Abstract
Current stratification of prognosis in patients with epithelioid sarcoma (ES) is based largely on data reported by individual centers with a limited number of patients. We sought to identify the important prognostic parameters using the Surveillance, Epidemiology, and End Results (SEER) database. We identified 441 patients with ES in the database and extracted information regarding patient demographics and clinical characteristics. Kaplan-Meier, log-rank, and Cox regression were used for analysis. Disease-specific survival declined until 100 months after diagnosis after which survival was unrelated to epithelioid sarcoma. The overall incidence of ES during 2005 was 0.041 per 100,000. The reported incidence has increased since 1973, with an annual percentage change of 5.217%. On multivariate analysis, only age younger than 16 years, local stage of disease, or negative nodes and surgical resection of the tumor predicted better disease-specific survival. We observed no increase in survival by comparing decades of diagnosis since 1986. The SEER database shows only age younger than 16 years, negative nodes, or local stage of disease and operability of primary disease independently predict survival in patients with ES.
Level of Evidence: Level II, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Unlike other sarcomas, ES displays a propensity to spread by way of the lymphatic system, and tenacious local and distant progression [6, 44]. ES generally is considered a high-grade soft tissue sarcoma; although grading currently is not recommended for this histologic type [3], some authors have graded this sarcoma [44]. Traditionally, wide surgical excision has been the mainstay treatment, and radiation and chemotherapy have been used occasionally as adjuvant therapy but have had limited success [44]. Chemotherapeutic agents used include ifosfamide and doxorubicin [36, 39].
Previous reports describing the outcomes of patients with ES have limited statistical power owing to the small number of patients in each series [3–7]. The largest series to date by Chase and Enzinger [8] in 1985 described 241 patients from multiple centers. Two other larger series came from the Royal Marsden Hospital and the Mayo Clinic [5, 27]. Although the numbers of patients are larger in these series than in other reported series, they are limited in statistical power. Thus, data from a population-based registry are more likely able to provide prognostic and survival factors. Previously, the SEER database has been used to describe the outcomes for breast, colorectal, prostate, lung, ovarian, and neuroectodermal cancers and has been validated for accuracy [9, 17–20, 24, 26, 31, 32].
We attempted to define current treatment outcomes and demographic characteristics for patients with ES using a large, population-based cancer registry to define prognostic factors and evaluate the impact of improvement in diagnostics and therapeutics by comparison of improvement in survival time over decades. Specifically, we sought to establish incidence rates and trends with time, identify independent predictors of survival among various demographic factors (race, ethnicity, age, or gender), clinical characteristics of disease (stage, nodal status, or anatomic location), and describe treatment strategies (surgical or radiation therapy).
Materials and Methods
We used the SEER database to identify all incident cases of ES diagnosed between 1973 and 2005 using the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) [25]. Four hundred forty-one patients were identified and information regarding patient demographics, stage at diagnosis, primary site and size, year of diagnosis, surgical and radiation treatment (if provided within 4 months of diagnosis), and survival time (months) until death or loss to followup was extracted (Table 1). Percentages were based on available data for each variable. Patients with missing data were excluded from each respective univariate and multivariate analysis. We retrospectively analyzed the data collected by the National Cancer Institute in a comprehensive fashion for survival for each demographic and clinical parameter including decade of diagnosis as mentioned above. The main cohort was divided into subgroups based on categories in each of the demographic and clinical variables. Survival times for subgroups were compared and statistically analyzed to establish the survival advantage. All data reported are in compliance with the Health Insurance Portability and Accountability Act of 1996.
Table 1.
Demographic and clinical characteristics of entire cohort of patients
| Characteristic | Number of patients | Valid % of total |
|---|---|---|
| Total | 441 | 100 |
| Age | ||
| 0–16 years | 40 | 9.1 |
| 17–30 years | 105 | 23.8 |
| 31–45 years | 97 | 22.0 |
| 46–60 years | 97 | 22.0 |
| 61–75 years | 56 | 12.7 |
| 75+ years | 46 | 10.4 |
| Gender | ||
| Male | 250 | 56.7 |
| Female | 191 | 43.3 |
| Race | ||
| White | 353 | 80.0 |
| Black | 53 | 12.0 |
| Other | 35 | 7.9 |
| Ethnicity | ||
| Hispanic | 39 | 8.8 |
| Non-Hispanic | 402 | 91.2 |
| Stage | ||
| Local | 134 | 47.2 |
| Regional | 78 | 27.5 |
| Distant | 72 | 25.4 |
| Nodes | ||
| None | 182 | 64.1 |
| Regional | 30 | 10.6 |
| Distant | 72 | 25.4 |
| Location | ||
| Superficial axial | 125 | 28.9 |
| Superficial appendicular | 223 | 51.6 |
| Deep axial | 74 | 17.1 |
| Deep appendicular | 10 | 2.3 |
| Surgery | ||
| Yes | 354 | 81.2 |
| No | 82 | 18.8 |
| Radiation | ||
| Yes | 143 | 33.2 |
| No | 288 | 66.8 |
| Year of diagnosis | ||
| 1973–1975 | 6 | 1.4 |
| 1976–1985 | 43 | 9.8 |
| 1986–1995 | 93 | 21.1 |
| 1996–2005 | 299 | 67.8 |
Data were extracted from the population-based cancer registries that participate in the National Cancer Institute’s SEER program. The SEER program was established as a direct result of the National Cancer Act of 1971. SEER started collecting information from nine registries across the United States in 1973; it was expanded to 13 in 1992 and 17 in 2000. Currently, the SEER program collects data from 17 population-based registries covering approximately 26% of the US population. It is the only comprehensive source of population-based data in the United States that includes the stage of cancer at the time of diagnosis and followup of all patients for survival data. In addition, each registry collects data on patient demographics, primary tumor site and morphology, and first course of treatment (occurring within 4 months of diagnosis) [2, 10, 12, 15, 22, 29, 33, 34, 37, 40–43]. The specific local registries were selected for their completeness and adequate representation of minority populations. The SEER program is regarded as the standard of quality among cancer registries around the world with case completeness of 98% [30].
More than ½ of the cases (67.8%) were diagnosed from 1996–2005, reflecting the expansion of data collection. Patients between 17 and 60 years of age at diagnosis comprised 67.8% of the cases. Males comprised 56.7% of the group; 80.0% and 91.2% of the patients were recognized as Caucasian and non-Hispanic, respectively. Just under ½ (47.2%) of the patient population had localized disease at the time of diagnosis. Sixty-four percent (284/441) of patients had the nodal metastasis status documented in the database and only 36% of these were recorded as positive. The most common anatomic primary tumor location was appendicular superficial (51.6%). Surgical resection was performed in 81.2% of patients and subsequently 66.8% of patients had no additional local therapy. No information regarding the specifics of chemotherapeutic agents or other medical therapies is included in the SEER database. Staging information (AJCC) was available for only 64.4% of the entire cohort owing to data omissions. Only cases with complete staging information were considered in the subsequent analysis.
The patients’ ages were converted to categorical variables with uniform intervals of 15 years for each category from 0 to 75+ years for analysis. Similarly, the categorical variables appendicular and axial skeleton were used for nonuniformly described tumor locations. Tumor location was further stratified as superficial or deep. Superficial appendicular location included lesions involving skin and subcutaneous tissue of the upper and lower limbs superficial to the fascia, and deep appendicular location included structures deeper to the fascia including short and long bones of upper and lower limbs. The scapula was included in the appendicular skeleton, whereas the pelvic bones and associated joints were categorized under the axial skeleton. Hip is categorized as part of the appendicular skeleton. Similarly, superficial axial locations included lesions involving skin and subcutaneous tissue of the torso, and deep axial lesions referred to lesions involving the viscera and axial skeleton. The axial skeleton included vertebrae, ribs, sternum, clavicle and associated joints, bones of the skull, face, and associated joints, and the mandible. Staging categories of local, regional, and distant were used according to the AJCC staging system for sarcomas [1]. Year of diagnosis was categorized in four categorical variables: 1973–1975, 1976–1985, 1986–1995, and 1996–2005.
SEER*Stat software (Version 6.4.4; National Cancer Institute, Bethesda, MD) was used to analyze incidence rates and trends from 1973 to 2005. Incidence rates were age adjusted and normalized using the 2000 US Standard population. The annual percentage change was calculated using weighted least-square method as described in the SEER Cancer Statistics Review, 1973–2005 [30]. The chi square test was used to make correlations between categorical variables. Overall and disease-specific survival from the time of initial diagnosis to the date of last contact (or the date of death, if the patient was deceased) was calculated using the Kaplan-Meier method [38]. The effects of demographic, clinical, pathologic, and treatment variables were tested using the log-rank test for categorical values. A multivariate analysis was performed to determine independent prognostic factors using the Cox proportional-hazards model. All prognostic factors significant in the univariate analysis (p < 0.05), namely, gender, stage, primary site, size, and surgical therapy, were included in the multivariate analysis. Statistical analysis was performed using the SPSS® statistical package Version 16.0 (SPSS Inc, Chicago, IL).
Results
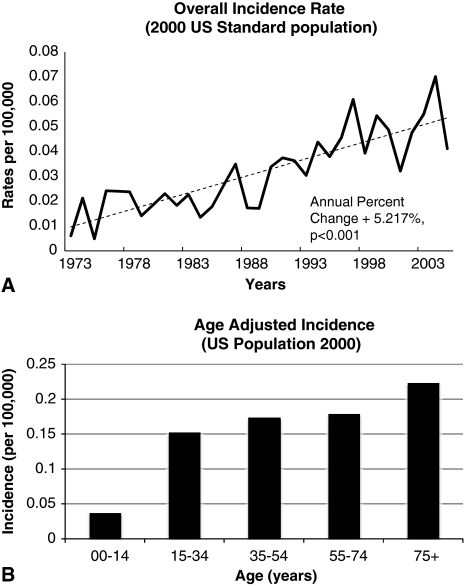
The overall incidence for ES in 2005 was 0.041 per 100,000 persons from a total population in the SEER registry of 75,304,906. The reported incidence has increased (p < 0.001) since 1973, with an annual percentage change of 5.2% (Fig. 1A). The peak incidence was 75 years of age (Fig. 1B).
Fig. 1A–B.
(A) A graph shows the overall incidence of ES plotted by year of diagnosis and normalized to the US 2000 census. (B) An age-adjusted histogram depicts the incidence rate of ES normalized to the US 2000 census.
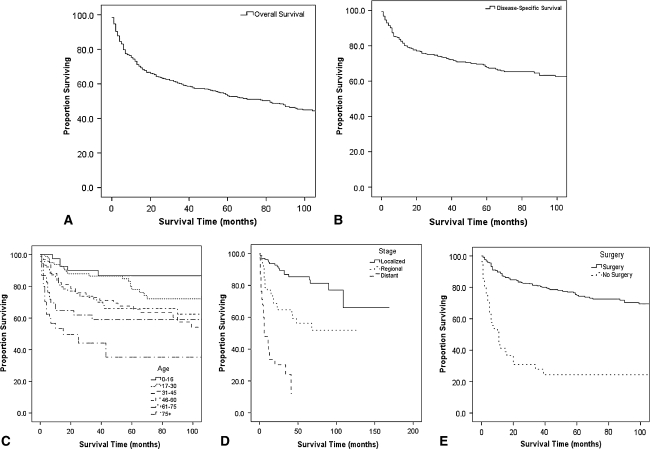
The disease-specific survival was 68% at 5 years and 61% at 10 years (Table 2; Fig. 2), and started to plateau at approximately 100 months (Fig. 2B), which suggests deaths occurring thereafter, as shown in the overall survival plot (Fig. 2A), can be attributed to factors other than ES. No survival advantage was associated with any gender, race, or ethnic group. The disease-specific survival worsened (p = 0.017) with time by decade from 1976–1985 to 1995–2005.
Table 2.
Disease-specific survival stratified by demographic variables and treatment characteristics
| Variable | 1-year survival | 5-year survival | 10-year survival | p Value* |
|---|---|---|---|---|
| Overall | 0.83 | 0.68 | 0.61 | NA |
| Age | ||||
| 0–16 years | 0.98 | 0.85 | 0.82 | < 0.001† |
| 17–30 years | 0.94 | 0.81 | 0.72 | |
| 31–45 years | 0.86 | 0.67 | 0.55 | |
| 46–60 years | 0.84 | 0.67 | 0.61 | |
| 61–75 years | 0.66 | 0.57 | 0.53 | |
| 75+ years | 0.54 | 0.38 | 0.36 | |
| Gender | ||||
| Male | 0.83 | 0.68 | 0.60 | 0.397 |
| Female | 0.82 | 0.68 | 0.62 | |
| Race | ||||
| White | 0.84 | 0.69 | 0.62 | 0.096‡ |
| Black | 0.81 | 0.59 | 0.51 | |
| Other | 0.72 | 0.64 | 0.56 | |
| Ethnicity | ||||
| Hispanic | 0.92 | 0.80 | 0.65 | 0.39 |
| Non-Hispanic | 0.82 | 0.67 | 0.60 | |
| Stage | ||||
| Local | 0.95 | 0.85 | 0.75 | < 0.001 |
| Regional | 0.78 | 0.57 | 0.49 | |
| Distant | 0.46 | ? | ? | |
| Nodes | ||||
| None | 0.92 | 0.80 | 0.70 | < 0.001§ |
| Regional | 0.72 | 0.42 | ? | |
| Distant | 0.46 | ? | ? | |
| Location | ||||
| Superficial axial | 0.80 | 0.65 | 0.58 | |
| Superficial appendicular | 0.91 | 0.78 | 0.71 | |
| Deep axial | 0.62 | 0.43 | 0.39 | < 0.001|| |
| Deep appendicular | 0.76 | 0.73 | 0.69 | |
| Surgery | ||||
| Yes | 0.90 | 0.75 | 0.68 | < 0.001 |
| No | 0.49 | 0.35 | 0.33 | |
| Radiation | ||||
| Yes | 0.82 | 0.60 | 0.53 | 0.033 |
| No | 0.83 | 0.72 | 0.65 | |
| Year of diagnosis | ||||
| 1973–1975 | 1.00 | 1.00 | 0.80 | |
| 1976–1985 | 0.95 | 0.85 | 0.78 | 0.017¶ |
| 1986–1995 | 0.89 | 0.70 | 0.63 | |
| 1996–2005 | 0.79 | 0.63 | ? | |
* p values shown for log-rank test between variables; †p < 0.001 for age 0–16 years versus 75+ years, 17–30 years versus 75+ years, 31–45 years versus 75+ years, and 46–60 years versus 75+ years; 0–16 years versus 17–30 years (p = 0.102); 0–16 years versus 31–45 years (p = 0.007); 0–16 years versus 46–60 years (p = 0.01); 0–16 years versus 61–75 years (p = 0.002); 17–30 years versus 31–45 years (p = 0.069); 17–30 years versus 46–60 years (p = 0.097); 17–30 years versus 61–75 years (p = 0.008); 31–45 years versus 46–60 years (p = 0.980); 31–45 years versus 61–75 years (p = 0.200); 46–60 years versus 61–75 years (p = 0.231); 61–75 years versus 75+ years (p = 0.067); ‡p = 0.096 for white versus black only, white versus other (p = 0.146); black versus other (p = 0.921); §p < 0.001 for all associations except distant versus regional (p = 0.004); ||p < 0.001 for superficial axial versus deep axial and superficial appendicular versus deep axial only, superficial axial versus superficial appendicular (p = 0.07); deep axial versus deep appendicular (p = 0.142); superficial appendicular versus deep appendicular (p = 0.636); superficial axial versus deep appendicular (p = 0.718); ¶p = 0.017 for 1976–1985 versus 1996–2005 only; 1973–1975 versus 1976–1985 (p = 0.806); 1973–1975 versus 1986–1995 (p = 0.360); 1973–1975 versus 1996–2005 (p = 0.293); 1976–1985 versus 1986–1995 (p = 0.058); 1986–1995 versus 1996–2005 (p = 0.212); NA = not applicable.
Fig. 2A–E.
Kaplan-Meier plots depict (A) overall survival (mean = 155 months, 95% CI = 134.98–175.01 months), (B) disease-specific survival (mean = 239.23 months, 95% CI = 217.45–261.01 months), and disease-specific survival estimates for patients with ES stratified by (C) age, (D) stage, and (E) surgery (corresponding data in Table 2).
Patients diagnosed with local disease had a better (p < 0.001) 5-year survival of 75% as compared with patients with regional disease who had a 5-year survival of 49%. None of the patients who presented with distant disease survived for 5 years, and 1-year survival was 46%. Deep axial lesions had the worst (p < 0.001) prognosis, with a 5-year survival of 43% as compared with 65% for superficial axial, 73% for deep appendicular, and 78% for superficial appendicular lesions.
Patients undergoing surgery fared better (p < 0.001) than patients with no surgical treatment (5-year survival 68% and 33%, respectively). Patients receiving no radiotherapy had a better (p = 0.033) 5-year survival than patients receiving radiotherapy (72% versus 60%). This could be a result of selection bias for patients receiving radiotherapy.
Among independent demographic variables, age older than 75 years, distant disease, and absence of surgical resection independently predicted lower overall survival (Table 3; Fig. 2C–E).
Table 3.
Cox proportional-hazards model for risk of death from epithelioid sarcoma using stage
| Variable | Number | Hazard ratio | 95% confidence interval | p Value |
|---|---|---|---|---|
| Age | ||||
| 0–16 years | 24 | 0.178 | 0.049–0.645 | 0.009 |
| 17–30 years | 55 | 0.284 | 0.124–0.648 | 0.003 |
| 31–45 years | 69 | 0.366 | 0.185–0.725 | 0.004 |
| 46–60 years | 62 | 0.346 | 0.174–0.690 | 0.003 |
| 61–75 years | 32 | 0.461 | 0.218–0.975 | 0.043 |
| 75+ years | 35 | Reference group | ||
| Stage | ||||
| Local | 132 | 0.174 | 0.084–0.359 | < 0.001 |
| Regional | 74 | 0.46 | 0.237–0.895 | 0.022 |
| Distant | 71 | Reference group | ||
| Location | ||||
| Superficial axial | 86 | 2.167 | 0.275–17.067 | 0.463 |
| Superficial appendicular | 132 | 1.491 | 0.191–11.626 | 0.703 |
| Deep axial | 51 | 3.011 | 0.382–23.723 | 0.295 |
| Deep appendicular | 8 | Reference group | ||
| Surgery | ||||
| Yes | 227 | 0.408 | 0.218–0.763 | 0.005 |
| No | 50 | Reference group | ||
| Radiation | ||||
| Yes | 102 | 1.168 | 0.736–1.852 | 0.511 |
| No | 175 | Reference group | ||
| Year of diagnosis | ||||
| 1973–1975 | ||||
| 1976–1985 | ||||
| 1986–1995 | 16 | 0.744 | 0.282–1.964 | 0.55 |
| 1996–2005 | 261 | Reference group | ||
A stepwise multivariate analysis using the nodal status instead of staging data was performed, and distant nodal metastasis also was identified as an independent prognostic factor in place of distant disease (Table 4).
Table 4.
Cox proportional-hazards model for risk of death from epithelioid sarcoma using nodal status
| Variable | Number | Hazard ratio | 95% confidence interval | p Value |
|---|---|---|---|---|
| Age | ||||
| 0–16 years | 24 | 0.138 | 0.038–0.504 | 0.003 |
| 17–30 years | 55 | 0.23 | 0.099–0.536 | 0.001 |
| 31–45 years | 69 | 0.29 | 0.141–0.597 | 0.001 |
| 46–60 years | 62 | 0.304 | 0.151–0.614 | 0.001 |
| 61–75 years | 32 | 0.432 | 0.204–0.914 | 0.028 |
| 75+ years | 35 | Reference group | ||
| Nodes | ||||
| Distant | 71 | 4.67 | 2.393–9.115 | < 0.001 |
| Regional | 29 | 3.97 | 1.975–7.980 | < 0.001 |
| None | 177 | Reference group | ||
| Location | ||||
| Superficial axial | 86 | 2.002 | 0.253–15.865 | 0.511 |
| Superficial appendicular | 132 | 1.394 | 0.179–10.858 | 0.751 |
| Deep axial | 51 | 2.498 | 0.313–19.931 | 0.388 |
| Deep appendicular | 8 | Reference group | ||
| Surgery | ||||
| Yes | 227 | 0.388 | 0.206–0.732 | 0.003 |
| No | 50 | Reference group | ||
| Radiation | ||||
| Yes | 102 | 1.169 | 0.733–1.864 | 0.513 |
| No | 175 | Reference group | ||
| Year of diagnosis | ||||
| 1973–1975 | ||||
| 1976–1985 | ||||
| 1986–1995 | 16 | 0.767 | 0.292–2.015 | 0.59 |
| 1996–2005 | 261 | Reference group | ||
Discussion
ES is an exceedingly rare malignancy, constituting less than 1% of all soft tissue sarcomas [11]. To understand outcomes for patients and potentially improve survival, we examined a population-based registry to identify global prognostic factors important in the treatment outcome for patients with ES.
Limitations of this approach are severalfold and include lack of any information in the SEER database regarding specific chemotherapy or any other medical therapy. We could not obtain information regarding extent of surgery from the SEER database, limiting our ability to comment on amputation versus conservative surgery or sentinel node dissection as the appropriate treatment modalities.
Our study is unmatched in length of followup and breadth of patients accrued and is inclusive of patients in all age groups. In addition, it provides a unique analysis of demographic and clinical prognostic factors across the demographic strata. Many previous studies attempting to identify the prognostic factors for ES were based on data from one large center. Many included only a particular subset of patients with respect to clinical features, resulting in potential bias and conflicting evidence for various prognostic factors. The small number of patients in many of the studies [3, 5–8, 11, 27, 36, 39, 44] limited their statistical power when identifying important prognostic factors affecting outcomes (Table 5). In a report from the Royal Marsden Hospital, Livi et al. [27] retrospectively reviewed 39 patients from 1978 to 2001. Patient followup time was limited in most of the studies and the largest study to date did not report 5- and/or 10-year survival [8]. Older age, male gender, proximal or axial location, depth, tumor size, mitotic figures, necrosis, vascular invasion, and tumor hemorrhage were identified as adverse prognostic factors in this study [8]. Ross et al. [36] and Callister et al. [6] reviewed mainly monoinstitutional series and often did not agree on several of the results.
Table 5.
Published prognostic factors for epithelioid sarcoma
| Study | Number of patients | Prognostic factors |
|---|---|---|
| Chase and Enzinger [8] | 241 | Location (axial vs appendicular) and limb location, size, depth, hemorrhage, mitotic figures, necrosis, vascular invasion, age, gender |
| Bos et al. [5] | 51 | Size, depth of lesion, focal necrosis |
| Ross et al. [36] | 16 | Gender, stage |
| Spillane et al. [39] | 37 | Regional nodal metastasis, recurrence, size |
| Callister et al. [6] | 24 | Size |
| Livi et al. [27] | 39 | Limb location |
| Casanova et al. [7] | 30 | Depth of lesion, limb location |
| de Visscher et al. [11] | 23 | Stage |
| Baratti et al. [3] | 54 | Stage, nodal metastasis, size, limb location |
| Wolf et al. [44] | 11 | Size |
| Current study | 441 | Age, stage, surgical resection |
Some authors have suggested most patients are between 10 and 35 years of age when diagnosed with ES [11]. Although a proportion of the disease population in our study was in these age limits, most patients were between 17 and 60 years (Table 1). Also, a peak in incidence was observed for the age group of 75+ years (Fig. 1B). Older age was recognized as a predictor of adverse outcome by Chase and Enzinger [8]. One of the monoinstitutional trials failed to recognize age as a predictor of outcome [3]. We found age older than 75+ years independently predicted poor survival. Moreover, as reported by Casanova et al. [7], we also found younger age did not predict improved survival.
A strong predilection of ES for the male gender has been reported and our analysis confirmed this finding [11, 36]. Male gender also has been associated with a poorer outcome, whereas others have reported conflicting evidence [5, 8, 13, 28, 36]. We observed no survival benefit associated with female gender on univariate analysis. The 5- and 10-year survival rates for male and female patients with ES were similar.
Distant metastasis at the time of diagnosis is widely accepted as a predictor of worse prognosis [3, 5, 6, 8, 11, 16, 21, 28, 36, 39]. Our analysis supported this commonly held belief and patients with local and regional disease did better than patients with distant disease. Also, patients with local disease did better than patients with regional disease on multivariate analysis (data not shown). Stage at the time of diagnosis was recognized as an independent predictor of survival on stepwise multivariate analysis.
Nodal involvement is a feature of ES that distinguishes it from most other types of soft tissue sarcomas. Lymph node metastasis occurs in 2.6% of patients with the majority of soft tissue sarcomas [14]. In contrast, lymphatic spread has been reported in 22% to 48% of patients with ES, leading some clinicians to recommend sentinel node biopsy for all patients [23, 44]. In our study, lymph node involvement was seen in 36% of cases among all cases for which information regarding nodal status was available. Nodal metastasis has been recognized as an independent predictor of adverse prognosis [3, 13, 35], with a notable exception in a series reported by Ross et al. [36] from Memorial Sloan-Kettering Cancer Center. We report nodal involvement of regional and/or distant disease as an independent prognostic factor for adverse outcomes. We observed no difference in outcome when regional and distant nodes were compared.
Livi et al. [27] studied 29 patients over a 23-year period and reported poor prognosis with axial location and increased depth of the primary lesion. This observation has been confirmed by others [7, 8, 39]. However, Ross et al. [36] reported no association of primary tumor site with patient survival. Our analysis suggested tumor location predicted survival on univariate analysis but not on multivariate analysis. Thus, in our study, tumor location was not an independent prognostic factor for patients with ES.
We found a higher proportion of patients diagnosed with either superficial appendicular (201/219) or deep appendicular (10/10) tumors undergoing surgical resection as compared with patients with superficial axial (95/125) and deep axial (45/73) tumors. Patients undergoing any sort of surgery had a better prognosis on univariate and multivariate analyses, confirming surgery as the mainstay of treatment for this disease [11, 44]. Absence of radiotherapy was associated with improved survival on univariate analysis but not on multivariate analysis. Further analysis revealed a higher proportion of patients with localized disease not undergoing radiotherapy (92/133, data not shown), suggesting a higher proportion of patients undergoing radiotherapy with regional or distant disease. This observation confirms the selection bias resulting in a survival benefit for patients not receiving radiotherapy on univariate analysis.
Survival analysis for year of diagnosis by decade revealed a decrease in survival from 1976–1985 to 1996–2005. On multivariate analysis, the year of diagnosis was not recognized as an independent predictor of prognosis. Further analysis revealed a gradual increase in the proportion of patients not undergoing surgery from 1976–1985 through 1996–2005 (3/41, 14/92, and 64/297, respectively, for the three decades).
Callister et al. [6] reported primary tumor size as one of the most important variables associated with improved survival as reported by others. The size of primary tumor could directly affect the ability to resect the tumor and ultimately survival. We did not include size as a variable in our study primarily for two reasons. First, the data for size of primary tumor were available for a small proportion of the cohort (266 patients, 60%). Inclusion of size as a variable in multivariate analysis would have resulted in a loss in statistical significance owing to the exclusion of a large number of patients. Second, multifocality of ES lesions precludes the determination of exact tumor size as suggested by Baratti et al. [3]. Similarly, we did not include grade as a variable for survival analysis as information regarding histologic grade was available only for 40.5% of the entire cohort (179 patients) and it has been suggested grading for ES currently is not recommended [3].
The reported 5-year overall survival ranges from 60% to 75% [3, 36, 39]. Our study showed a 5-year disease-specific survival (68%) in this range. Retrospective statistical analysis of the SEER data identified younger age group, local stage of disease, and surgical resection as independent demographic and clinical characteristics associated with improved survival for the entire cohort of patients with ES. Although demographic analysis revealed 80.0% of all identified patients as Caucasians, there was no difference in incidence with respect to race (data not shown). Despite the limitations, we believe our study constitutes an important step toward identifying independent demographic and clinical factors associated with improved survival and clarifies some of the associated controversies.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved or does not require approval for the protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer; 2003.
- 2.Bach PB, Guadagnoli E, Schrag D, Schussler N, Warren JL. Patient demographic and socioeconomic characteristics in the SEER-Medicare database applications and limitations. Med Care. 2002;40(8 suppl):IV-19–25. [DOI] [PubMed]
- 3.Baratti D, Pennacchioli E, Casali PG, Bertulli R, Lozza L, Olmi P, Collini P, Radaelli S, Fiore M, Gronchi A. Epithelioid sarcoma: prognostic factors and survival in a series of patients treated at a single institution. Ann Surg Oncol. 2007;14:3542–3551. [DOI] [PubMed]
- 4.Bliss BO, Reed RJ. Large cell sarcomas of tendon sheath. Am J Clin Pathol. 1968;49:776–781. [DOI] [PubMed]
- 5.Bos GD, Pritchard DJ, Reiman HM, Dobyns JH, Ilstrup DM, Landon GC. Epithelioid sarcoma: an analysis of fifty-one cases. J Bone Joint Surg Am. 1988;70:862–870. [PubMed]
- 6.Callister MD, Ballo MT, Pisters PW, Patel SR, Feig BW, Pollock RE, Benjamin RS, Zagars GK. Epithelioid sarcoma: results of conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:384–391. [DOI] [PubMed]
- 7.Casanova M, Ferrari A, Collini P, Bisogno G, Alaggio R, Cecchetto G, Gronchi A, Meazza C, Garaventa A, Di Cataldo A, Carli M. Epithelioid sarcoma in children and adolescents: a report from the Italian Soft Tissue Sarcoma Committee. Cancer. 2006;106:708–717. [DOI] [PubMed]
- 8.Chase DR, Enzinger FM. Epithelioid sarcoma: diagnosis, prognostic indicators, and treatment. Am J Surg Pathol. 1985;9:241–263. [DOI] [PubMed]
- 9.Cheung MC, Perez EA, Molina MA, Jin X, Gutierrez JC, Franceschi D, Livingstone AS, Koniaris LG. Defining the role of surgery for primary gastrointestinal tract melanoma. J Gastrointest Surg. 2008;12:731–738. [DOI] [PubMed]
- 10.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40(8 suppl):IV-43–48. [DOI] [PubMed]
- 11.de Visscher SA, van Ginkel RJ, Wobbes T, Veth RP, Ten Heuvel SE, Suurmeijer AJ, Hoekstra HJ. Epithelioid sarcoma: still an only surgically curable disease. Cancer. 2006;107:606–612. [DOI] [PubMed]
- 12.Earle CC, Nattinger AB, Potosky AL, Lang K, Mallick R, Berger M, Warren JL. Identifying cancer relapse using SEER-Medicare data. Med Care. 2002;40(8 suppl):IV-75–81. [DOI] [PubMed]
- 13.Evans HL, Baer SC. Epithelioid sarcoma: a clinicopathologic and prognostic study of 26 cases. Semin Diagn Pathol. 1993;10:286–291. [PubMed]
- 14.Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults: analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72–77. [DOI] [PMC free article] [PubMed]
- 15.Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003;8:541–552. [DOI] [PubMed]
- 16.Gross E, Rao BN, Pappo A, Bowman L, Shearer P, Kaste S, Greenwald C, Michalkiewicz E, Pratt C. Epithelioid sarcoma in children. J Pediatr Surg. 1996;31:1663–1665. [DOI] [PubMed]
- 17.Gutierrez JC, De Oliveira LO, Perez EA, Rocha-Lima C, Livingstone AS, Koniaris LG. Optimizing diagnosis, staging, and management of gastrointestinal stromal tumors. J Am Coll Surg. 2007;205:479–491 (Quiz 524). [DOI] [PubMed]
- 18.Gutierrez JC, Fischer AC, Sola JE, Perez EA, Koniaris LG. Markedly improving survival of neuroblastoma: a 30-year analysis of 1,646 patients. Pediatr Surg Int. 2007;23:637–646. [DOI] [PubMed]
- 19.Gutierrez JC, Franceschi D, Koniaris LG. How many lymph nodes properly stage a periampullary malignancy? J Gastrointest Surg. 2008;12:77–85. [DOI] [PubMed]
- 20.Gutierrez JC, Perez EA, Franceschi D, Moffat FL Jr, Livingstone AS, Koniaris LG. Outcomes for soft-tissue sarcoma in 8249 cases from a large state cancer registry. J Surg Res. 2007;141:105–114. [DOI] [PubMed]
- 21.Halling AC, Wollan PC, Pritchard DJ, Vlasak R, Nascimento AG. Epithelioid sarcoma: a clinicopathologic review of 55 cases. Mayo Clin Proc. 1996;71:636–642. [DOI] [PubMed]
- 22.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20–37. [DOI] [PubMed]
- 23.Herr MJ, Harmsen WS, Amadio PC, Scully SP. Epithelioid sarcoma of the hand. Clin Orthop Relat Res. 2005;431:193–200. [DOI] [PubMed]
- 24.Hodgson N, Koniaris LG, Livingstone AS, Franceschi D. Gastric carcinoids: a temporal increase with proton pump introduction. Surg Endosc. 2005;19:1610–1612. [DOI] [PubMed]
- 25.International Agency for Research on Cancer. International Classification of Diseases for Oncology. 3rd ed. Geneva, Switzerland: World Health Organization; 2000.
- 26.Lee DJ, Voti L, Mackinnon J, Koniaris LG, Fleming LE, Huang Y, Wohler B, Franceschi D, Dietz NA, Sherman R, Soler-Vila H. Gender- and race-specific comparison of tobacco-associated cancer incidence trends in Florida with SEER regional cancer incidence data. Cancer Causes Control. 2008;19:711–723. [DOI] [PubMed]
- 27.Livi L, Shah N, Paiar F, Fisher C, Judson I, Moskovic E, Thomas M, Harmer C. Treatment of epithelioid sarcoma at the Royal Marsden Hospital. Sarcoma. 2003;7:149–152. [DOI] [PMC free article] [PubMed]
- 28.Matsushita Y, Ahmed AR, Kawaguchi N, Matsumoto S, Manabe J. Epithelioid sarcoma of the extremities: a dismal long-term outcome. J Orthop Sci. 2002;7:462–466. [DOI] [PubMed]
- 29.Merrill RM, Dearden KA. How representative are the surveillance, epidemiology, and end results (SEER) program cancer data of the United States? Cancer Causes Control. 2004;15:1027–1034. [DOI] [PubMed]
- 30.National Cancer Institute. Available at: http://seer.cancer.gov/csr/1975_2005/index.html. Accessed December 1, 2008.
- 31.Perez EA, Gutierrez JC, Jin X, Lee DJ, Rocha-Lima C, Livingstone AS, Franceschi D, Koniaris LG. Surgical outcomes of gastrointestinal sarcoma including gastrointestinal stromal tumors: a population-based examination. J Gastrointest Surg. 2007;11:114–125. [DOI] [PubMed]
- 32.Perez EA, Livingstone AS, Franceschi D, Rocha-Lima C, Lee DJ, Hodgson N, Jorda M, Koniaris LG. Current incidence and outcomes of gastrointestinal mesenchymal tumors including gastrointestinal stromal tumors. J Am Coll Surg. 2006;202:623–629. [DOI] [PubMed]
- 33.Petrelli NJ. SEER data: it can be thought provoking, but where do we go from here? Ann Surg Oncol. 2007;14:2173–2174. [DOI] [PubMed]
- 34.Potosky AL, Warren JL, Riedel ER, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40(8 suppl):IV-62–68. [DOI] [PubMed]
- 35.Prat J, Woodruff JM, Marcove RC. Epithelioid sarcoma: an analysis of 22 cases indicating the prognostic significance of vascular invasion and regional lymph node metastasis. Cancer. 1978;41:1472–1487. [DOI] [PubMed]
- 36.Ross HM, Lewis JJ, Woodruff JM, Brennan MF. Epithelioid sarcoma: clinical behavior and prognostic factors of survival. Ann Surg Oncol. 1997;4:491–495. [DOI] [PubMed]
- 37.Schrag D, Bach PB, Dahlman C, Warren JL. Identifying and measuring hospital characteristics using the SEER-Medicare data and other claims-based sources. Med Care. 2002;40(8 suppl):IV-96–103. [DOI] [PubMed]
- 38.Shwartz M, Pliskin JS, Grondahl HG, Boffa J. Use of the Kaplan-Meier estimate to reduce biases in estimating the rate of caries progression. Community Dent Oral Epidemiol. 1984;12:103–108. [DOI] [PubMed]
- 39.Spillane AJ, Thomas JM, Fisher C. Epithelioid sarcoma: the clinicopathological complexities of this rare soft tissue sarcoma. Ann Surg Oncol. 2000;7:218–225. [DOI] [PubMed]
- 40.Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40(8 suppl):IV-49–54. [DOI] [PubMed]
- 41.Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL, Klabunde CN, Cooper GS, Knopf KB. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(8 suppl):IV-55–61. [DOI] [PubMed]
- 42.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 suppl):IV-3–18. [DOI] [PubMed]
- 43.Wingo PA, Jamison PM, Hiatt RA, Weir HK, Gargiullo PM, Hutton M, Lee NC, Hall HI. Building the infrastructure for nationwide cancer surveillance and control: a comparison between the National Program of Cancer Registries (NPCR) and the Surveillance, Epidemiology, and End Results (SEER) Program (United States). Cancer Causes Control. 2003;14:175-193. [DOI] [PubMed]
- 44.Wolf PS, Flum DR, Tanas MR, Rubin BP, Mann GN. Epithelioid sarcoma: the University of Washington experience. Am J Surg. 2008;196:407–412. [DOI] [PubMed]