Abstract
Viscosupplementation is a symptomatic treatment of osteoarthritis (OA) intended to restore rheologic homeostasis of the synovial fluid by injecting hyaluronic acid intraarticularly. Despite the long history of this therapy, little is known about its mechanisms of action and differences between commercial preparations. We investigated the rheologic behavior of OA synovial fluid with time, when stored at 4°C, before and after the addition of two hyaluronic acid commercial preparations (linear and cross-linked). Thirteen OA synovial fluids were stored at 4°C and assayed using steric exclusion chromatography, which allows hyaluronic acid to be separated from the remaining pool of proteins and its molecular weight and concentration to be determined without any pretreatment and calibration. The synovial fluid rheology also was studied in vitro, before and after addition of two viscosupplements, over 6 weeks. The non-Newtonian behavior of synovial fluid throughout followup appears to be the result of loose interactions between proteins and hyaluronic acid. When mixed with the linear hyaluronic acid, synovial fluid becomes less non-Newtonian whereas the non-Newtonian behavior was reinforced when mixed with the cross-linked hyaluronic acid. The rheology was nearly unchanged for all synovial fluids over 6 weeks. Our preliminary trial shows it is possible to study synovial fluid, stored at 4°C, over a long time and suggests the enzymatic degradation of hyaluronic acid is negligible under these experimental conditions.
Introduction
OA is the most common disease affecting synovial joints and is one of the most prevalent chronic conditions in Western populations [16]. Balazs [6] and Balaz and Denlinger [7] proposed correcting OA synovial fluid (SF) disorders by injecting high-molecular-weight hyaluronan acid (HA) and thereby introduced the concept of viscosupplementation (VS). VS by intraarticular injections of HA is used for reducing pain and improving joint mobility in patients with OA of the knee and other synovial joints [5, 11] by restoring SF rheologic properties affected in OA. HA is a natural polysaccharide existing in the animal body [28, 31, 35] and especially in the joints where it plays an important role in lubrication, shock absorption, and viscoelastic behavior of the synovial fluid [8, 17, 22, 39, 58].
The viscoelastic behavior involves entanglements in HA and/or protein-HA associations based mainly on electrostatic interactions [46]. As HA is an anionic polyelectrolyte able to interact with positive charges on proteins, the viscoelasticity of SF may be related to the formation of a complex of HA and proteins forming a physical three-dimensional network [17, 29, 57, 58]. Inflammatory and degenerative joint diseases, mainly OA, are responsive to modifications of the SF characterized by a decrease in the amount of HA molecules of high molecular weight and in the concentration of HA at least partly as a result of exudation [9, 55]. The mechanisms of degradation of HA have been described [3, 41, 45, 49–51]. These changes reduce the viscoelasticity of the SF and its ability to protect the joint [5, 6, 21]. HA in healthy adults is claimed to have a molecular weight in the range of 4 to 5 × 106 Da [7]. Its synovial concentration ranges from 2.5 to 4 mg/mL [7, 9, 14, 48, 58], which grossly corresponds to a total amount of 4 to 8 mg HA in a healthy person in which the volume of SF is assumed to be 0.5 to 4 mL [7, 30].
Rheology, which includes intrinsic viscosity and dynamic and steady viscoelastic measurements (ie, the measurement of the storage modulus G′ reflecting the elasticity and the loss modulus G″ reflecting the viscosity), is a convenient technique to evaluate the behavior of SF, especially in view of the evolution of its viscoelasticity in OA when compared with normal SF [8, 39]. The rheologic behavior of linear HA solutions is strictly related to the product of molar mass and HA concentration, which also controls HA’s biologic effects [12, 13, 18, 19, 22, 23, 54]. The injection of very high-molecular-weight HA, and especially of partially cross-linked HA, may allow recovery of desirable viscoelastic behavior (especially elastic contribution) [7, 36, 37]. Other biologic roles for exogenous HA in OA include an analgesic effect by interaction with pain receptors [23], promotion of endogenous HA production [4, 38], and various antiinflammatory effects [10, 15, 24, 34, 42, 43, 56]. Nevertheless, the biologic effects of VS last much longer than its presence in SF, leading to the concept of “viscoinduction” effects extending beyond the effects of viscosupplementation. In an open-label study, Bagga et al. [4] reported an increase from baseline in SF HA concentration (13%) and complex shear modulus at 3 months after HA injection, suggesting VS could promote endogenous HA production. This effect could be mediated through interactions between HA and its receptors CD44 and RHAMM (receptor for hyaluronan-mediated motility) [20].
Despite its widespread use, the magnitude of the clinical effect of VS remains controversial and appears different according to the VS formulations, which differ widely in molecular weight, origin (animal or bacterial), and residence time in the joint [4, 20, 25, 27, 29]. The advantage of cross-linkage seems mainly to increase the stability and residence time of the VS in the joint. However, other studies suggest the noncross-linked VS effect is comparable with that of the cross-linked system [27, 43], and to date, no definitive conclusion has been reached regarding any advantage of cross-linkage on the clinical benefit [2, 27, 29, 33, 52, 59]. Therefore, techniques aimed at investigating variations of HA (concentration and molecular weight) and rheologic changes attributable to VS are needed to better understand its mechanisms of action and to determine possible differences between different HA commercial formulations.
The objectives of this study therefore were to (1) compare in vitro the changes attributable to the addition of two different formulations of VS (medium-molecular-weight linear HA and high-molecular-weight cross-linked HA) on SF rheology and (2) evaluate the stability of the HA-modified SF with time.
Materials and Methods
The primary aim of the study being the modifications induced by exogenous HA addition in OA SF, we obtained synovial fluid by sterile aspiration of the affected joint in 12 patients (nine women; mean age, 68 years; mean body mass index, 27.4) with knee OA and one patient (male; age, 63 years; body mass index, 28.2) with shoulder OA. All patients were referred to the rheumatology unit for moderate (five patients) to severe (eight patients) OA, according to the Kellgren-Lawrence [26] radiographic classifications (Grades 1–2 and 3–4, respectively), with clinical evidence of SF effusion. None was affected by any other arthritic condition (inflammatory or crystal deposition disease). Arthrocentesis was performed by an experienced rheumatologist using a 50-mm 20-gauge needle to remove as much SF as possible before corticosteroid or HA intraarticular injection. The SF was collected in sterile tubes and the volume recorded. Each SF sample was aliquoted into three tubes. One (2 mL) contained SF alone and was used for steric exclusion chromatography (SEC) experiments and rheology; in the other two (each 1 mL), a VS was added in a volume ratio of 1:1 before rheologic examination. Such a ratio was chosen because it corresponds to that obtained in vivo in the usual clinical conditions of VS (ie, injection of 2 to 6 mL VS into a knee without effusion) [15]. Two distinct HA commercial formulations, representative of those used in clinical practice as VS, were used to compare their effect on SF behavior. All three samples then were stored at 4°C.
The HA used as a VS consisted of (1) a linear HA of bacterial origin (ARD, Pomacle, France) and (2) a cross-linked HA, Hylan G-F 20 (Synvisc®; Genzyme Corp, Cambridge, MA) The linear HA solution at 10 g/L was prepared by direct dissolution of HA in 0.15 mol/L NaCl. The weight-averaged molecular weight of the linear HA was 1.14 × 106 Da, with an intrinsic viscosity of approximately 1000 mL/g. Hylan G-F 20 has a concentration of 8 ± 2 g/L containing 80% Hylan A, obtained by extraction from rooster combs, and 20% chemically cross-linked Hylan A, characterized by sulfonyl-bis-ethyl cross-links between the hydroxyl groups of polymer chains, named Hylan B. This structure gives a chemical network leading to a substantially different rheology compared with that of linear HA [37, 39]. For example, a solution of Hylan G-F 20 at 1% is 15-fold more viscous and ninefold more elastic than unmodified HA at the same concentration. The cross-linking also allows a longer residence time in the joint than that of linear HA products, particularly for Hylan B, whose insolubility delays its removal from the joint. Only an apparent molecular weight (approximately 8 × 106 Da) may be used to characterize such systems [37].
Each SF was assayed twice by SEC using an Alliance GPCV2000 system (Waters Corp, Boston, MA) equipped with three detectors online: a differential refractometer, a viscometric detector, and a multiangle laser light scattering detector from Wyatt Technology Corp (Santa Barbara, CA) [37, 44, 47]. The concentration of HA polymer in the sample injected was lower than 0.5 g/L with an injection volume of 108 μL using two columns in series (Shodex OH-pack 805 and 806; Showa Denko KK, Tokyo, Japan); these columns allow separation of high-hydrodynamic-volume HA from the lower-hydrodynamic-volume proteins. Knowing the volume injected and the refractive index increment dn/dc, we could determine the polymer concentration eluted. Before injection, all samples were filtrated on a 0.2-μm-pore cellulose acetate filter (Sartorius AG, Göttingen, Germany) to retain large aggregates (or flocculated proteins). The eluent used was a 0.1-mol/L NaNO3 aqueous solution at an elution temperature of 30°C and a flow rate of 0.5 mL/minute. Molecular weight distribution and weight-averaged molecular weight of HA and proteins could be determined separately from light scattering detection eliminating the need for molecular weight calibration. The signal of the differential refractometer is related to the polymer concentration eluted through the coefficient dn/dc (0.153 for HA [37] and 0.190 for the protein pool [53]).
Only soluble proteins were detected in this assay. Because of the large dilution used, we assumed no protein-HA complexes perturbed the quantitative determination of the polymers. The amount of soluble proteins was determined using the Bradford titration after dilution of the SF (ratio 1:20). The interference with HA was taken into account in a calibration curve performed with bovine serum albumin. The HA content of SF is much lower than that of proteins and its contribution does not exceed 2% in the protein determination.
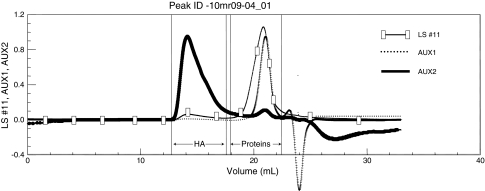
The mean concentration of HA was 0.86 g/L (range, 0.2–2.5 g/L) and that of soluble proteins was 29.9 g/L (range, 25–35 g/L). The mean weight-averaged molecular weight of HA was 1.45 × 106 Da (range, 1 × 106 to 3 × 106 Da), whereas the weight-average molecular weight of the proteins was 118,547 Da (range, 85,000–135,000 Da) (Fig. 1). The values of protein concentration were in good agreement with the direct titration using the Bradford method.
Fig. 1.
The SEC of SF allows for determination of soluble protein and HA molecular weights and concentrations. Temperature = 30°C; eluent = 0.1 mol/L NaNO3. LS = light-scattering signal; AUX 1 = refractive index signal; AUX 2 = viscometric signal.
The rheologic behavior of SF was determined using an AR 1000 Rheometer (TA Instruments, New Castle, DE) at 25°C using a cone and plate geometry (4-cm-diameter plate with 3.59° cone). Dynamic experiments were performed in the linear viscoelastic region where G′ and G″ are independent of the stress applied at a given frequency. Dynamic moduli (storage modulus G′ and loss modulus G″) and complex viscosity ∣η*∣ were determined as a function of the angular frequency (ω) expressed in Hertz. Steady-state viscosity η was determined as a function of the shear rate γ covering the range from 0.1 to 50 s−1.
Dynamic moduli (storage modulus G′ and loss modulus G″, complex viscosity ∣η*∣, and steady-state viscosity η) were tested on SF alone and on SF with the two studied VSs added in a volume ratio 1:1. Equal volumes of each component were mixed at ambient temperature under magnetic stirring 15 minutes before the experiment. The stability of the rheologic behavior in SF and HA-modified SF samples was obtained by measurements at baseline and then 6, 15, and 45 days later. Between successive experiments, the samples (SF and HA-modified SF) were stored at 4°C in a refrigerator without any pretreatment.
Results
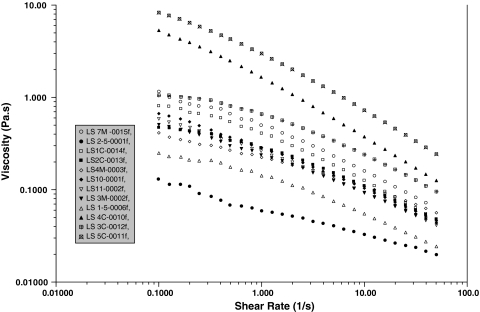
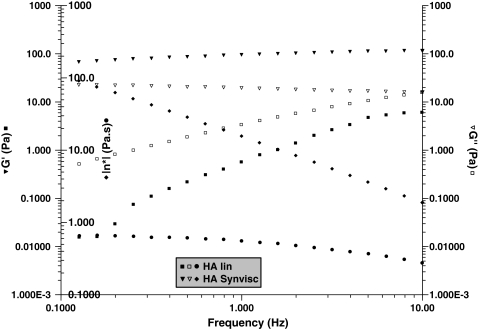
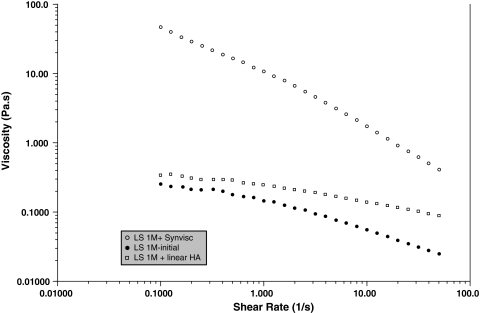
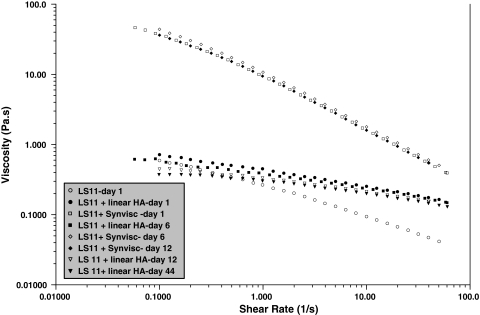
In steady-state flow experiments, all SFs (in the absence of added HA) were non-Newtonian (ie, viscosity decreases when the shear rate increases), the viscosity at 0.1 s−1 varying from 0.1 to 10 Pa · s (Fig. 2). The dynamic rheology of the VSs showed gel-like behavior (with G′ > G″ over a large range of frequency) for the cross-linked HA but not for the linear HA (with G″ > G′ over a large range of frequency), corresponding to the behavior of a viscous solution (Fig. 3). The behavior of Hylan G-F 20 in a steady-state experiment was non-Newtonian throughout the range of shear rates. The linear HA had Newtonian behavior up to 10 s−1 (not shown). Addition of the linear HA slightly modified the behavior of SF, increasing the viscosity at higher shear rates and decreasing the shear rate dependence of the SF, suggesting SF/HA interaction (Fig. 4). The addition of Hylan G-F 20 led to considerable non-Newtonian behavior in the supplemented SF and a large increase in the viscosity over the entire range of shear rates.
Fig. 2.
A graph shows the steady-state viscosity as a function of the shear rate for the different SFs (in the absence of added HA) at 25°C.
Fig. 3.
A graph shows the rheologic behavior of cross-linked (Synvisc®) and linear HA at 10 g/L in 0.15 mol/L NaCl. Temperature = 25°C. Cross-linked: G′ (▼), G″ (∇), |η*| (♦); linear HA: G′ (■), G″ (□), |η*| (●).
Fig. 4.
A graph shows the steady-state viscosity as a function of the shear rate for a SF in the presence of cross-linked (Synvisc®) and linear HA. Temperature = 25°C. SF only (●); linear HA-modified SF (□); cross-linked HA-modified SF (○).
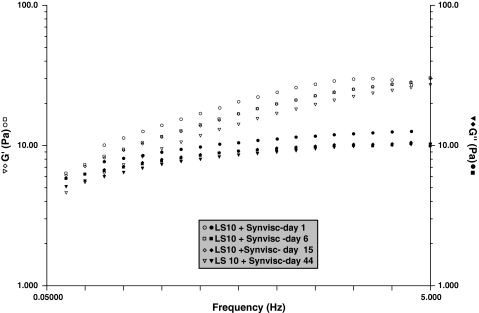
A slight decrease in the rheologic behavior appeared in the very first hours of aging and then stabilized (Fig. 5). We observed nearly no change at any subsequent time in either of the HA-treated SFs. The addition of Hylan G-F 20 clearly induced a gel-like behavior (G′ > G″) in the SF over the range of frequency (Fig. 6). G′ modulus decreased slowly with time but remained larger than G″ over the entire range of frequency.
Fig. 5.
A graph shows a test of the stability of steady-state viscosity as a function of shear rate for SFs modified by cross-linked (Synvisc®) and linear HA during a 44-day period. Temperature = 25°C.
Fig. 6.
A graph shows the dynamic rheology as a function of the frequency for SFs modified by cross-linked HA (Synvisc®) during a 44-day period. Temperature = 25°C. ω0 = 0.054 Hz at Day 15 and 0.08 Hz at Day 44. G′ (open symbols); G″ (filled symbols).
Discussion
VS is a symptomatic treatment of OA aimed to restore rheologic homeostasis of the SF by injecting HA intraarticularly. Despite the long history of this therapy, little is known about its mechanisms of action and differences between commercial preparations. We therefore (1) compared in vitro the changes attributable to the addition of two different formulations of VS (medium-molecular-weight linear HA and high-molecular-weight cross-linked HA) on SF rheology and (2) evaluated the stability of the HA-modified SF with time.
Despite the fact this was only a pilot study, performed on a limited number of SFs obtained from patients with knee OA of various severity, our results showed good agreement between SFs for composition and rheologic parameters. Furthermore, the accuracy of HA content determination by SEC may be slightly limited because of the large ratio of protein concentration/HA concentration (approximately 30 g/L for protein and 0.5 to 1 g/L for HA in SF). Finally, although these data strongly suggest linear and cross-linked VSs induce large differences in the OA SF rheologic behavior, the results of this in vitro study cannot be applied to in vivo situations. Further studies specially designed for that goal must be performed in vivo.
Our data show non-Newtonian behavior in SF whatever the level of viscosity and the HA concentration. This behavior can be attributed to electrostatic interactions between anionic HA and cationic sites of proteins that form a loose three-dimensional network, which reinforces the rheologic behavior. Hydrogen bonds also can be involved to stabilize these interactions. The main lesson from this study is that the cross-linked HA is much more efficient in improving the rheologic behavior of the OA SF than the linear one. In comparison to linear HA, cross-linked HA favors the elasticity of the HA-modified SF. However, the rheologic modifications induced by VS are likely not the only parameters explaining the clinical benefit [13, 38] of the treatment, as pointed out in the Introduction. The effective HA concentration and the content in proteins are other major factors playing a role in SF rheology; if the HA molecular weight is not clearly depressed, as has been seen previously, the concentration is important because the viscosity is related in priority to the product molecular weight × concentration. The main factor influencing the SF rheologic behavior seems to be the level of dilution as a consequence of joint effusion [40, 55]. One other important factor may be the interaction between proteins and HA, leading to complex formations whose structure may be influenced by the composition of the proteins [46].
Our data suggest the rheologic properties of SF are unchanged over 6 weeks. This suggests there is no ongoing HA degradation in isolated SF and the enzymatic mechanisms may be negligible in the current experimental conditions. However, one cannot presume this absence of degradation occurs in an arthritic joint in vivo. In vivo studies are needed to better understand the mechanisms that lead to the elimination of the VS injected in an OA joint. Our results show our protocol of SF removal and storage (dry tube at 4°C) could be usable in further in vivo studies.
SEC and rheology may be combined to characterize SFs and their evolution during VS treatment. Their use could be helpful to better understand the mechanisms of action of VS and to explain a patient’s response to treatment. The method we are proposing allows analysis of the composition of SF without any pretreatment or calibration. Our assay gives a direct molecular weight determination by light scattering and determination of concentration by differential refractometry. A similar technique showed good agreement between SEC estimation after incubation with pronase to hydrolyze proteins [40]; direct determination by SEC seems to be a useful technique to determine molecular weight and concentration of HA in SF. The elution of HA in SEC is perfectly dissociated from that of the protein pool. The peak appearing first in SEC corresponds to HA in direct relation with its large hydrodynamic volume. A low concentration is associated with high viscosity and high light-scattering signals. The weight-averaged molecular weight of HA ranged from 1 to 3 × 106 Da (but more generally approximately 1.5 × 106 Da), in agreement with previous results obtained with a similar technique [1, 40]. This range of molecular weight values is lower than published results but similar to those obtained using other techniques [9, 14, 32].
Our data suggest it is possible to assess HA concentration and viscoelastic properties of OA SF by the combination of SEC and rheologic experiments. Linear and cross-linked HA induce different changes in the OA SF rheologic properties when added in vitro. This modification of SF behavior is related to VS rheology but may also be attributable to interactions between SF proteins and exogenous HA. In vivo studies are needed to investigate the changes of SF occurring after VS and consequently to better understand its mechanisms of action.
Footnotes
One of the authors (TC) previously received payments from Genzyme Orthopaedics Laboratory, Cambridge, MA, for studies other than the current one and as an occasional consultant.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Centre de Recherches sur les Macromolécules Végétales (CERMAV-CNRS).
References
- 1.Adam N, Ghosh P. Hyaluronan molecular weight and polydispersity in some commercial intra-articular injectable preparations and in synovial fluid. Inflamm Res. 2001;50:294–299. [DOI] [PubMed]
- 2.Adams ME, Lussier AJ, Peyron JG. A risk-benefit assessment of injections of hyaluronan and its derivatives in the treatment of osteoarthritis of the knee. Drug Saf. 2000;23:115–130. [DOI] [PubMed]
- 3.Al-Assaf S, Phillips GO, Deeble DJ, Parsons B, Starnes H, Von Sonntag C. The enhanced stability of the crosslinked hylan structure to hydroxyl (OH) radicals compared with the uncrosslinked hyaluronan. Radiat Phys Chem. 1995;46:207–217. [DOI]
- 4.Bagga H, Burkhardt D, Sambrook P, March L. Long term effects of intraarticular hyaluronan on synovial fluid in osteoarthritis of the knee. J Rheumatol. 2006;33:946–950. [PubMed]
- 5.Balazs EA. Analgesic effect of elastoviscous hyaluronan solutions and the treatment of arthritic pain. Cells Tissues Organs. 2003;174:49–62. [DOI] [PubMed]
- 6.Balazs EA. Viscosupplementation for treatment of osteoarthritis: from initial discovery to current status and results. Surg Technol Inter. 2004;12:278–289. [PubMed]
- 7.Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3–9. [PubMed]
- 8.Balazs EA, Gibbs DA. The rheological properties and biological function of hyaluronic acid. In: Balazs EA, ed. Chemistry and Molecular Biology of the Intercellular Matrix. London, UK: Academic Press; 1970:1241–1254.
- 9.Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritic human fluids. Arthritis Rheum. 1967;10:357–376. [DOI] [PubMed]
- 10.Band PA. Molecular strategies for the therapeutic utilization of hyaluronan. In: Kennedy JF, Phillips GO, Williams PA, Hascall VS, eds. Hyaluronan, Volume 2: Biomedical, Medical and Clinical Aspects. Cambridge, UK: Woodhead Publishing; 2002:427–440.
- 11.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2005;2:CD005321. [DOI] [PubMed]
- 12.Berriaud N, Milas M, Rinaudo M. Rheological study on mixtures of different molecular weight hyaluronates. Int J Biol Macromol. 1994;16:137–142. [DOI] [PubMed]
- 13.Camenish TD, McDonald JA. Hyaluronan: is bigger better? Am J Resp Cell Mol Biol. 2000;23:431–433. [DOI] [PubMed]
- 14.Dahl LB, Dahl IM, Engström-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985;44:817–822. [DOI] [PMC free article] [PubMed]
- 15.Dougados M, Nguyen M, Listrat V, Amor B. High molecular weight sodium hyaluronate (hyalectin) in osteoarthritis of the knee: a 1 year placebo-controlled trial. Osteoarthritis Cartilage. 2006;14:814–822. [DOI] [PubMed]
- 16.Felson DT. The epidemiology of knee osteoarthritis: results from the Framingham Osteoarthritis Study. Semin Arthritis Rheum. 1990;20(suppl 1):42–50. [DOI] [PubMed]
- 17.Fergusson J, Boyle JA, McSween NM, Jasani MK. Observations on the flow properties of the synovial fluid from patients with rheumatoid arthritis. Biorheology. 1968;5:119–131. [DOI] [PubMed]
- 18.Fouissac E, Milas M, Rinaudo M. Shear-rate, concentration, molecular weight, and temperature viscosity dependences of hyaluronates, a wormlike polyelectrolyte. Macromolecules. 1993;26:6945–6951. [DOI]
- 19.Fouissac E, Milas M, Rinaudo M, Borsali R. Influence of the ionic strength on the dimensions of sodium hyaluronate. Macromolecules. 1992;25:5613–5617. [DOI]
- 20.Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32:10–37. [DOI] [PubMed]
- 21.Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthritis Cartilage. 2005;13:216–224. [DOI] [PubMed]
- 22.Gomez JE, Thurston GB. Comparisons of the oscillatory shear viscoelasticity and composition of pathological synovial fluids. Biorheology. 1993;30:409–427. [DOI] [PubMed]
- 23.Gomis A, Pawlak M, Balazs EA, Schmidt RF, Belmonte C. Effects of different molecular weight elastoviscous hyaluronan solutions on articular nociceptive afferents. Arthritis Rheum. 2004;50:314–326. [DOI] [PubMed]
- 24.Greenberg DD, Stoker A, Kane S, Cockrell M, Cook JL. Biochemical effects of two different hyaluronic acid products in a co-culture model of osteoarthritis. Osteoarthritis Cartilage. 1993;1:97–103. [DOI] [PubMed]
- 25.Jackson DW, Simon TM. Intra-articular distribution and residence time of Hylan A and B: a study in the goat knee. Osteoarthritis Cartilage. 2006;14:1248–1257. [DOI] [PubMed]
- 26.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–501. [DOI] [PMC free article] [PubMed]
- 27.Kirchner M, Marshall D. A double-blind randomized controlled trial comparing alternate forms of the high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14:154–162. [DOI] [PubMed]
- 28.Kogan G, Soltes L, Stern R, Gemeiner P. Hyaluronic acid: a natural biopolymer with abroad range of biomedical and industrial applications. Biotechnol Lett. 2007;29:17–25. [DOI] [PubMed]
- 29.Kotevoglu N, Lyibozkurt PC, Hiz O, Toktas H, Kuran B. A prospective randomised controlled clinical trial comparing the efficacy of different molecular weight hyaluronan solutions in the treatment of knee osteoarthritis. Rheumatol Int. 2006;26:325–330. [DOI] [PubMed]
- 30.Kraus VB, Stabler TV, Kong SY, Varju G, McDaniel G. Measurement of synovial fluid volume using urea. Osteoarthritis Cartilage. 2007;15:1217–1220. [DOI] [PMC free article] [PubMed]
- 31.Lapcik L Jr, Lapcik L, de Smedt S, Demeester J, Chabrecek P. Hyaluronan: preparation, structure, properties and applications. Chem Rev. 1998;98:2663–2684. [DOI] [PubMed]
- 32.Lee HG, Cowman MK. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal Biochem. 1994;219:278–287. [DOI] [PubMed]
- 33.Lee S, Park D, Chmell S. Viscosupplementation with hylan G-F 20 (Synvisc): pain and mobility observations from 74 consecutive patients. J Knee Surg. 2004;17:73–77. [DOI] [PubMed]
- 34.Marshall KW. Intra-articular hyaluronan therapy. Curr Opin Rheumatol. 2000;12:468–474. [DOI] [PubMed]
- 35.Milas M, Rinaudo M. Characterization and properties of hyaluronic acid (hyaluronan). In: Dimitriu S, ed. Polysaccharides: Structural Diversity and Functional Versatility. New York, NY: Marcel Dekker; 2002:535–549.
- 36.Milas M, Rinaudo M, Roure I, Al-Assaf S, Phillips GO, Williams PA. Comparative rheological behavior of hyaluronan from bacterial and animal sources with cross-linked hyaluronan (hylan) in aqueous solution. Biopolymers. 2001;59:191–204. [DOI] [PubMed]
- 37.Milas M, Rinaudo M, Roure I, Al-Assaf S, Phillips GO, Williams PA. Rheological behaviour of hyaluronan, healon and hylan in aqueous solution. In: Kennedy JF, Phillips GO, Williams PA, Hascall VC, eds. Hyaluronan, Volume 1: Chemical, Biochemical and Biological Aspects. Cambridge, UK: Woodhead Publishing; 2002:181–193.
- 38.Moreland LW. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: mechanisms of action. Arthritis Res Theor. 2003;5:54–57. [DOI] [PMC free article] [PubMed]
- 39.Nuki G, Ferguson J. Studies on the nature and significance of macromolecular complexes in the rheology of synovial fluid from normal and diseased human joints. Rheol Acta. 1971;10:8–14. [DOI]
- 40.Praest BM, Greiling H, Kock R. Assay of synovial fluid parameters: hyaluronan concentration as a potential marker for joint diseases. Clin Chim Acta. 1997;266:117–128. [DOI] [PubMed]
- 41.Praest BM, Greiling H, Kock R. Effects of oxygen-derived free radicals on the molecular weight and the polydispersity of hyaluronan solutions. Carbohydr Res. 1997;303:153–157. [DOI]
- 42.Raynauld JP, Torrance GW, Band PA, Goldsmith CH, Tugwell P, Walker V, Schultz M, Bellamy N. A prospective, randomized, pragmatic, health outcomes trial evaluating the incorporation of hylan G-F 20 into the treatment paradigm for patients with knee osteoarthritis (Part 1 of 2): clinical results. Osteoarthritis Cartilage. 2002;10:506–517. [DOI] [PubMed]
- 43.Reichenbach S, Blank S, Rutjes AW, Shang A, King EA, Dieppe PA, Juni P, Trelle S. Hylan versus hyaluronic acid for osteoarthritis of the knee: a systematic review and meta-analysis. Arthritis Rheum. 2007;57:1410–1418. [DOI] [PubMed]
- 44.Rinaudo M. Advances in characterisation of polysaccharides in aqueous solution and gel state. In: Dimitriu S, ed. Polysaccharides: Structural Diversity and Functional Versatility. Ed 2. New York, NY: Marcel Dekker; 2004:237–252.
- 45.Rinaudo M. Properties and degradation of selected polysaccharides. Corros Eng Sci Technol. 2007;42:324–334. [DOI]
- 46.Rinaudo M. Rheological investigation on hyaluronan-fibrinogen interaction. Int J Biol Macromol. 2008;43:444–450. [DOI] [PubMed]
- 47.Rinaudo M, Roure I, Milas M. Use of steric exclusion chromatography to characterize hyaluronan, a semi-rigid polysaccharide. Int J Polym Anal Charact. 1999;5:277–287. [DOI]
- 48.Saari H, Konttinen YT. Determination of synovial fluid hyaluronate concentration and polymerisation by high performance liquid chromatography. Ann Rheum Dis. 1989;48:565–570. [DOI] [PMC free article] [PubMed]
- 49.Schiller J, Fuchs B, Arnhold J, Arnold K. Contribution of reactive oxygen species to cartilage degradation in rheumatic diseases: molecular pathways, diagnosis and potential therapeutic strategies. Curr Med Chem. 2003;10:2123–2145. [DOI] [PubMed]
- 50.Soltes L, Kogan G, Stankovska M, Mendichi R, Rychly J, Schiller J, Gemeiner P. Degradation of high-molar-mass hyaluronan and characterization of fragments. Biomacromolecules. 2007;8:2697–2705. [DOI] [PubMed]
- 51.Soltes L, Stankovska M, Kogan G, Mendichi R, Volpi N, Sasinkova V, Gemeiner P. Degradation of high-molar-mass hyaluronan by an oxidative system comprising ascorbate, Cu(II), and hydrogen peroxide: inhibitory action of antiinflammatory drugs Naproxen and acetylsalicylic acid. J Pharm Biomed Anal. 2007;44:1056–1063. [DOI] [PubMed]
- 52.Tamir E, Robinson D, Koren R, Agar G, Halperin N. Intra-articular hyaluronan injections for the treatment of osteoarthritis of the knee: a randomized, double blind, placebo controlled study. Clin Exp Rheumatol. 2001;19:265–270. [PubMed]
- 53.Vendrely C, Valadie H, Bednarova L, Cardin L, Pasdeloup M, Cappadoro J, Bednar J, Rinaudo M, Jamin M. Assembly of the full-length recombinant mouse prion protein I: formation of soluble oligomers. Biochim Biophys Acta. 2005;1724:355–366. [DOI] [PubMed]
- 54.Vitanzo PC, Sennett BJ. Hyaluronans: is clinical effectiveness dependent on molecular weight? Am J Orthop. 2006;35:421-428. [PubMed]
- 55.Waddell DD, Marino AA. Chronic knee effusions in patients with advanced osteoarthritis: implications for functional outcome of viscosupplementation. J Knee Surg. 2007;20:181–184. [DOI] [PubMed]
- 56.Wang CT, Lin YT, Chiang BL, Lin YH, Hou SM. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage. 2006;14:1237–1247. [DOI] [PubMed]
- 57.Weigel PH, Fuller GM, LeBoeuf RD. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J Theor Biol. 1986;119:219–234. [DOI] [PubMed]
- 58.Weiss C. Why viscoelasticity is important for the medical uses of hyaluronan and Hylans. In: Abatangelao G, Veigel PH, eds. New Frontiers in Medical Sciences: Redefining Hyaluronan. Amsterdam, The Netherlands: Elsevier Science BV; 2000:89–103.
- 59.Wobig M, Bach G, Beks P, Dickhut A, Runzheimer J, Schwieger G, Vetter G, Balazs E. The role of elastoviscosity in the efficacy of viscosupplementation for osteoarthritis of the knee: a comparison of hylan G-F 20 and a lower-molecular-weight hyaluronan. Clin Ther. 1999;21:1549–1562. [DOI] [PubMed]