenkov I. V., Anderson R. A. The sequence of phosphatidylinositol-4-phosphate 5-kinase defines a novel family of lipid kinases. J Biol Chem. 1995 Feb 17;270(7):2881–2884. doi: 10.1074/jbc.270.7.2881. [DOI] [PubMed] [Google Scholar]
- Chong L. D., Traynor-Kaplan A., Bokoch G. M., Schwartz M. A. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994 Nov 4;79(3):507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Cochet C., Chambaz E. M. Catalytic properties of a purified phosphatidylinositol-4-phosphate kinase from rat brain. Biochem J. 1986 Jul 1;237(1):25–31. doi: 10.1042/bj2370025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Bokoch G. M., Carpenter C. L., Janmey P. A., Taylor L. A., Toker A., Stossel T. P. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995 Aug 25;82(4):643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Jenkins G. H., Fisette P. L., Anderson R. A. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J Biol Chem. 1994 Apr 15;269(15):11547–11554. [PubMed] [Google Scholar]
- Kachintorn U., Vajanaphanich M., Barrett K. E., Traynor-Kaplan A. E. Elevation of inositol tetrakisphosphate parallels inhibition of Ca(2+)-dependent Cl- secretion in T84 cells. Am J Physiol. 1993 Mar;264(3 Pt 1):C671–C676. doi: 10.1152/ajpcell.1993.264.3.C671. [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Yamamoto K., Fujita T., Takai Y. ADP-ribosylation of the bovine brain rho protein by botulinum toxin type C1. J Biol Chem. 1988 Nov 5;263(31):16303–16308. [PubMed] [Google Scholar]
- Knaus U. G., Heyworth P. G., Kinsella B. T., Curnutte J. T., Bokoch G. M. Purification and characterization of Rac 2. A cytosolic GTP-binding protein that regulates human neutrophil NADPH oxidase. J Biol Chem. 1992 Nov 25;267(33):23575–23582. [PubMed] [Google Scholar]
- Lee S. B., Rhee S. G. Significance of PIP2 hydrolysis and regulation of phospholipase C isozymes. Curr Opin Cell Biol. 1995 Apr;7(2):183–189. doi: 10.1016/0955-0674(95)80026-3. [DOI] [PubMed] [Google Scholar]
- Ling L. E., Schulz J. T., Cantley L. C. Characterization and purification of membrane-associated phosphatidylinositol-4-phosphate kinase from human red blood cells. J Biol Chem. 1989 Mar 25;264(9):5080–5088. [PubMed] [Google Scholar]
- Liscovitch M., Chalifa V., Pertile P., Chen C. S., Cantley L. C. Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J Biol Chem. 1994 Aug 26;269(34):21403–21406. [PubMed] [Google Scholar]
- Malcolm K. C., Ross A. H., Qiu R. G., Symons M., Exton J. H. Activation of rat liver phospholipase D by the small GTP-binding protein RhoA. J Biol Chem. 1994 Oct 21;269(42):25951–25954. [PubMed] [Google Scholar]
- Manser E., Leung T., Salihuddin H., Zhao Z. S., Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994 Jan 6;367(6458):40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- McNamee H. P., Ingber D. E., Schwartz M. A. Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-induced inositol lipid breakdown. J Cell Biol. 1993 May;121(3):673–678. doi: 10.1083/jcb.121.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moodie S. A., Willumsen B. M., Weber M. J., Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993 Jun 11;260(5114):1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- Morii N., Kawano K., Sekine A., Yamada T., Narumiya S. Purification of GTPase-activating protein specific for the rho gene products. J Biol Chem. 1991 Apr 25;266(12):7646–7650. [PubMed] [Google Scholar]
- Moritz A., De Graan P. N., Ekhart P. F., Gispen W. H., Wirtz K. W. Purification of a phosphatidylinositol 4-phosphate kinase from bovine brain membranes. J Neurochem. 1990 Jan;54(1):351–354. doi: 10.1111/j.1471-4159.1990.tb13322.x. [DOI] [PubMed] [Google Scholar]
- Moritz A., De Graan P. N., Gispen W. H., Wirtz K. W. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem. 1992 Apr 15;267(11):7207–7210. [PubMed] [Google Scholar]
- Narumiya S., Morii N. rho gene products, botulinum C3 exoenzyme and cell adhesion. Cell Signal. 1993 Jan;5(1):9–19. doi: 10.1016/0898-6568(93)90003-5. [DOI] [PubMed] [Google Scholar]
- Olson M. F., Ashworth A., Hall A. An essential role for Rho, Rac, and Cdc42 GTPases in cell cycle progression through G1. Science. 1995 Sep 1;269(5228):1270–1272. doi: 10.1126/science.7652575. [DOI] [PubMed] [Google Scholar]
- Paterson H. F., Self A. J., Garrett M. D., Just I., Aktories K., Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990 Sep;111(3):1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilliam L. A., Lacal J. C., Bokoch G. M. Identification of rho as a substrate for botulinum toxin C3-catalyzed ADP-ribosylation. FEBS Lett. 1989 Apr 24;247(2):221–226. doi: 10.1016/0014-5793(89)81339-0. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992 Aug 7;70(3):389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P., Warne P. H., Dhand R., Vanhaesebroeck B., Gout I., Fry M. J., Waterfield M. D., Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994 Aug 18;370(6490):527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Schwartz M. A., Lechene C. Adhesion is required for protein kinase C-dependent activation of the Na+/H+ antiporter by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6138–6141. doi: 10.1073/pnas.89.13.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self A. J., Paterson H. F., Hall A. Different structural organization of Ras and Rho effector domains. Oncogene. 1993 Mar;8(3):655–661. [PubMed] [Google Scholar]
- Singer W. D., Brown H. A., Bokoch G. M., Sternweis P. C. Resolved phospholipase D activity is modulated by cytosolic factors other than Arf. J Biol Chem. 1995 Jun 23;270(25):14944–14950. doi: 10.1074/jbc.270.25.14944. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tolias K. F., Cantley L. C., Carpenter C. L. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995 Jul 28;270(30):17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- Tominaga T., Sugie K., Hirata M., Morii N., Fukata J., Uchida A., Imura H., Narumiya S. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J Cell Biol. 1993 Mar;120(6):1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]




