Abstract
Curettage is the most attractive procedure for surgically treating a giant cell tumor because it preserves joint function. However, since many giant cell tumors compromise subchondral bone this technique can jeopardize the articular surface with subsequent fractures or collapse. We asked whether intralesional curettage of a giant cell tumor close to the knee that combined morselized bone and cortical structural allograft would preserve joint function. We retrospectively reviewed 22 patients treated with that approach. The minimum followup was 2 years (average, 48 months; range, 24–80 months). The distal femur was involved in 12 patients and proximal tibia in 10. Complications and failures were recorded and functional results evaluated with Musculoskeletal Tumor Society score. We determined survivorship using the Kaplan-Meier technique using removal of the implant as the endpoint. The survival was 85% and the average functional score 28 points. Three of the 22 patients had a local tumor recurrence and one had a partial subchondral collapse not requiring further treatment. Among the remaining patients, none had fracture, infection, or knee instability. The combination of fragmented and cortical allograft allows reconstructing the bone defect and ligaments created after extensive curettage of a knee giant cell tumor obtaining normal joint function and a high survival rate with minimal complications in a high percentage of the patients.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The treatment of giant cell tumors (GCTs) remains a difficult problem and surgical options are intralesional excision (curettage) or en bloc resection of the tumor [6, 7, 9, 11, 14, 16, 20, 22]. Curettage is the most attractive method because it preserves autologous structures, bone architecture, and native joint cartilage; however, a higher rate of local tumor recurrence has been reported with this method [11]. Most surgeons agree that the major factor in the success of local tumor control is how thoroughly the tumor is excised [1, 2, 18, 21]; for this reason, a large cortical window is necessary for appropriate visualization of the entire tumor cavity to obtain extensive curettage.
Because many GCTs compromise subchondral bone, curettage may jeopardize the articular surface or increase stresses in either the cortical bone (with the defect for curettage) or the subcortical bone (from the curettage itself) leading to fracture or joint collapse. In this situation, the defect cavity can be reconstructed with polymethylmethacrylate [3, 7, 13], bone graft [2, 4, 5, 12, 23], or a bone substitute [19]. The use of cement has the potential advantage of restoring stability immediately but the long-term presence of cement in a weightbearing subchondral location may lead to degenerative changes in the articular surface [21]. Morselized bone allograft or bone substitutes may restore bone stock, improving future options after long-term followup. However, they do not provide prompt restoration of the cortical bone and therefore often required long periods of load protection to prevent fractures or joint collapse [18].
To avoid fractures or collapse, we previously described a combination of morselized bone allograft to reconstruct the cavity and cortical bone allograft to reconstruct the cortical window [2]. The intention of this combination is to reconstruct the cortical window allowing mechanical support and load transmission for the joint, buttressing the reconstruction while maintaining the morselized allograft in place.
We therefore asked whether this combined reconstruction method after extensive curettage of knee GCTs would allow: (1) survival without the need for removal for fracture or joint collapse; (2) high functional (MSTS) scores; and (3) high radiographic (ISOLS) scores.
Materials and Methods
We retrospectively reviewed all 22 patients who underwent knee reconstruction with a combination of cancellous bone and structural allograft after resection of a GCT between July 2002 and August 2006. The diagnosis was confirmed by preoperative fine-needle percutaneous biopsy. All reconstructions were performed after an intralesional resection of a primary (19 cases) or recurrent GCT (three cases). Twelve were distal femur and 10 proximal tibia reconstructions. Six patients were female and 16 male with an average age of 31 years at the time of surgery (range, 17–66 years). The minimum followup was 24 months (average, 48 months; range, 24–80 months) unless failure or local recurrence occurred earlier. No patient was excluded or lost to followup in this series. We had prior approval of our Institutional Review Board.
We performed staging studies in all patients, including plain radiography, computed tomography (CT), and MRI images of the affected limb. CT studies of the chest were also performed in all patients. According to the grading system of Campanacci et al. [6] or GCT of bone, 18 tumors were classified as grade 2 and four as grade 3.
The main indication for this reconstruction technique was when enough remaining subchondral bone is present to support joint loading. In addition, the absence of cortical bone due to tumor growth, or the need for an extensive surgical window in order to excise the lesion, creates a massive cortical defect that may indicate mechanical weakening and a reconstruction failure. We believe the technique is contraindicated for patients in whom preoperative imaging studies demonstrate evidence of intraarticular compromise of the tumor, or intraarticular fracture due to tumor growth. It is also contraindicated in patients in whom there is inadequate remaining subchondral bone to resist the normal loading, since that would jeopardize the reconstruction. In addition, we considered gross degenerative changes of the joint surface a contraindication.
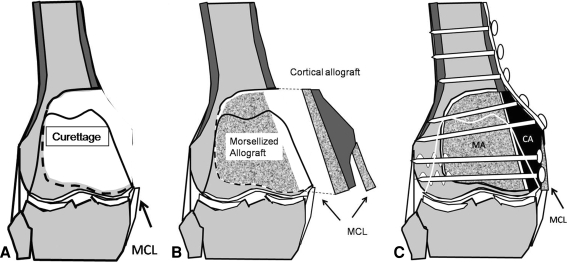
We exposed the distal femur through a medial or lateral longitudinal incision with its corresponding muscle flaps. A large cortical window allowed wide exposure of the lesion (Fig. 1A). We included, when necessary, the lateral or medial collateral ligament insertions. We packed the tumor perimeter with sponges to avoid spilling tumor-tissue during the procedure. The tumor bulk was removed with a curette using an intralesional technique. In weightbearing areas we attempted to retain at least a thin layer of native subchondral bone underneath the articular cartilage for packing the graft without alteration of the joint shape. Care was taken to excise all potentially involved bone and soft tissue. A phenol solution was topically used in the subchondral and cortical bone of the cavity in an attempt to eliminate remnants of the tumor after curettage. After three topical applications of phenol, we irrigated the cavity with sterile water.
Fig. 1A–C.
The surgical technique of distal femoral reconstruction is shown. (A) Tumor bulk was removed with a curette using an intralesional technique through a large cortical window that includes, when necessary, the lateral or medial collateral ligament insertions. (B) Residual space between the preserved articular cartilage and the cortical strut is then filled with morselized bone allograft. The host cortical bone requires cortical window resizing with the intention to obtain a more geometric configuration of the defect. This type of window shape will allow us to obtain better cortical contact between the allograft and the host. Collateral ligaments can be reconstructed with the corresponding allograft ligament. (C) A locking compression plate (LCP) buttressing the reconstruction was put in place.
Corticocancellous and structural cortical bone allograft obtained and stored according to established criteria were selected from our hospital bone bank [15]. The subchondral region of the cavity was packed with morselized bone allograft as was the rest of the cavity (Fig. 1B).
The bone reconstruction required cortical window resizing by performing straight cuts of the cortex with the saw with the intention to obtain a more geometric configuration of the defect. This type of window shape allows us to tailor the cortical allograft and obtain better cortical contact between the allograft and the host (Fig. 1C). Cortical allograft selection was based on the same anatomic segment side as that required by the recipient. We then introduced the structural cortical allograft in the defect and compression forces were obtained with cancellous screws reducing the strut while maintaining the morselized allograft in place. We then implanted a locking compression plate (LCP; Synthes, Paoli, PA) buttressing the reconstruction (Fig. 2A–D). Any residual space between the preserved articular cartilage and the cortical strut is filled with morselized bone allograft. The muscle flaps were repaired and subcutaneous tissues were closed with absorbable suture with a running subcutaneous closure.
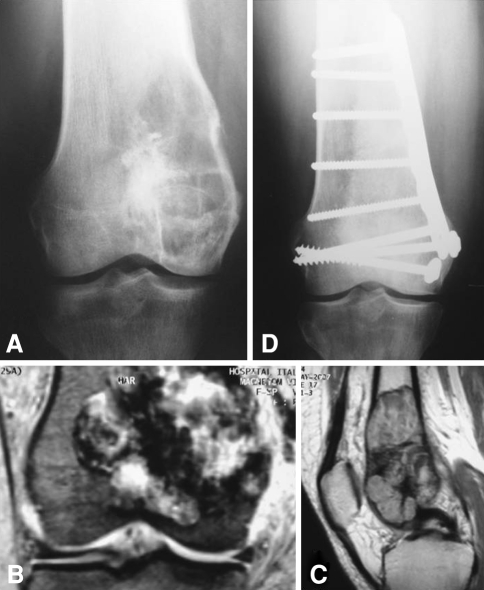
Fig. 2A–D.
An example of distal femoral reconstruction is shown. (A) The preoperative radiograph shows a distal femur giant cell tumor. (B) The coronal MRI view shows invasion of the distal femur compromising the medial cortex. (C) Lateral MRI view shows intramedullary extension with preservation of both anterior and posterior cortices. (D) The 2-year postoperative radiograph shows the reconstructed femur with no joint line narrowing.
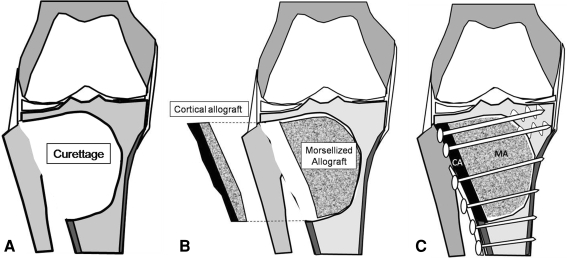
In the proximal tibia, we performed an anterolateral or anteromedial incision of the knee according to the tumor location. We never excised the proximal fibula (in resection of the lateral tibial plateau). The joint capsule was incised longitudinally by cutting the anterior horn of the corresponding meniscus. The joint capsule, ipsilateral meniscus, and collateral ligament were retracted upward, exposing the corresponding tibial plateau. We made a wide cortical window preserving the rim of the tibial plateau similar to that in the distal femur (Fig. 3A). After extensive curettage of the tumor, we also used phenol adjuvant on the tibial side. We then packed the cavity with morselized bone as in the distal femur (Fig. 3B) and fitted the cortical allograft and applied the LCP plate (Fig. 3C, Fig. 4A–C). In one of the 10 patients with a GCT of the tibia, there was no remaining native subchondral bone after the curettage. In this particular patient, we reconstructed the subchondral bone with a thin layer of cancellous allograft maintaining the shape of the tibia plateau by packing more morselized allograft underneath.
Fig. 3A–C.
The surgical technique of proximal tibial reconstruction is shown. (A) Tumor bulk was removed with a curette using a similar technique as for the distal femur. The joint capsule, ipsilateral meniscus, and collateral ligament were retracted upward, exposing the corresponding tibial plateau. A wide cortical window preserving the rim of the tibial plateau was made similar to the distal femur location. We did not excise the proximal fibula (in resection of the lateral tibial plateau). (B) In the proximal tibia, we used a similar reconstruction technique as described for the distal femur. (C) A locking compression plate (LCP) buttressing the reconstruction was used.
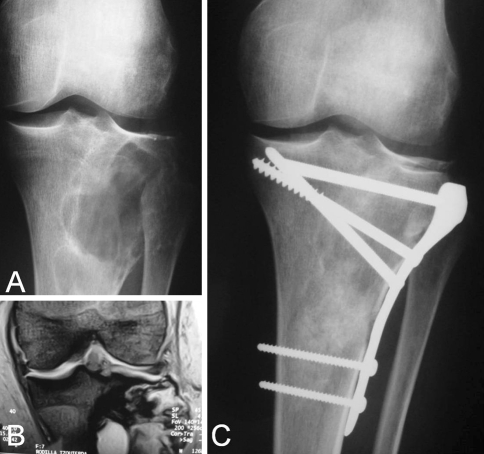
Fig. 4A–C.
An example of proximal tibia reconstruction is shown. (A) A preoperative radiograph and (B) MRI of the giant cell tumor affecting the proximal tibia with partial subchondral bone compromise is shown. (C) A 4-year postoperative radiograph shows the reconstructed defect.
Postoperatively a brace was applied with the knee in full extension. Range-of-motion knee exercises were started 48 hours postoperatively. Partial weightbearing started during the second week postoperatively. The brace is removed in 4 to 6 weeks depending on intraoperative stability, and the patient is allowed to continue partial weightbearing with crutches. Patients were allowed to bear weight as tolerated 3 to 4 months postoperatively when radiographs showed healing of the cortical window. We considered the window healed when there was bridging trabecular callus across the proximal and distal window junction sites.
All patients were seen postoperatively at 1 week, 2 weeks, 1 month, 2 months, and 3 months; every 3 months thereafter until 2 years; and then annually. Plain AP and lateral radiographs were made at every visit, beginning one month after the operation. We recorded complications such as local recurrences, instability, infection, fractures, subchondral collapse, and joint narrowing. The functional evaluation was performed in both groups using the revised 30-point functional classification system established by the Musculoskeletal Tumor Society [8], which assesses pain, functional limitation, walking distance, the use of a support, emotional acceptance, and gait. Each variable was assessed on a 5-point scale.
The plain radiographs were evaluated by two of the authors (GLF, CALR) according to the system established by the International Society of Limb Salvage [10], which is based on seven criteria: fusion, resorption, fracture, graft shortening, fixation, joint narrowing, and changes in subchondral bone. Each parameter is given a value ranging from 0 to 5 according to specific criteria. We calculated the score by adding the value for each criterion and dividing by the total maximum attainable score. The score is expressed as a percentage; the maximum possible score was 100%.
We estimated survival of the allograft with the Kaplan-Meier method using the date of allograft removal as the endpoint. Because of the small sample size and low power, no other statistical analysis was considered.
Results
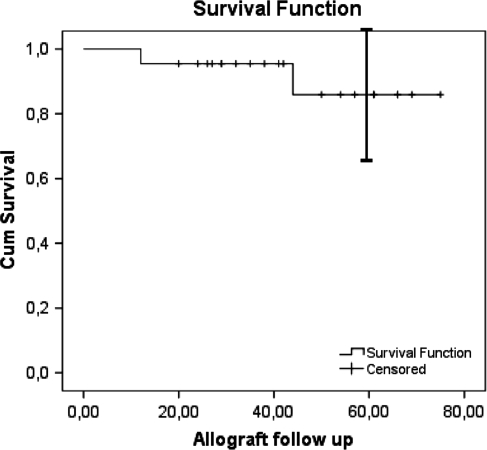
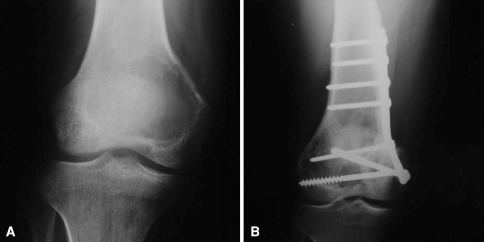
The overall reconstruction survival was 85% at 5 years (95% confidence interval, 65%–100%). The mean reconstruction duration was 69 months for all patients (95% confidence interval, 61–77 months) (Fig. 5). No patients were revised for fracture, massive bone resorption, instability, or infection. One patient had minimal subchondral collapse without joint narrowing. This was the patient with no remaining subchondral bone after the curettage of a GCT of the tibia and was reconstructed with a thin layer of cancellous allograft. No additional surgical treatment had been performed for this patient at last followup. Two of the 22 reconstructions were removed as a result of local recurrences. One was a proximal tibia at 13 months after surgery and the other a distal femur reconstruction at 44 months after surgery. Both recurrences were located in metaphyseal bone (Fig. 6A–B). The patient with a proximal tibial recurrence was treated originally because of a recurrent GCT. In this case, we performed en bloc resection and reconstruction with a knee allograft-prosthesis composite. The patient with a distal femur recurrence was treated with a new curettage and reconstructed with the same procedure. Another patient had a soft tissue recurrence treated with resection but did not require removal of the allograft.
Fig. 5.
The Kaplan-Meier curve for survival of the allografts is shown. The I-bar indicates the 95% confidence interval.
Fig. 6A–B.
This case shows a distal femoral recurrence located at metaphyseal bone. (A) A preoperative radiograph of the giant cell tumor affecting the distal femur is shown. (B) A postoperative radiograph shows the metaphyseal recurrence.
For the patients who retained the reconstruction (20 cases), the mean Musculoskeletal Tumor Society functional score at last followup was 28.6 of 30 (94.3%; range, 24–30). For distal femoral reconstructions, the mean score was 28.6 (95.3%; range, 24–30) and 28.7 (95.6%; range, 26–30) for proximal tibial reconstructions. Physical examination revealed the arc of active motion of the knee averaged 119° (range, 100°–130°). All patients returned to their full preoperative level of function after this reconstruction.
The mean radiographic score for the 20 allografts evaluated was 96% with all grafts having scores between 85% and 100%.
Discussion
Giant cell tumor is difficult to treat, but preservation of the joint through extensive curettage should be considered a priority [11]. This condition usually leaves a large cortical defect originating either by aggressive tumor growth or by the surgeon in an attempt to obtain adequate access to the tumor cavity while performing the curettage. Our technique allows joint preservation while adding more stability with a cortical allograft and plate than would be achieved by morselized allograft alone. We presume the allograft will incorporate and remodel providing bone stock for subsequent joint reconstruction if required. We therefore asked whether this combined reconstruction method after extensive curettage of knee GCTs would allow: (1) survival without the need for removal for fracture or joint collapse; (2) high functional (MSTS) scores; and (3) high radiographic (ISOLS) scores.
We note several limitations. First, due to the small number of patients, statistical analysis was not possible for identifying factors that might predict survival. Second, the survival analysis had a wide confidence interval also owing to the small number of patients. Also, our results should be considered preliminary and longer followup is necessary. Third, the minimum followup was 2 years and, although most local recurrences occur during this period [6], the followup was too short to definitively assess recurrences. Nonetheless, the time was sufficient to evaluate joint collapse. To evaluate potential joint degeneration, in the future we intend to follow these patients longer. Finally, the difference in size and location of the primary tumor resulted in a heterogeneous population with differing amounts of segmental defects. Nonetheless, this is a relatively large number of patients given the problem is uncommon and the treatment approach was similar in all of them.
Our findings suggest when this combined reconstruction procedure is indicated after an extensive curettage, it can restore normal knee function with minimum complications and can survive without fractures or articular collapse in a high percentage of patients. However, reconstruction of the weightbearing subchondral bone after intralesional curettage of a GCT of the knee is controversial [1, 5, 11, 12, 18]. Prosser et al. [18] in a recent article observed the empty cavity after curettage gradually fills with new bone and consolidates with time. Although they concluded filling agents are unnecessary after curettage, they reported 3.6% of patients required surgical treatment for fracture through the cavity. It has been also proposed that cement filling alone provides immediate mechanical stability and can be tolerated close to the joint surface [11]. Nevertheless, recent reports suggested cement can induce heat necrosis a few millimeters to the adjacent articular cartilage [21]. Other studies suggest cement constructs are less rigid than normal subchondral bone, leading to articular cartilage resorption and fragmentation [21]. One alternative to this problem is to interpose bone graft between the cartilage and the cement with the intention of reducing heat damage or early degenerative changes [6]. However, the presence of polymethylmethacrylate in a weightbearing location may still lead to development of long-term degenerative changes [17] or alter future options if the primary reconstruction fails as a result of arthritis or mechanical failure [21]. Filling the cavity with morselized bone graft [4, 23] may also restore the subchondral bone and remaining bone defects, obtaining close to normal biomechanical stress distribution and potentially decreasing the chance of joint cartilage deterioration. However, the remodeling into a mechanically appropriate cortical bone of this morselized allograft may take a long time with an increased risk of cortical fracture.
To avoid this problem, we previously described [2] the use of a structural cortical allograft to reconstruct the cortical window and buttress the affected bone segment. It was published as a technical note and reported only our initial experience in a limited number of patients. Other authors [5, 12] also have noted the advantages of adding strut allograft to the morselized graft after curettage of bone tumors to prevent fractures or collapse. Buecker and Gebhardt [5] used strut fibula allograft inside the cavity with the intention to buttress the reconstruction and to perform biologically active reconstruction. Seventeen of the 22 patients (77.3%) returned to their full level of preoperative function, having excellent/good functional recovery by the first year. Li et al. [12] reported 13 patients with a GCT at the proximal tibia treated with en bloc resection of the affected plateau and reconstructed with iliac crest autograft and vertical autologous fibula graft for buttressing the reconstruction. The main limitation of that technique is the loss of articular cartilage. However, the authors concluded the autologous meniscus is preserved, absorbing shock, buffer stress, and allowing stress transfer between the femoral condyle and the reconstructed plateau transfer. In our study, we preserved autologous meniscus in all patients and we concur that preservation of native meniscus is crucial for articular cartilage preservation.
Based on our observations we recommend this combined reconstruction procedure for most grade 2 and some grade 3 GCTs of the knee when there is enough remaining subchondral bone is present to support loading, when there is absence of cortical bone due to tumor growth, or when there is a need for an extensive surgical window in order to excise the lesion. This technique can restore normal limb function with minimum complications and can survive without fracture or articular collapse in a high percentage of patients.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his institution has approved the reporting of this case report and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Arbeitsgemeinschaft Knochentumoren, Becker WT, Dohle J, Bernd L, Braun A, Cserhati M, Enderle A, Hovy L, Matejovsky Z, Szendroi M, Trieb K, Tunn PU. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. J Bone Joint Surg Am. 2008;90:1060–1067. [DOI] [PubMed]
- 2.Ayerza MA, Aponte-Tinao LA, Muscolo DL, Abalo ED. Morsellized and structural cortical allograft reconstruction after intralesional curettage of a distal femoral giant-cell tumor. Orthopedics. 2006;29:679–682. [DOI] [PubMed]
- 3.Balke M, Schremper L, Gebert C, Ahrens H, Streitbuerger A, Koehler G, Hardes J, Gosheger G. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol. 2008;134:969–978. [DOI] [PubMed]
- 4.Blackley HR, Wunder JS, Davis AM, White LM, Kandel R, Bell RS. Treatment of giant-cell tumors of long bones with curettage and bone-grafting. J Bone Joint Surg Am. 1999;81:811–820. [DOI] [PubMed]
- 5.Buecker PJ, Gebhardt MC. Are fibula strut allografts a reliable alternative for periarticular reconstruction after curettage for bone tumors? Clin Orthop Relat Res. 2007;461:170–174. [DOI] [PubMed]
- 6.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–114. [PubMed]
- 7.Eckardt JJ, Grogan TJ. Giant cell tumor of bone. Clin Orthop Relat Res. 1986;204:45–58. [PubMed]
- 8.Enneking WF, Dunham W, Gebhardt MC, Malawer M, Prichard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed]
- 9.Gitelis S, Mallin BA, Piasecki P, Turner F. Intralesional excision compared with en bloc resection for giant-cell tumor of bone. J Bone Joint Surg Am. 1993;75:1648–1655. [DOI] [PubMed]
- 10.Glasser D, Langlais F. The ISOLS radiological implant evaluation system. In: Langlais F, Tomeno B, eds. Limb Salvage: Major Reconstructions in Oncologic and Nontumoral Conditions. Heidelberg, Germany: Springer-Verlag; 1991.
- 11.Kivioja AH, Blomqvist C, Hietaniemi K, Trovik C, Walloe A, Bauer HC, Jorgensen PH, Bergh P, Follerås G. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian Sarcoma Group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008;79:86–93. [DOI] [PubMed]
- 12.Li JM, Yang ZP, Li ZF, Li X, Carter SR. Knee reconstruction with preservation of the meniscus in tibial giant cell tumor. Clin Orthop Relat Res. 2008;466:3101–3107. [DOI] [PMC free article] [PubMed]
- 13.Malawer MM, Bickels J, Meller I, Buch RG, Henshaw RM, Kollender Y. Cryosurgery in the treatment of giant cell tumor: a long-term followup study. Clin Orthop Relat Res. 1999;359:176–188. [DOI] [PubMed]
- 14.McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68:235–242. [PubMed]
- 15.Muscolo DL, Ayerza MA, Aponte-Tinao LA. Massive allograft use in orthopedic oncology. Orthop Clin North Am. 2006;37:65–74. [DOI] [PubMed]
- 16.Muscolo DL, Ayerza MA, Calabrese ME, Gruenberg M. The use of a bone allograft for reconstruction after resection of giant-cell tumor close to the knee. J Bone Joint Surg Am. 1993;75:1656–1662. [DOI] [PubMed]
- 17.Persson BM, Ekelund L, Lövdahl R, Gunterberg B. Favourable results of acrylic cementation for giant cell tumors. Acta Orthop Scand. 1984;55:209–214. [DOI] [PubMed]
- 18.Prosser GH, Baloch KG, Tillman RM, Carter SR, Grimer RJ. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop Relat Res. 2005;435:211–218. [DOI] [PubMed]
- 19.Schindler OS, Cannon SR, Briggs TW, Blunn GW. Use of a novel bone graft substitute in peri-articular bone tumours of the knee. Knee. 2007;14:458–464. [DOI] [PubMed]
- 20.Sung HW, Kuo DP, Shu WP, Chai YB, Liu CC, Li SM. Giant cell tumor of bone: analysis of two hundred and eight cases in Chinese patients. J Bone Joint Surg Am. 1982;64:755–761. [PubMed]
- 21.Turcotte RE, Wunder JS, Isler MH, Bell RS, Schachar N, Masri BA, Moreau G, Davis AM, Canadian Sarcoma Group. Giant cell tumor of long bone: a Canadian Sarcoma Group study. Clin Orthop Relat Res. 2002;397:248–258. [DOI] [PubMed]
- 22.Ward WG Sr, Li G 3rd. Customized treatment algorithm for giant cell tumor of bone: report of a series. Clin Orthop Relat Res. 2002;397:259–270. [DOI] [PubMed]
- 23.Zhen W, Yaotian H, Songjian L, Ge L, Qingliang W. Giant-cell tumour of bone: the long-term results of treatment by curettage and bone graft. J Bone Joint Surg Br. 2004;86:212–216. [DOI] [PubMed]