Abstract
Allograft safety is a great concern owing to the risk of disease transmission from nonsterile tissues. Radiation sterilization is not used routinely because of deleterious effects on the mechanical integrity and stability of allograft collagen. We previously reported several individual cross-linking or free radical scavenging treatments provided some radioprotective effects for tendons. We therefore asked whether a combination of treatments would provide an improved protective effect after radiation exposure regarding mechanical properties and enzyme resistance. To address this question we treated 90 rabbit Achilles tendons with a combination of cross-linking (1-ethyl-3-[3-dimethyl aminopropyl] carbodiimide [EDC]) and one of three scavenging regimens (mannitol, ascorbate, or riboflavin). Tendons then were exposed to one of three radiation conditions (gamma or electron beam irradiation at 50 kGy or unsterilized). Combination-treated tendons (10 per group) had increases in mechanical properties and higher resistance to collagenase digestion compared with EDC-only and untreated tendons. Irradiated tendons treated with EDC-mannitol, -ascorbate, and -riboflavin combinations had comparable strength to native tendon and had averages of 26%, 39%, and 37% greater, respectively, than those treated with EDC-only. Optimization of a cross-linking protocol and free radical scavenging cocktail is ongoing with the goal of ensuring sterile allografts through irradiation while maintaining their structure and mechanical properties.
Introduction
Allograft use in replacement and reconstruction procedures has become more common among orthopaedic surgeons [11, 24, 27]. Allografts can provide comparable mechanical and physiologic function relative to autograft tissues. An estimated 1.5 million bone and tissue allografts were implanted in 2006 [9], and the amount of allograft donors increased from 6,000 in 1994 to 22,000 in 2005 [43]. Consequently, a major concern associated with increased use of allografts is the danger for disease transmission [11, 44]. Concerns exist regarding sensitivity of culture testing [19, 45] and screening against emerging pathogens [19, 41]. Also, allografts harvested from donors who become infected just before death pose a major obstacle to screening [17, 19]. Infection during this ‘window period’ could pass undetected through serologic testing, and unsafe allografts may be approved mistakenly for implantation [17, 23, 44]. Despite tissue bank harvesting and processing protocols, which greatly minimize possible bacterial and viral transfer, the threat of transmission has not been eliminated [2, 11, 41]. In 2002, the American Association of Tissue Banks (AATB) reported two bacterial infections among 900,000 distributed allografts [7]. These data provide only an estimation of infection risk given that 43% of all tissue banks are not members of the AATB and reporting infections to the AATB is voluntary [7]. The risk of implanting tissues from an HIV-infected donor, considering current safety protocols, was estimated to be one in one million [5, 15]. This accentuates the importance of standardizing tissue banking and sterilization procedures to further reduce infection risks.
To improve allograft safety, some tissue banks use secondary sterilization methods along with medical history and serologic evaluation [41, 44]. Sterilization is accomplished predominantly with ionizing radiation, specifically gamma and electron beam (ebeam) radiation derived from gamma rays and high-energy electrons, respectively. Unfortunately, these sources of energy cause damage to collagenous structures [4, 10, 17, 22, 28, 36]. The primary cause of damage is modification of collagen molecules by free radicals generated from irradiation of water and oxygen [4, 37]. Tissue banks that perform radiation sterilization commonly expose allografts to doses from 10 kGy to 35 kGy [41, 44]. These doses are insufficient to neutralize radiation-resistant bacteria and viruses such as HIV [17, 40]. The use of the higher doses of irradiation necessary for sterilization will correspond to more collagen damage. On a tissue level, these radiation effects would compromise mechanical performance and postimplantation stability, likely resulting in failure. As a result, terminal sterilization of allografts cannot be performed using appropriately high doses of irradiation.
Free radical scavenging reportedly provides partial radioprotection of bone and tendon allografts [1, 17]. We previously compared free radical scavenging and cross-linking as radioprotective methods [39]. Cross-linking was aimed at increasing collagen cross-link density to compensate for radiation-induced damage. Scavengers included ascorbate, mannitol, and riboflavin; cross-linkers included 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and glucose [39]. Cross-linkers showed protective effects with respect to mechanical stability and resistance to collagenase degradation up to 50 kGy, and free radical scavengers provided similar benefits up to 25 kGy [39]. At 50 kGy, EDC treatment showed the most protection of these parameters but was unsuccessful in maintaining the properties of native tendon [39]. Consequently, efforts to improve radioprotective techniques are continuing.
We therefore hypothesized (1) protection against ionizing radiation damage, specifically regarding mechanical properties and enzyme resistance, would occur by treating allografts with a combined cross-linking and scavenging method; and (2) this dual radioprotective treatment would be superior to previously investigated single treatments.
Materials and Methods
We explored means of improving radioprotective treatments of allografts by combining previously reported [39] EDC cross-linking and free radical scavenging methods. Specifically, this involved initial cross-linking with EDC followed by soaking in mannitol, ascorbate, or riboflavin solution. This method intended to (1) bolster graft properties before irradiation through EDC precross-linking while (2) providing protective benefits of scavenging during irradiation. We evaluated mechanical properties and collagenase resistance of 90 rabbit Achilles tendons treated with combined radioprotection methods and exposure to radiation. There were three dual radioprotection treatments (EDC with mannitol, ascorbate, or riboflavin) and three radiation conditions (0 kGy, gamma, or ebeam radiation at 50 kGy) with 10 tendons per group of each of the nine combinations. Eight tendons were used for mechanical testing and the remaining two were reserved for enzyme resistance testing.
We harvested Achilles tendons from mature (5–8 months) New Zealand White rabbits; these were selected for their large size and availability. Tendons were obtained from frozen hindlimbs purchased from Pel-Freeze Bio (Rogers, AR). Achilles tendons are used commonly as allografts for ligament replacement in humans [41, 44]. We dissected the tendons from the hindlimbs, then wrapped each in phosphate buffer solution (PBS)-soaked gauze, and stored them in a −20°C freezer. Tendons were separated arbitrarily into experimental groups.
Dual radioprotection treatment first involved cross-linking in a solution made from EDC (Sigma-Aldrich, St Louis, MO) and N-hydroxyl succinimide (Sigma-Aldrich). EDC cross-linking was selected as a protection method for its ability to introduce cross-links in collagen [31, 32]. This cross-linking protocol was developed for collagen scaffolds [6]. We placed 30 tendons in 225 mL of 10 mmol/L EDC and 5 mmol/L N-hydroxyl succinimide solution in deionized water for 24 hours with agitation. On removal from EDC solution, they were washed three times with deionized water at 10-minute intervals. This was followed by soaking in 100 mmol/L Na2HPO4 (Sigma-Aldrich) solution in deionized water for 2 hours. The last step involved washing in deionized water for 24 hours. This was the same procedure used for EDC cross-linking only (single treatment).
After cross-linking, tendons were soaked in free radical scavenger solution for 36 hours at 2°C. Three different free radical scavengers were investigated including D-mannitol (M), ascorbate (AS), and riboflavin (RB) (all from Sigma-Aldrich). The dual-treated groups were designated as EDCM, EDCAS, and EDCRB, respectively. Tendons in each group were soaked in free radical scavenger solutions, each of which was made at 100 mmol/L with PBS at a pH of 7.4. We selected these concentrations from previous single-treatment pilot studies [39]. Tendons were packaged in pairs in 15-mL polystyrene tubes filled with respective free radical scavenger solutions.
Radiation was performed using gamma and ebeam radiation, which allowed for comparison of the two most common forms of sterilization. Gamma irradiation was applied using a Co60 source at an average received dose of 50 kGy by Sterigenics Inc (Rockaway, NJ). Ebeam irradiation was performed using a 5-MeV electron accelerator by Ebeam Services Inc (Cranbury, NJ) at the same dose. A dose of 50 kGy was chosen as a more effective sterilization dose and closer to terminal sterilization ranges [7, 8, 40]. All treated tendons were irradiated in free radical scavenger solutions at room temperature (22–25°C) and shipped back to us in polystyrene tubes. Tendons not subjected to irradiation also were in solution for the same amount of time for shipping and processing. After tendons were shipped back to the laboratory, the solution in the tubes was removed, and the tendons were wrapped in PBS-soaked gauze and returned to −20°C.
We performed tensile testing using a mechanical tester (Model 4204; Instron, Canton, MA) to compare irradiation effects on mechanical properties for different treatment methods. Before testing, tendons were thawed in PBS and allowed to soak for 30 minutes. Dimensional measurements were taken using a laser micrometer (Z-Mike 1202 series, Dayton, OH). Two width and thickness measurements were taken for each tendon assuming a rectangular cross-sectional area.
Tendons then were mounted on cryogenic freeze clamps (Enduratec, Eden Prairie, MN) at 1 cm of tendon ends and were allowed to freeze until 1 mm was visibly frozen outside the clamps. Gauge length (3 cm) was measured as the distance between frozen ends with an aspect ratio of 10:1. PBS was applied regularly to maintain hydration. Tendons then were preconditioned in tension at approximately 1.5 to 3.5 N for five cycles. Tendons then were pulled in tension at a speed of 100 mm/minute to failure. Test data were collected using a Smart Motherboard data collector (Microstrain Inc, Williston, VT). Strength, elastic modulus, failure strain, and toughness were calculated using a custom-written MATLAB® program (The Mathworks, Norwood, MA). Elastic modulus calculations were performed on the linear regions of the stress verses strain curve. Parameters were represented as mean ± standard deviation.
We performed collagenase resistance testing to determine the state of collagen nativity and cross-link density in response to irradiation and treatments. Bacterial collagenase derived from Clostridium histolyticum, which cleaves the bond between the “X” and glycine amino acids in backbone -X-glycine-proline-Y- sequences [46], was used for this experiment. We then lyophilized the tendons and cut 3.5-mg segments. These segments were placed in test tubes and immersed in 10 mL collagenase solution (400 units/mL). This solution was made using 1 mol/L Tris buffer and clostridiopeptidase A (Sigma-Aldrich). Collagenase resistance testing was performed in triplicate for each group with collagen sponges included as positive controls. Test tubes were placed in a 37°C water bath and evaluated hourly for the first 24 hours and then every 6 hours for the final 24 hours. Average dissolution time was recorded and represented as mean ± standard deviation.
We used Student-Neuman-Keuls two-factor analysis of variance to identify differences in the mechanical properties (strength, elastic modulus, failure strain, and toughness) and the state of collagen nativity and cross-link density among four treated groups and the untreated group. The two factors were irradiation (gamma, ebeam, or unirradiated) and treatment (three dual treatments, single treatment, or untreated). Statistical analysis of dual-treated groups also included comparison to previous EDC single treatment and untreated data [39]. We used SigmaStat® (Systat Software Inc, San Jose, CA) to perform all analyses.
Results
We observed radioprotection of mechanical properties for tendons treated with a combination of free radical scavenging and cross-linking with EDC. At 0 kGy, none of the combined treatments influenced strength, elastic modulus, and strain (Figs. 1–3). The only notable effect was a decrease in toughness, which also was observed with EDC-only (single treatment) (Fig. 4). After 50 kGy gamma or ebeam irradiation, protective effects of combined treatments were most evident for strength, elastic modulus, and collagenase resistance. Strength values were higher for dual-treated tendons compared with untreated and, for several conditions, approached values close to native tendon (Fig. 1). Elastic modulus values also were greater than for untreated tendons for the majority of the groups (Fig. 2). Dual-treated groups had greater toughness than untreated, but not to the same degree as strength and modulus values. There was little change between irradiated and nonirradiated conditions (Fig. 4). Differences among strain values were the least distinguishable (Fig. 3). Changes in collagenase resistance were most evident for conditions involving cross-linking. At 50 kGy, dual-treated tendons displayed some degradation during the 48-hour study, although at the conclusion, these tendons were still intact. There were no major differences in mechanical properties or collagenase resistance among dual treatments (Fig. 5).
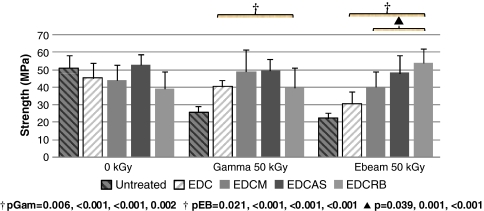
Fig. 1.
After 50 kGy of gamma or ebeam irradiation, radioprotective effects by dual treatments were most evident for strength and elastic modulus. Comparisons were made with previous EDC-only treatments and untreated data (striped bars) [39]. EDC treatment previously possessed the best combination of mechanical properties and enzyme resistance. Dual-treated tendons had greater strength than EDC-treated tendons, which supports an additive radioprotective effect. The strengths of several of the dual-treatment conditions were close to native tendon values. Reported p values correspond to figure bars from left to right. †Compared with untreated; ▲compared with EDC. EDC = 1-ethyl-3-[3-dimethyl aminopropyl] carbodiimide.
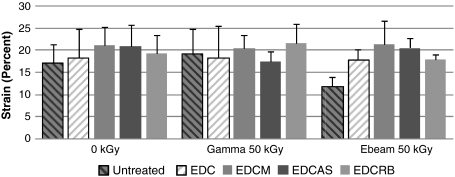
Fig. 3.
Effects of irradiation and radioprotective treatments were least distinguishable for failure strain values. There were no identifiable differences between radiation conditions and treatment conditions.
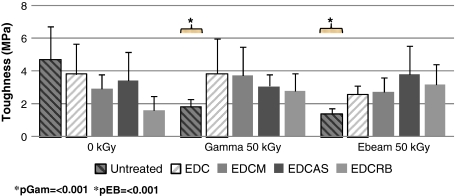
Fig. 4.
Radiation at 50 kGy caused a decrease in toughness for untreated tendons. Conversely, the effects of radiation were minimal for treated tendons. There were no differences between dual-treated and EDC-only treated tendons. Before irradiation, there was a decrease in toughness corresponding with EDC cross-linking. The decrease in toughness may be the consequence of cross-linking causing pliability of nonirradiated tendons to be reduced. *Compared with native. EDC = 1-ethyl-3-[3-dimethyl aminopropyl] carbodiimide.
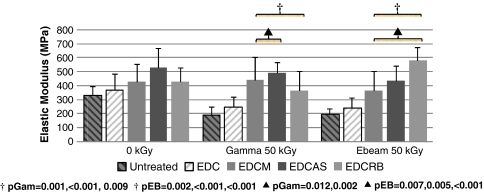
Fig. 2.
Similar trends seen with strength values were observed with elastic modulus. Elastic moduli of dual-treated tendons were comparable or greater than native tendon. Again, higher modulus was observed with tendons treated with dual radioprotection compared with EDC-only (single) treatment after irradiation. Reported p values correspond to figure bars from left to right. †Compared with untreated; ▲compared with EDC. EDC = 1-ethyl-3-[3-dimethyl aminopropyl] carbodiimide.
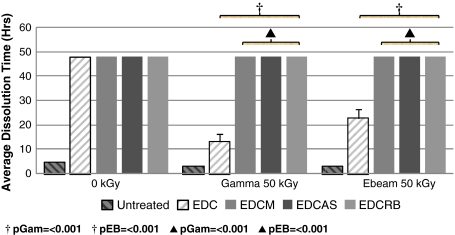
Fig. 5.
Collagenase resistance data support added radioprotection as observed with mechanical testing. Dual-treated tendons possessed higher collagenase resistance than untreated and EDC-only treatment. Dual-treated tendons remained intact throughout the 48-hour study, whereas EDC-treated tendons dissolved at 14 and 22 hours. Reported p values correspond to figure bars from left to right. †Compared with untreated; ▲compared with EDC. EDC = 1-ethyl-3-[3-dimethyl aminopropyl] carbodiimide.
When comparing dual-treated with EDC-only (single treatment) -treated tendons at 50 kGy, tendons treated with dual radioprotection had greater strength and elastic modulus; and toughness and strain values were consistent. Strength of EDCM-, EDCAS-, and EDCRB-treated tendons were an average 26%, 39%, and 37% greater, respectively, compared with those treated with EDC-only. Similarly, for elastic modulus, these groups were 64%, 89%, and 93% greater, respectively, than EDC-only-treated tendons. These trends of enhanced radioprotection observed with mechanical properties also were reflected in collagenase resistance. Specifically, collagenase resistance of tendons treated only with EDC cross-linking was surpassed by all dual-treated groups. Previously, tendons treated with EDC-only were dissolved completely after 14 and 22 hours for gamma and ebeam irradiation at 50 kGy, respectively (Fig. 5). These dual-treated tendons were considerably more resistant to degradation compared with EDC and with untreated tendons (Fig. 5).
Discussion
Currently, there is concern among surgeons, tissue banks, and regulatory agencies regarding assurance that implanted allografts are disease-free [24, 27, 42, 44]. Complete sterilization of infective tissues is difficult, and a multitude of techniques have been proposed to achieve this goal [42, 43]. Effective sterilization must be successful against a wide variety of pathogens, exhibit high penetrability through tissue structure, and preserve native properties and functionality [1]. Ionizing irradiation is capable of meeting the first two requirements [4, 12, 34, 35], but at the high doses required to inactivate resistant pathogens, tissue mechanical properties are known to deteriorate [1, 17, 36]. We hypothesized that a combined cross-linking and free radical scavenging treatment would produce an improved protective effect beyond single treatments previously examined [39]. Protection of tissues against adverse radiation effects would allow regular use of ionizing irradiation for allograft sterilization.
There were experimental limitations associated with this study and several that are potential sources of variability, which could be complicated further by comparison to controls from our previous study [39]. First, as a precursor to implantation studies, rabbit tissues were tested. Although we ordered only skeletally mature rabbits (validation was not performed in this study), animal-to-animal differences were a possibility. Second, as an animal model, these results provide an estimation of treatment effects on Achilles tendons in humans. Therefore, further optimization of radioprotective processing regarding diffusion and mechanical properties is needed for human tissue owing to anatomic differences. Third, sterilization at radiation facilities was performed at different times. Both radiation facilities maintain strict regulations to reduce variability between sterilizations and are tested frequently for reproducible results. Fourth, resistance to proteolytic degradation has been used as a method to indirectly measure cross-linking and denaturation of collagen-based materials [29, 30, 46]. We used this established method to detect similar outcomes in collagenous tissues. Although it is clear that collagenase resistance data were not as sensitive as the mechanical data for observing differences between treatment groups, there were major differences between cross-linked and noncross-linked groups after irradiation. Exposure to collagenase also provides a rudimentary simulation of the postimplantation environment. Despite this, in vivo studies must be conducted for accurate determination of the stability of irradiated allografts. Fifth, it is unknown if these treatments have long-term effects on resorption and remodeling of allografts. Sixth, it was reported that assuming rectangular cross-sectional area has limited accuracy [16, 21, 47]. Image reconstruction using triangular segments in polar coordinates apparently measures standard geometric shapes to 2% accuracy or less, measuring in 1°-increments [21]. Measurement of porcine anterior cruciate ligament cross-sectional areas required 15 minutes using this method [21], which is not feasible for large-quantity testing. Accuracy relative to true mechanical values is lost by assuming a rectangular shape; however, precision regarding differences between radioprotective effects still is preserved. Addressing these concerns is essential for the ongoing development of radioprotective methods.
There are several precautions associated with cross-linking and free radical scavenging that also must be recognized. Although EDC is an effective cross-linker of collagenous tissues, two constraints associated with cross-linking are excessive surface cross-linking and limitation of cross-linking sites [3]. Higher concentrations of EDC may not correspond to greater bulk cross-linking if surface cross-linking dominates. In this case, surface resistance to enzyme interaction may have increased, but with limited enhancement of bulk strength. Also, the extracellular matrix of tendon possesses a higher organized structure and does not have the number of available cross-linking sites compared with milled or partially solubilized collagen. The current EDC cross-linking procedure originally was optimized for collagen constructs made from milled dermal collagen [6]. Additional alteration of the EDC cross-linking procedure is needed. We observed a decrease in the preirradiation toughness and a slight increase in elastic modulus possibly resulting from cross-linking. This may have been the result of tendons becoming more brittle, but the trend was not observed after irradiation. The primary objective of radioprotective treatments is to halt immediate irradiation damage to tissue properties. Regarding free radical scavengers, it has been acknowledged that their use potentially could impede sterilization by protecting pathogens and extracellular matrix molecules [1]. Sterility assurance testing is required to determine appropriate radiation doses required for sufficient sterilization in the presence and absence of the scavengers.
Gamma irradiation reduces mechanical properties of tissues at radiation doses ranging from 25 kGy to 80 kGy on bone-patellar-bone, bone and tendon allografts [1, 13, 14, 17, 36, 38]. After 50 kGy of radiation, reductions in strength, elastic modulus, and toughness were observed for rabbit tendon allografts [39]. More specifically, parameters dependent on break load were most affected and strain least affected [39]. Salehpour et al. [36] reported a corresponding relationship between reduction of hydropyridinium cross-links and break load and strength with increased radiation dose. Evidence of radioprotection was characterized by limitation of radiation effects on mechanical properties and collagenase resistance. Resultant protective effects are likely to be attributed to the introduction of cross-links in collagen, which compensate for the reduction in cross-link density caused by irradiation. In addition, free radical scavengers acted to sequester free radicals generated from the irradiation of oxygen and water.
Free radical scavenging has been studied for radioprotection of biologic materials [20, 25, 26]. Akkus et al. [1] showed free radical scavengers can be partially successful in counteracting radiation effects in bone allografts. Our previous comparison of scavengers and cross-linkers showed radioprotective effects over a range of radiation doses [39]. These studies were unable to maintain native graft properties after exposure at 36 kGy for the bone allografts [1] and 50 kGy for tendon allografts [39]. Others have reported more successful radioprotection after combining radioprotective treatments [17, 18]. A patented procedure known as the Clearant Process® (Clearant, Inc, Los Angeles, CA) reportedly provides full protection of musculoskeletal tissue using a scavenger cocktail and tightly regulated processing conditions, although it has not gained widespread acceptance [17, 18], Also, Konopacka et al. [20] and Packer et al. [33] suggested vitamin combinations could act synergistically by regenerating molecular conformations capable of scavenging through free radical exchange. Our data suggest the combination of cross-linking and free radical scavengers has further improved protection against irradiation beyond that seen with single treatments [39]. Comparison of mechanical properties between EDC-only treated and dual-treated tendons is suggestive of an additive protective effect. Additive radioprotection also was supported by resistance to collagenase. Tendons treated with dual radioprotection were more resistant to collagenase than the untreated and the EDC-only-treated group. After 50 kGy of gamma or ebeam irradiation, dual-treatment groups showed strength, modulus, and strain greater than or comparable to native (untreated, unirradiated) tendon.
Mechanical data suggest EDC cross-linking limits the majority of irradiation damage. We observed no differences among combined treatments composed of different free radical scavengers. Previous comparison of free radical scavengers and cross-linkers showed EDC provided the most protection of graft properties [39]. Alternatively, collagenase data indicate a great radioprotective contribution is made by free radical scavengers compared with mechanical data. Regardless, it is clear single treatment is insufficient, and supplemental protection is required for full protection of native properties. Simply increasing the concentration of EDC is not a possible option as mentioned previously.
Tissue banks have greatly reduced the potential for disease transmission through current safety protocols [44]. Secondary sterilization procedures using ionizing radiation could further ensure allograft safety. Gamma and ebeam irradiation are effective at sterilization with sufficient penetration through soft tissues. The main drawback of ionizing irradiation has been its detrimental effect on mechanical properties. Our data suggest a combined cross-linking and free radical scavenging procedure provides added protection to initial mechanical integrity and resistance to enzyme digestion at 50 kGy. Because concerns still remain, more studies are needed to optimize the cross-linking protocol and free radical scavenging cocktail. The focus then will move to validation, bioburden testing, and implantation models to determine safety and efficacy of radioprotected sterilized allografts.
Footnotes
One or more of the authors (MGD) have received funding from a grant from the Musculoskeletal Transplant Foundation Peer Reviewed Scientific Grants Program (January–December 2005).
Each author certifies that his or her institution has approved or waived approval for the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Akkus O, Belaney RM, Das P. Free radical scavenging alleviates the biomechanical impairment of gamma radiation sterilized bone tissue. J Orthop Res. 2005;23:838–845. [DOI] [PubMed]
- 2.Beynnon BD, Johnson RJ, Abate JA, Fleming BC, Nichols CE. Treatment of anterior cruciate ligament injuries, part I. Am J Sports Med. 2005;33:1579–1602. [DOI] [PubMed]
- 3.Billiar K, Murray J, Laude D, Abraham G, Bachrach N. Effects of carbodiimide crosslinking conditions on the physical properties of laminated intestinal submucosa. J Biomed Mater Res. 2001;56:101–108. [DOI] [PubMed]
- 4.Block SS. Disinfection, Sterilization, and Preservation. Ed 4. Philadelphia, PA: Lea & Febiger; 1991.
- 5.Buck BE, Malinin TI. Human bone and tissue allografts: preparation and safety. Clin Orthop Relat Res. 1994;303:8–17. [PubMed]
- 6.Caruso AB, Dunn MG. Changes in mechanical properties and cellularity during long-term culture of collagen fiber ACL reconstruction scaffolds. J Biomed Mater Res A. 2005;73:388–397. [DOI] [PubMed]
- 7.Centers for Disease Control and Prevention. Update: allograft-associated bacterial infections—United States, 2002. JAMA. 2002;287:1642–1644. [DOI] [PubMed]
- 8.Centers for Disease Control and Prevention (CDC). Invasive Streptococcus pyogenes after allograft implantation—Colorado, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:1174–1176. [PubMed]
- 9.Centers for Disease Control and Prevention (CDC). About tissue transplants. Available at http://www.cdc.gov/ncidod/dhqp/tissuetransplantsfaq.html. Accessed April 6, 2009.
- 10.Cheung DT, Perelman N, Tong D, Nimni ME. The effect of gamma-irradiation on collagen molecules, isolated alpha-chains, and crosslinked native fibers. J Biomed Mater Res. 1990;24:581–589. [DOI] [PubMed]
- 11.Cohen SB, Sekiya JK. Allograft safety in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:597–605. [DOI] [PubMed]
- 12.Dziedzic-Goclawska A, Kaminski A, Uhrynowska-Tyszkiewicz I, Stachowicz W. Irradiation as a safety procedure in tissue banking. Cell Tissue Bank. 2005;6:201–219. [DOI] [PubMed]
- 13.Fideler BM, Vangsness CT Jr, Lu B, Orlando C, Moore T. Gamma irradiation: effects on biomechanical properties of human bone-patellar tendon-bone allografts. Am J Sports Med. 1995;23:643–646. [DOI] [PubMed]
- 14.Gibbons MJ, Butler DL, Grood ES, Bylski-Austrow DI, Levy MS, Noyes FR. Effects of gamma irradiation on the initial mechanical and material properties of goat bone-patellar tendon-bone allografts. J Orthop Res. 1991;9:209–218. [DOI] [PubMed]
- 15.Gocke DJ. Tissue donor selection and safety. Clin Orthop Relat Res. 2005;435:17–21. [DOI] [PubMed]
- 16.Goodship AE, Birch HL. Cross sectional area measurement of tendon and ligament in vitro: a simple, rapid, non-destructive technique. J Biomech. 2005;38(3):605–608. [DOI] [PubMed]
- 17.Grieb TA, Forng RY, Bogdansky S, Ronholdt C, Parks B, Drohan WN, Burgess WH, Lin J. High-dose gamma irradiation for soft tissue allografts: high margin of safety with biomechanical integrity. J Orthop Res. 2006;24:1011–1018. [DOI] [PubMed]
- 18.Grieb TA, Forng RY, Stafford RE, Lin J, Almeida J, Bogdansky S, Ronholdt C, Drohan WN, Burgess WH. Effective use of optimized, high-dose (50 kGy) gamma irradiation for pathogen inactivation of human bone allografts. Biomaterials. 2005;26:2033–2042. [DOI] [PubMed]
- 19.Hernigou P. Allograft sterility as exemplified by human immunodeficiency virus and sterilization by irradiation. J Arthroplasty. 2000;15:1051–1058. [DOI] [PubMed]
- 20.Konopacka M, Widel M, Rzeszowska-Wolny J. Modifying effect of vitamins C, E and beta-carotene against gamma-ray-induced DNA damage in mouse cells. Mutat Res. 1998;417:85–94. [DOI] [PubMed]
- 21.Lee TQ, Woo SL. A new method for determining cross-sectional shape and area of soft tissues. J Biomech Eng. 1988;110:110–114. [DOI] [PubMed]
- 22.Maeda A, Inoue M, Shino K, Nakata K, Nakamura H, Tanaka M, Seguchi Y, Ono K. Effects of solvent preservation with or without gamma irradiation on the material properties of canine tendon allografts. J Orthop Res. 1993;11:181–189. [DOI] [PubMed]
- 23.Marrale J, Morrissey MC, Haddad FS. A literature review of autograft and allograft anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2007;15:690–704. [DOI] [PubMed]
- 24.McAllister DR, Joyce MJ, Mann BJ, Vangsness CT Jr. Allograft update: the current status of tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2007;35:2148–2158. [DOI] [PubMed]
- 25.Monboisse JC, Borel JP. Oxidative damage to collagen. EXS. 1992;62:323–327. [DOI] [PubMed]
- 26.Monboisse JC, Gardes-Albert M, Randoux A, Borel JP, Ferradini C. Collagen degradation by superoxide anion in pulse and gamma radiolysis. Biochim Biophys Acta. 1988;965:29–35. [DOI] [PubMed]
- 27.Mroz TE, Lin EL, Summit MC, Bianchi JR, Keesling JE Jr, Roberts M, Vangsness CT Jr, Wang JC. Biomechanical analysis of allograft bone treated with a novel tissue sterilization process. Spine J. 2006;6:34–39. [DOI] [PubMed]
- 28.Nguyen H, Morgan DA, Forwood MR. Sterilization of allograft bone: effects of gamma irradiation on allograft biology and biomechanics. Cell Tissue Bank. 2007;8:93–105. [DOI] [PubMed]
- 29.Ohan MP, Dunn MG. Glucose stabilizes collagen sterilized with gamma irradiation. J Biomed Mater Res A. 2003;67:1188–1195. [DOI] [PubMed]
- 30.Ohan MP, Weadock KS, Dunn MG. Synergistic effects of glucose and ultraviolet irradiation on the physical properties of collagen. J Biomed Mater Res. 2002;60:384–391. [DOI] [PubMed]
- 31.Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17:765–773. [DOI] [PubMed]
- 32.Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. In vitro degradation of dermal sheep collagen cross-linked using a water-soluble carbodiimide. Biomaterials. 1996;17:679–684. [DOI] [PubMed]
- 33.Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979;278:737–738. [DOI] [PubMed]
- 34.Petrigliano FA, McAllister DR, Wu BM. Tissue engineering for anterior cruciate ligament reconstruction: a review of current strategies. Arthroscopy. 2006;22:441–451. [DOI] [PubMed]
- 35.Russell AD. Principles and Practice of Disinfection, Preservation, and Sterilization. Ed 2. Oxford, England: Blackwell Scientific Publications; 1992.
- 36.Salehpour A, Butler DL, Proch FS, Schwartz HE, Feder SM, Doxey CM, Ratcliffe A. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone-patellar tendon-bone allografts. J Orthop Res. 1995;13:898–906. [DOI] [PubMed]
- 37.Schreiner LJ, Cameron IG, Funduk N, Miljkovic L, Pintar MM, Kydon DN. Proton NMR spin grouping and exchange in dentin. Biophys J. 1991;59:629–639. [DOI] [PMC free article] [PubMed]
- 38.Schwartz HE, Matava MJ, Proch FS, Butler CA, Ratcliffe A, Levy M, Butler DL. The effect of gamma irradiation on anterior cruciate ligament allograft biomechanical and biochemical properties in the caprine model at time zero and at 6 months after surgery. Am J Sports Med. 2006;34:1747–1755. [DOI] [PubMed]
- 39.Seto A, Gatt CJ Jr, Dunn MG. Radioprotection of tendon tissue via crosslinking and free radical scavenging. Clin Orthop Relat Res. 2008;466:1788–1795. [DOI] [PMC free article] [PubMed]
- 40.Smith RA, Ingels J, Lochemes JJ, Dutkowsky JP, Pifer LL. Gamma irradiation of HIV-1. J Orthop Res. 2001;19:815–819. [DOI] [PubMed]
- 41.Strickland SM, MacGillivray JD, Warren RF. Anterior cruciate ligament reconstruction with allograft tendons. Orthop Clin North Am. 2003;34:41–47. [DOI] [PubMed]
- 42.Suarez LS, Richmond JC. Overview of procurement, processing, and sterilization of soft tissue allografts for sports medicine. Sports Med Arthrosc. 2007;15:106–113. [DOI] [PubMed]
- 43.Vangsness CT Jr. Soft-tissue allograft processing controversies. J Knee Surg. 2006;19:215–219. [DOI] [PubMed]
- 44.Vangsness CT Jr, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31:474–481. [DOI] [PubMed]
- 45.Veen MR, Bloem RM, Petit PL. Sensitivity and negative predictive value of swab cultures in musculoskeletal allograft procurement. Clin Orthop Relat Res. 1994;300:259–263. [PubMed]
- 46.Weadock KS, Miller EJ, Keuffel EL, Dunn MG. Effect of physical crosslinking methods on collagen-fiber durability in proteolytic solutions. J Biomed Mater Res. 1996;32:221–226. [DOI] [PubMed]
- 47.Woo SL, Danto MI, Ohland KJ, Lee TQ, Newton PO. The use of a laser micrometer system to determine the cross-sectional shape and area of ligaments: a comparative study with two existing methods. J Biomech Eng. 1990;112(4):426–431. [DOI] [PubMed]