Abstract
Several lines of evidence suggest cyclooxygenase-2 (COX-2) overexpression may be a causal factor for tumor growth and metastasis. However, there is no evidence COX-2 expression in a primary tumor correlates with clinical outcome of osteosarcoma. We examined expression levels of COX-2 immunohistochemically in 51 patients with extremity osteosarcoma who completed standard therapy and obtained complete initial regression of the tumor. Correlation of the positivity of staining with prognosis was analyzed. COX-2 was expressed in most of the cases. We found no correlation between COX-2 staining intensity and variables such as gender, age, anatomic site, necrosis after chemotherapy, and surgical stage. Strong COX-2 expression was associated with low metastasis-free survival. Age older than 20 years and strong COX-2 expression independently predicted increased risk of metastasis. Among seven patients with resectable lung metastasis, all three with greater COX-2 expression in the metastatic lesion than that in a primary site died of the disease. Our preliminary data suggest COX-2 overexpression in the primary tumor correlates with the occurrence of distant metastasis in patients with osteosarcoma and also may affect postmetastatic survival.
Level of Evidence: Level IV, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Osteosarcoma is the most common primary bone tumor in children and adolescents. It is a highly metastatic tumor, and its most common metastatic site is the lung [1]. Adjuvant and neoadjuvant chemotherapy, introduced in the early 1970s, have considerably improved the long-term survival rate for patients with osteosarcoma. Nevertheless, recurrent disease still occurs in 30% to 40% of patients, more than 70% of whom die of their tumor despite second-line treatment [1]. Therefore, the identification of risk factors for recurrence would be of major importance in the development of new and risk-adapted strategies of treatment. Chemotherapy regimens based on risk evaluation would be important to limit the incidence of side effects and substantial economic and social consequences of an intensive chemotherapy program. Because chemotherapy is indispensable for the treatment of osteosarcoma, the means to predict the course of the tumor would help guide treatment, and a reliable prognostic factor would identify patients at low risk of metastases who would not benefit from toxic chemotherapy.
Cyclooxygenase (COX) is an enzyme implicated in the conversion of arachidonic acid into prostaglandin. There are two isoforms of COX, designated COX-1 and COX-2. COX-1 is constitutively expressed in most tissues, and COX-2 is an inducible enzyme associated with inflammatory disease and cancer. Various reports have indicated COX-2 is overexpressed in many types of malignant tumors such as colorectal [5], prostate [8], breast [10], and lung [21], and several lines of evidence suggest COX-2 overexpression may be a causal factor for tumor growth and metastasis [12, 15, 17, 19].
Numerous studies have shown COX-2 is expressed in osteosarcoma cell lines [16] and clinical specimens obtained from osteosarcomas [4, 14]. Recently, Rodriguez et al. [18] reported COX-2 expression of lung metastasis tissue correlated with disease-specific survival in patients with metastatic osteosarcoma. However, there is little evidence COX-2 overexpression in primary tumors correlates with the clinical outcome of patients with osteosarcoma after surgical treatment combined with neoadjuvant and adjuvant chemotherapy. The relation of COX-2 expression in primary and metastatic osteosarcoma also is unknown.
We therefore first determined whether COX-2 expression in the primary tumor predicted metastasis-free and overall survival in patients with initially nonmetastatic osteosarcoma arising in the extremity and treated surgically and chemotherapy. Second, we determined COX-2 expression in resected lung metastasis, compared with that in the primary lesion, and determined whether the difference predicted postmetastatic survival.
Materials and Methods
We have diagnosed and treated 139 patients with high-grade conventional osteosarcoma since 1987 at our institutions. Complete clinical records were unavailable for 12 patients. We excluded 14 patients with an unresectable primary site. Among the remaining 113 patients, we had tumor tissue samples from 55 patients before any chemotherapy. Of the 55 patients, three patients with metastasis at the first visit and one patient with clavicle involvement were excluded. One patient with Rothmund-Thomson syndrome was included. This left 51 patients for review. There were 33 males and 18 females with a median age of 15 years (range, 4–57 years) (Table 1). There were 29 cases with femur, 11 with tibia, five with humerus, four with fibula, and two with radius involvement. We reviewed all slides of the cases to confirm the diagnosis by pathologists. According to the American Joint Committee on Cancer (AJCC) Staging System, 32 tumors were Stage IIA and 19 were Stage IIB. Followup data were obtained in June 2008, allowing a minimum followup of 3.8 months (mean, 67.4 months; range, 3.8–162.6 months). All patients gave signed consent.
Table 1.
Clinical features and treatments of study group
| Characteristics or treatment | Number of patients |
|---|---|
| Gender | |
| Male | 33 (65%) |
| Female | 18 (35%) |
| Age (years)* | 15 (4–57) |
| Anatomic site | |
| Femur | 29 (57%) |
| Tibia | 11 (22%) |
| Humerus | 5 (10%) |
| Fibula | 4 (8%) |
| Radius | 2 (4%) |
| AJCC surgical stage | |
| IIA | 32 (63%) |
| IIB | 19 (37%) |
| Chemotherapy before and after surgery | |
| C/D/M/I | 27 (53%) |
| C/D/M | 18 (35%) |
| Others | 6 (12%) |
| Surgery | |
| Amputation | 3 (6%) |
| Limb salvage | 48 (94%) |
| Followup (months)* | 67.4 (3.8–162.6) |
* Values are expressed as median, with range in parentheses; AJCC = American Joint Committee on Cancer Staging System; C = cisplatin; D = doxorubicin; M = methotrexate; I = ifosfamide.
We obtained the samples of primary tumor before chemotherapy and subjected them to analysis. The biopsy was performed from the nonnecrotic part of the primary osteosarcoma based on MRI and immediately subjected to formalin fixation. The samples of lung metastasis were obtained at the operation of first-time lung metastasis.
We carried the formalin-fixed samples to the laboratory. After formalin fixation for 24 hours, a paraffin block of the tumor was made. Conventional immunohistochemical studies were performed using a streptavidin-biotin complex technique on the formalin-fixed, paraffin-embedded sections (8 μm thick) to detect COX-2 expression. We immersed deparaffinized and rehydrated sections three times in phosphate-buffered saline (PBS). Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in methanol for 15 minutes at room temperature and rinsed in PBS. We then soaked the slides for 10 minutes in 10% normal rabbit serum as a blocking agent. The slides then were incubated at room temperature for 1 hour with primary polyclonal goat antibody for the carboxy terminus of rat COX-2 (sc-1747; Santa Cruz Biotechnology, Santa Cruz, CA; 1:500 dilution), which crossreacts with human COX-2. After rinsing with PBS, we applied biotinylated peroxidase conjugated anti-goat rabbit IgG conjugated with peroxidase as the second antibody, and the reaction products were observed using 3,3′-diaminobenzidine tetrahydrochloride. Slides were counterstained with hematoxylin, dehydrated, and mounted. Nonimmune goat serum was substituted for the primary antibody to serve as a negative control. Two orthopaedic surgeons (YN, HU) without knowledge of the clinicopathologic information evaluated the results of immunohistochemical staining on a 4-point scale: 0% to 9% for positive stainable cell number (negative), 10% to 39% (low), 40% to 79% (moderate), and 80% to 100% (strong) on four different high-power fields without necrosis. For statistical analysis, the cutoff used was 80%; strong staining was classified as overexpression of the respective antigen. Using these criteria, the two observers could come to agreement on the degree of positivity or negativity in all cases.
We collected clinical data from the patients’ clinical records. Fisher’s exact test and Pearson chi square test were used to examine correlations between COX-2 expression of primary tumor and each clinical variable, gender, age, site, necrosis after chemotherapy, and surgical stage. Survival periods were counted (months) from the date of the first visit to the date of death or last followup before study closure, and the metastasis-free period was counted in months from the date of operation to the date of detection of the first metastasis. Because there were no local relapses, not event-free but metastasis-free survival was analyzed in this study. We used the Kaplan-Meier product limit method to estimate the metastasis-free survival and the overall survival for the group and to illustrate the effect of each protein expression. The log-rank test was used to evaluate the differences between survival curves. For the multivariate analysis of metastasis, confidence intervals (CIs) for relative risks of metastasis were derived from the Cox proportional-hazards regression model with forward selection of variables.
Results
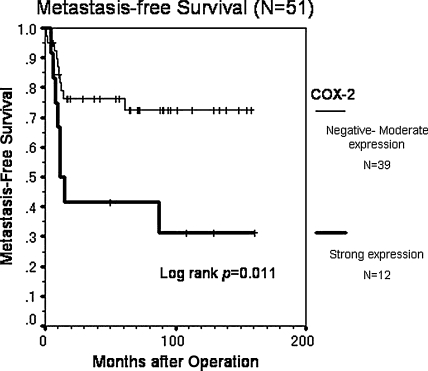
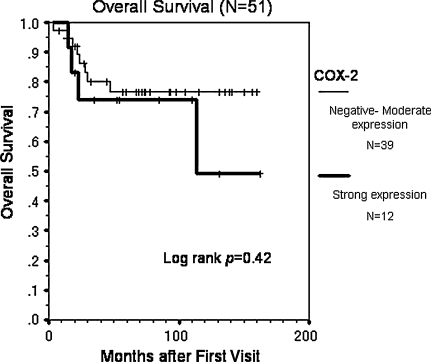
Forty-eight (94%) of the 51 patients underwent limb-sparing surgery with curative margins (Table 1). There were no local recurrences. At the last followup, 33 (65%) of the 51 patients remained continuously disease-free. Eighteen (35%) of 51 patients had distant metastasis and 12 (67%) of these 18 patients died of the disease. The expression levels of COX-2 varied widely. Overall, 47 (92%) of 51 patients showed positive immunoreactivity for COX-2 in neoplastic cells, and 12 patients (24%) showed strong positive immunostaining for COX-2 (Table 2). COX-2 overexpression was associated (p = 0.011) with a decreased probability of metastasis-free survival (Fig. 1), but not with overall survival (p = 0.42) (Fig. 2). The estimated metastasis-free survival at 5 years was 42% in patients with strong expression and 76% in patients with lower expression, and the estimated overall survival at 5 years was 74% for the patients with strong expression and 77% in patients with lower expression. Age older than 20 years and strong COX-2 expression independently predicted metastasis with relative risks of 3.20 (95% CI, 1.17–8.76; p = 0.024) and 3.64 (95% CI, 1.14–11.60; p = 0.029), respectively (Table 3). COX-2 staining intensity did not vary with gender, age (younger than 20 years versus 20 years or older), anatomic site (proximal versus distal extremity), necrosis after chemotherapy (< 90% versus ≥ 90%), and surgical stage (AJCC Stage IIA versus Stage IIB) (Table 4). COX-2 staining intensity tended to be greater (p = 0.056) in patients with necrosis after chemotherapy than in those without.
Table 2.
Expression of COX-2 in primary site and clinical outcomes
| Positive cell ratio | Number of cases | Number with metastasis | Number dead |
|---|---|---|---|
| Negative (0%–9%) | 4 | 2 | 2 |
| Low (10%–39%) | 18 | 5 | 4 |
| Moderate (40%–79%) | 17 | 3 | 2 |
| Strong (80%–100%) | 12 | 8 | 4 |
| Total | 51 | 18 | 12 |
COX-2 = cyclooxygenase-2.
Fig. 1.
The graph shows the cumulative metastasis-free survival rate of patients who achieved initial complete remission after neoadjuvant chemotherapy and curative surgery followed by adjuvant chemotherapy (n = 51) using the Kaplan-Meier method. COX-2 overexpression was associated (p = 0.011) with a decreased probability of metastasis-free survival.
Fig. 2.
The graph shows the cumulative overall survival rate of all patients (n = 51) using the Kaplan-Meier method. COX-2 overexpression was not associated (p = 0.42) with a decreased probability of overall survival.
Table 3.
Multivariate Cox regression analysis of clinical variables for metastasis
| Variable | Metastasis | ||
|---|---|---|---|
| Relative risk | 95% CI | p Value | |
| Gender | 0.98 | ||
| Male | 1.00 | ||
| Female | 0.99 | 0.32–3.01 | |
| Age | 0.024 | ||
| < 20 years | 1.00 | ||
| ≥ 20 years | 3.20 | 1.17–8.76 | |
| Site | 0.13 | ||
| Proximal extremity | 1.00 | ||
| Distal extremity | 2.47 | 0.77–7.98 | |
| Necrosis* | 0.34 | ||
| < 90% | 1.00 | ||
| ≥ 90% | 0.52 | 0.14–1.96 | |
| AJCC surgical stage | 0.18 | ||
| IIA | 1.00 | ||
| IIB | 2.18 | 0.70–6.75 | |
| Expression of COX-2 in primary site | 0.029 | ||
| Negative-moderate | 1.00 | ||
| Strong | 3.64 | 1.14–11.60 | |
* Necrosis of the resected section after chemotherapy; 95% CI = 95% confidence interval; AJCC = American Joint Committee on Cancer Staging System; COX-2 = cyclooxygenase-2.
Table 4.
Association between COX-2 overexpression in primary site and each clinical variable
| Variable | COX-2 expression | p Value* | |
|---|---|---|---|
| Negative–moderate | Strong | ||
| Gender | 0.50 | ||
| Male | 24 | 9 | |
| Female | 15 | 3 | |
| Age | 0.55 | ||
| < 20 years | 24 | 7 | |
| ≥ 20 years | 15 | 5 | |
| Site | 0.36 | ||
| Proximal extremity | 27 | 7 | |
| Distal extremity | 12 | 5 | |
| Necrosis† | 0.056 | ||
| < 90% | 28 | 5 | |
| ≥ 90% | 11 | 7 | |
| AJCC surgical stage | 0.51 | ||
| IIA | 24 | 8 | |
| IIB | 15 | 4 | |
* Fisher’s exact test and Pearson chi square test were used to examine correlations with COX-2 strong expression and each clinical variable; †necrosis of the resected section after chemotherapy; COX-2 = cyclooxygenase-2; AJCC = American Joint Committee on Cancer Staging System.
The initial sites of metastases were isolated to lung in 15 patients, bone in one patient, lung and bone in one patient, and lung and skin in one patient. The treatment for these 18 patients with postoperative metastases was resection of metastases with adjuvant chemotherapy in 10 patients, chemotherapy alone in five patients, radiation plus chemotherapy in two patients, and unknown in one patient (Table 5). Among seven patients with resectable lung metastasis, three had increased COX-2 expression in the lung metastatic lesion compared with that in the primary site and all three died of the disease (Fig. 3). Two patients with decreased COX-2 expression in the lung metastatic lesion compared with that in the primary site were alive with no evidence of disease after resection of the lung metastasis. Two patients with unchanged levels of COX-2 expression in the lung metastatic lesion compared with that in the primary site also were alive with no evidence of disease after resection of lung metastasis (Table 5).
Table 5.
Treatment of primary metastases and end result with comparison of COX-2 expression
| Patient | Age (years)* | Gender | Site of metastasis (number) | Treatment for metastases | Expression of COX-2 in primary site | Expression of COX-2 in lung metastasis | End result |
|---|---|---|---|---|---|---|---|
| 1 | 22 | Male | Lung (1) | Resection, chemotherapy | Negative | Moderate | DOD |
| 2 | 32 | Male | Lung (1) | Resection, chemotherapy | Negative | Moderate | DOD |
| 3 | 10 | Male | Lung (2) | Resection, chemotherapy | Low | Not available | NED |
| 4 | 24 | Male | Lung (≥ 4) | Chemotherapy | Low | DOD | |
| 5 | 35 | Male | Lung (2) | Chemotherapy | Low | DOD | |
| 6 | 42 | Male | Lung + skin (≥ 4) | Unknown | Low | DOD | |
| 7 | 57 | Male | Spine (unknown) | Chemotherapy, radiation | Low | DOD | |
| 8 | 13 | Female | Lung (1) | Resection, chemotherapy | Moderate | Strong | DOD |
| 9 | 20 | Female | Lung (1) | Resection, chemotherapy | Moderate | Moderate | NED |
| 10 | 56 | Male | Lung (≥ 4) | Chemotherapy | Moderate | DOD | |
| 11 | 10 | Male | Lung (2) | Resection, chemotherapy | Strong | Not available | NED |
| 12 | 12 | Female | Lung (2) | Resection, chemotherapy | Strong | Moderate | NED |
| 13 | 13 | Female | Lung (3) | Resection, chemotherapy | Strong | Strong | NED |
| 14 | 14 | Male | Lung (≥ 4) | Chemotherapy | Strong | DOD | |
| 15 | 22 | Female | Lung + spine + clavicle (≥ 4) | Chemotherapy, radiation (spine) | Strong | DOD | |
| 16 | 30 | Male | Lung (2) | Resection, chemotherapy | Strong | Not available | DOD |
| 17 | 38 | Male | Lung (≥ 4) | Chemotherapy | Strong | DOD | |
| 18 | 57 | Male | Lung (2) | Resection, chemotherapy | Strong | Low | NED |
* Age at first visit; COX-2 = cyclooxygenase-2; DOD = dead of disease; NED = no evidence of disease.
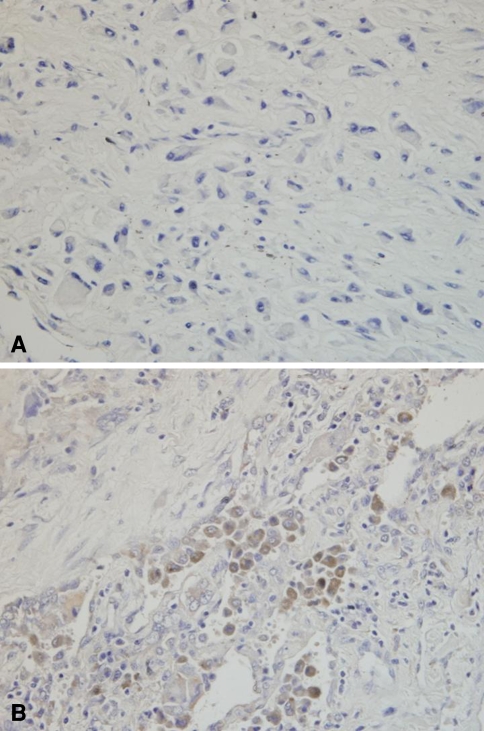
Fig. 3A–B.
Photomicrographs show (A) negative COX-2 expression in primary tumor and (B) moderate COX-2 expression in lung metastasis in the same patient with high-grade osteosarcoma (Stain, immunostain for COX-2 and counterstain with hematoxylin; original magnification, ×400). The osteosarcoma of the tibia occurred in a 32-year-old man.
Discussion
COX-2 overexpression is reportedly a causal factor for tumor growth and metastasis [12, 15, 17, 19]. However, there is little evidence COX-2 overexpression in primary tumors correlates with the clinical outcome of the patients with osteosarcoma, and the relation of COX-2 expression in primary and metastatic osteosarcoma also is unknown. Therefore, we assessed the prognostic value of COX-2 expression in primary osteosarcoma arising in the extremities and also examined COX-2 expression in resected lung metastasis in osteosarcoma and assessed the prognostic value of COX-2 expression for postmetastatic survival.
We note some limitations. First, we studied comparatively few patients and the study may be underpowered to detect differences between groups and between metastasis-free survival and overall survival. However, we consider our results meaningful as a pilot study. Future studies with more accumulated cases will clarify the association of COX-2 and the prognosis. Second, the results of immunohistochemical analysis may be influenced by sensitivity of the antibody, but the results of immunohistochemistry should be stable if it is performed with the same antibody and same protocol in one institution. The examination of COX-2 expression by immunohistochemistry is easy and can be performed routinely. Finally, quite a few patients without strong COX-2 expression also died with lung metastasis. Although the number of lung metastasis samples was small in our study, additional comparisons of COX-2 expression in primary tumors and metastatic lesions as a prognostic factor in osteosarcoma might be of interest.
Our data suggest COX-2 expression in biopsy samples of a primary site inversely correlates with metastasis-free survival but not with overall survival. In a previous investigation of pediatric osteosarcomas, COX-2 immunostaining was not correlated with disease-free survival or overall survival in primary conventional high-grade osteosarcoma [4]. This discrepancy may be attributable partly to differences in the sensitivity of the anti-COX-2 antibody used, the cutoff value, or various aspects of treatments such as surgical margin and chemotherapy protocols. We observed a higher frequency of cases with COX-2 positivity than reported in other studies (92% in our study versus 67% to 86%) [4, 14, 18], leading us to set a higher cutoff value. The study of pediatric osteosarcomas did not describe the demographic data, including the grade of osteosarcoma, resectability of the tumors, or therapeutic methods, including surgical margin and chemotherapy protocols [4]. Another possibility is the heterogeneity of each patient group contributed to the inaccurate results in the studies [4, 14, 18]. Several prognostic risk factors have been proposed, including tumor size, site, age, history of symptoms, treatment delay, margin, and response to chemotherapy [1, 3]. The presence or absence of other molecules in osteosarcoma cells appear to predict outcome [2, 6, 9, 11, 20]. To know the posttherapeutic clinical course of patients with osteosarcoma may lead physicians to adequate followup for the patients. We found no correlation between COX-2 strong expression and clinicopathologic variables such as gender, age, tumor location, and clinical stage, which is consistent with other reports of osteosarcoma [4, 18]. Histologic response to chemotherapy apparently correlates with outcome in osteosarcoma [1, 3]. We found no correlation between chemosensitivity and metastasis-free survival and between COX-2 expression and necrosis, likely owing to the small number of cases.
Results of our study also suggest alteration of COX-2 expression in the same patients between primary site and metastatic lesion may affect their postmetastatic survival. The alteration of COX-2 positivity between the primary site and lung metastatic lesion may be affected by some factors, such as the microenvironment of lung metastases, effect of chemotherapy, and aggressive change of osteosarcoma cells. Rodriguez et al. [18] suggested high COX-2 expression in lung metastases of osteosarcoma was correlated with poor disease-specific survival. However, they did not analyze COX-2 expression in the primary site nor determine its importance for survival. Dickens et al. [4] reported the COX-2-positive rate in metastatic lesions was greater than that of biopsy and/or resected samples of the primary site in osteosarcoma. They also did not analyze paired samples from the same patients; moreover, alteration of expression levels was not mentioned. An accumulated number of cases and paired samples of primary sites and metastatic lesions will clarify the prognostic importance of COX-2 expression in primary and metastatic tumors in the future.
Some reports suggest the possibility of COX-2 involvement in the tumorigenicity and/or metastasis of osteosarcoma [13, 16]. COX-2 expression may have roles in these processes; however, there is a possibility COX-2 is a secondary molecule of a crucial prognostic factor or a molecule upstream of critical prognostic factors. For example, PGE2, downstream of COX-2, can regulate immune function through inhibition of dendritic cell differentiation and T cell proliferation and suppression of the antitumor activity of natural killer cells and macrophages [7, 22].
We found patients with strong COX-2 expression had a poor prognosis with regard to metastasis, and all patients with increased COX-2 expression in lung metastases died of the disease. These results support our speculation that COX-2 could be one of the key enzymes directly or indirectly involved in the process of metastasis in osteosarcoma. COX-2 expression can be analyzed easily and is relevant for clinical use. The change of COX-2 expression between the primary site and lung metastasis seemed important for the survival of osteosarcoma after lung metastasis in our study patients. Additional comparisons are needed of COX-2 expression in primary tumors and metastasis as a prognostic factor in osteosarcoma.
Acknowledgments
We thank Drs. Shigeo Nakamura and Yoshie Shimoyama for their expert opinions as pathologists. We also thank Dr. Mitsutoshi Uchibori and Eri Ishihara for help during the study.
Footnotes
One or more of the authors (YN) have received funding from The Nakatomi Foundation and the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid 20591751 for Scientific Research [C]).
Each author certifies that his institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
This work was performed at Nagoya University Graduate School and School of Medicine.
References
- 1.Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. [DOI] [PubMed]
- 2.Baldini N, Scotlandi K, Serra M, Picci P, Bacci G, Sottili S, Campanacci M. P-glycoprotein expression in osteosarcoma: a basis for risk-adapted adjuvant chemotherapy. J Orthop Res. 1999;17:629–632. [DOI] [PubMed]
- 3.Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20:776–790. [DOI] [PubMed]
- 4.Dickens DS, Kozielski R, Leavey PJ, Timmons C, Cripe TP. Cyclooxygenase-2 expression does not correlate with outcome in osteosarcoma or rhabdomyosarcoma. J Pediatr Hematol Oncol. 2003;25:282–285. [DOI] [PubMed]
- 5.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, Dubois RN. Up-regulation of cyclooxygenase-2 gene-expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–1188. [DOI] [PubMed]
- 6.Foukas AF, Deshmukh NS, Grimer RJ, Mangham DC, Mangos EG, Taylor S. Stage-IIB osteosarcomas around the knee: a study of MMP-9 in surviving tumour cells. J Bone Joint Surg Br. 2002;84:706–711. [DOI] [PubMed]
- 7.Goodwin JS, Ceuppens J. Regulation of the immune-response by prostaglandins. J Clin Immunol. 1983;3:295–315. [DOI] [PubMed]
- 8.Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–78. [DOI] [PubMed]
- 9.Himelstein BP, Asada N, Carlton MR, Collins MH. Matrix metalloproteinase-9 (MMP-9) expression in childhood osseous osteosarcoma. Med Pediatr Oncol. 1998;31:471–474. [DOI] [PubMed]
- 10.Hwang D, Scollard D, Byrne J, Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998;90:455–460. [DOI] [PubMed]
- 11.Kaya M, Wada T, Akatsuka T, Kawaguchi S, Nagoya S, Shindoh M, Higashino F, Mezawa F, Okada F, Ishii S. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res. 2000;6:572–577. [PubMed]
- 12.Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Lippman SM, Feng L, Hong WK, Xu XC. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res. 2001;7:861–867. [PubMed]
- 13.Lee EJ, Choi EM, Kim SR, Park JH, Kim H, Ha KS, Kim YM, Kim SS, Choe M, Kim JI, Han JA. Cyclooxygenase-2 promotes cell proliferation, migration and invasion in U2OS human osteosarcoma cells. Exp Mol Med. 2007;39:469–476. [DOI] [PubMed]
- 14.Masi L, Recenti R, Silvestri S, Pinzani P, Pepi M, Paglierani M, Brandi ML, Franchi A. Expression of cyclooxygenase-2 in osteosarcoma of bone. Appl Immunohistochem Mol Morphol. 2007;15:70–76. [DOI] [PubMed]
- 15.Masunaga R, Kohno H, Dhar DK, Ohno S, Shibakita M, Kinugasa S, Yoshimura H, Tachibana M, Kubota H, Nagasue N. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000;6:4064–4068. [PubMed]
- 16.Naruse T, Nishida Y, Hosono K, Ishiguro N. Meloxicam inhibits osteosarcoma growth, invasiveness and metastasis by COX-2-dependent and independent routes. Carcinogenesis. 2006;27:584–592. [DOI] [PubMed]
- 17.Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876–1881. [DOI] [PubMed]
- 18.Rodriguez NI, Hoots WK, Koshkina NV, Morales-Arias JA, Arndt CA, Inwards CY, Hawkins DS, Munsell MF, Kleinerman ES. COX-2 expression correlates with survival in patients with osteosarcoma lung metastases. J Pediatr Hematol Oncol. 2008;30:507–512. [DOI] [PMC free article] [PubMed]
- 19.Shono T, Tofilon PJ, Bruner JM, Owolabi O, Lang FF. Cyclooxygenase-2 expression in human gliomas: prognostic significance and molecular correlations. Cancer Res. 2001;61:4375–4381. [PubMed]
- 20.Uchibori M, Nishida Y, Nagasaka T, Yamada Y, Nakanishi K, Ishiguro N. Increased expression of membrane-type matrix metalloproteinase-1 is correlated with poor prognosis in patients with osteosarcoma. Int J Oncol. 2006;28:33–42. [PubMed]
- 21.Watkins DN, Lenzo JC, Segal A, Garlepp MJ, Thompson PJ. Expression and localization of cyclo-oxygenase isoforms in non-small cell lung cancer. Eur Respir J. 1999;14:412–418. [DOI] [PubMed]
- 22.Yang L, Yamagata N, Yadav R, Brandon S, Courtney RL, Morrow JD, Shyr Y, Boothby M, Joyce S, Carbone DP, Breyer RM. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J Clin Invest. 2003;111:727–735. [DOI] [PMC free article] [PubMed]