Abstract
Based on sequence homologies with leguminous lectins, the intermediate compartment marker ERGIC-53 was proposed to be a member of a putative new class of animal lectins associated with the secretory pathway. Independent, a promyelocytic protein, MR60, was purified by mannose-column chromatography, and a cDNA was isolated that matched MR60 peptide sequences. This cDNA was identical to that of ERGIC-53 and homologies with the animal lectin family of the galectins were noticed. Not all peptide sequences of MR60, however, were found in ERGIC-53, raising the possibility that another protein associated with ERGIC-53 may possess the lectin activity. Here, we provide the first direct evidence for a lectin function of ERGIC-53. Overexpressed ERGIC-53 binds to a mannose column in a calcium-dependent manner and also co-stains with mannosylated neoglycoprotein in a morphological binding assay. By using a sequential elution protocol we show that ERGIC-53 has selectivity for mannose and low affinity for glucose and GlcNAc, but no affinity for galactose. To experimentally address the putative homology of ERGIC-53 to leguminous lectins, a highly conserved protein family with an invariant asparagine essential for carbohydrate binding, we substituted the corresponding asparagine in ERGIC-53. This mutation, as well as a mutation affecting a second site in the putative carbohydrate recognition domain, abolished mannose-column binding and co-staining with mannosylated neoglycoprotein. These findings establish ERGIC-53 as a lectin and provide functional evidence for its relationship to leguminous lectins. Based on its monosaccharide specificity, domain organization, and recycling properties, we propose ERGIC-53 to function as a sorting receptor for glyco-proteins in the early secretory pathway.
Full text
PDF




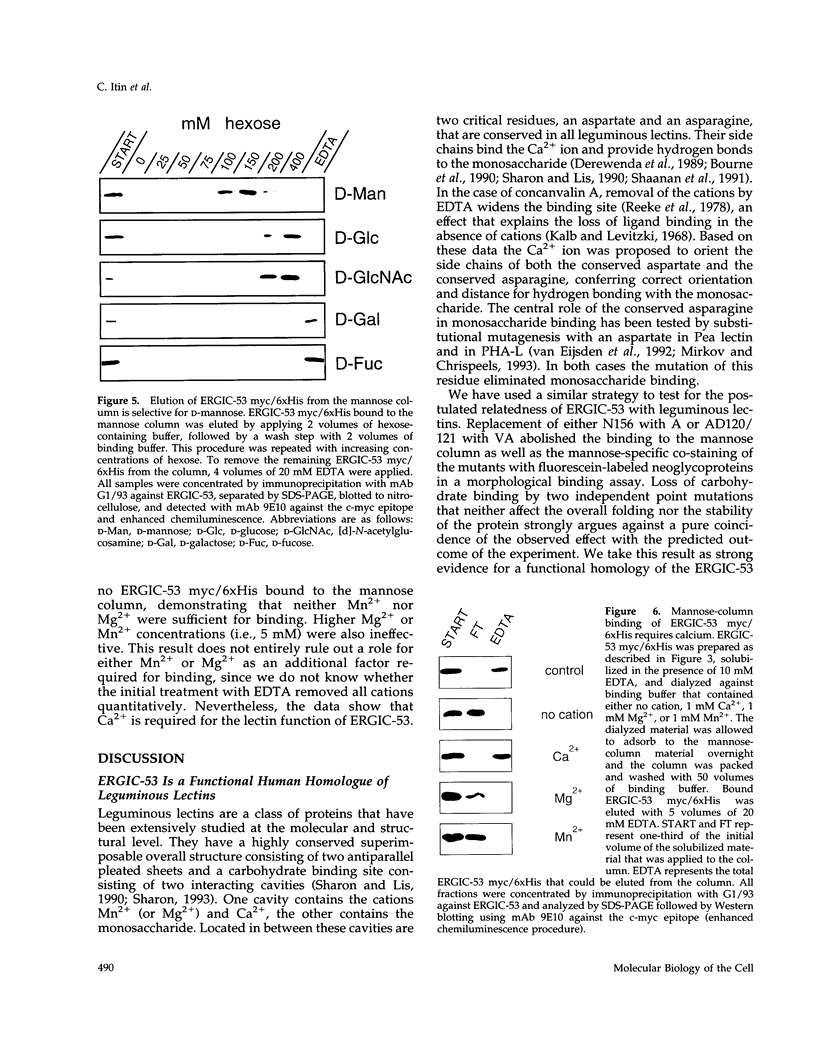



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arar C., Carpentier V., Le Caer J. P., Monsigny M., Legrand A., Roche A. C. ERGIC-53, a membrane protein of the endoplasmic reticulum-Golgi intermediate compartment, is identical to MR60, an intracellular mannose-specific lectin of myelomonocytic cells. J Biol Chem. 1995 Feb 24;270(8):3551–3553. doi: 10.1074/jbc.270.8.3551. [DOI] [PubMed] [Google Scholar]
- Balch W. E., McCaffery J. M., Plutner H., Farquhar M. G. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994 Mar 11;76(5):841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Barondes S. H., Cooper D. N., Gitt M. A., Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994 Aug 19;269(33):20807–20810. [PubMed] [Google Scholar]
- Bause E., Breuer W., Schweden J., Roeser R., Geyer R. Effect of substrate structure on the activity of Man9-mannosidase from pig liver involved in N-linked oligosaccharide processing. Eur J Biochem. 1992 Sep 1;208(2):451–457. doi: 10.1111/j.1432-1033.1992.tb17207.x. [DOI] [PubMed] [Google Scholar]
- Bischoff J., Liscum L., Kornfeld R. The use of 1-deoxymannojirimycin to evaluate the role of various alpha-mannosidases in oligosaccharide processing in intact cells. J Biol Chem. 1986 Apr 5;261(10):4766–4774. [PubMed] [Google Scholar]
- Bourne Y., Roussel A., Frey M., Rougé P., Fontecilla-Camps J. C., Cambillau C. Three-dimensional structures of complexes of Lathyrus ochrus isolectin I with glucose and mannose: fine specificity of the monosaccharide-binding site. Proteins. 1990;8(4):365–376. doi: 10.1002/prot.340080410. [DOI] [PubMed] [Google Scholar]
- Carpentier V., Vassard C., Plessis C., Motta G., Monsigny M., Roche A. C. Characterization and cellular localization by monoclonal antibodies of the 60 kDa mannose specific lectin of human promyelocytic cells, HL60. Glycoconj J. 1994 Aug;11(4):333–338. doi: 10.1007/BF00731206. [DOI] [PubMed] [Google Scholar]
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990 Jul 27;62(2):317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- Cosson P., Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994 Mar 18;263(5153):1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Degen E., Cohen-Doyle M. F., Williams D. B. Efficient dissociation of the p88 chaperone from major histocompatibility complex class I molecules requires both beta 2-microglobulin and peptide. J Exp Med. 1992 Jun 1;175(6):1653–1661. doi: 10.1084/jem.175.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derewenda Z., Yariv J., Helliwell J. R., Kalb A. J., Dodson E. J., Papiz M. Z., Wan T., Campbell J. The structure of the saccharide-binding site of concanavalin A. EMBO J. 1989 Aug;8(8):2189–2193. doi: 10.1002/j.1460-2075.1989.tb08341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K., Taylor M. E. Biology of animal lectins. Annu Rev Cell Biol. 1993;9:237–264. doi: 10.1146/annurev.cb.09.110193.001321. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K., Parton R. G., Kellner R., Etzold T., Simons K. VIP36, a novel component of glycolipid rafts and exocytic carrier vesicles in epithelial cells. EMBO J. 1994 Apr 1;13(7):1729–1740. doi: 10.1002/j.1460-2075.1994.tb06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K., Simons K. A putative novel class of animal lectins in the secretory pathway homologous to leguminous lectins. Cell. 1994 Jun 3;77(5):625–626. doi: 10.1016/0092-8674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Fujimoto K., Kornfeld R. alpha-Glucosidase II-deficient cells use endo alpha-mannosidase as a bypass route for N-linked oligosaccharide processing. J Biol Chem. 1991 Feb 25;266(6):3571–3578. [PubMed] [Google Scholar]
- Hammond C., Braakman I., Helenius A. Role of N-linked oligosaccharide recognition, glucose trimming, and calnexin in glycoprotein folding and quality control. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):913–917. doi: 10.1073/pnas.91.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Schweizer A. The endoplasmic reticulum-Golgi intermediate compartment. Curr Opin Cell Biol. 1992 Aug;4(4):600–608. doi: 10.1016/0955-0674(92)90078-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert D. N., Foellmer B., Helenius A. Glucose trimming and reglucosylation determine glycoprotein association with calnexin in the endoplasmic reticulum. Cell. 1995 May 5;81(3):425–433. doi: 10.1016/0092-8674(95)90395-x. [DOI] [PubMed] [Google Scholar]
- Helenius A. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol Biol Cell. 1994 Mar;5(3):253–265. doi: 10.1091/mbc.5.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin C., Kappeler F., Linstedt A. D., Hauri H. P. A novel endocytosis signal related to the KKXX ER-retrieval signal. EMBO J. 1995 May 15;14(10):2250–2256. doi: 10.1002/j.1460-2075.1995.tb07219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin C., Schindler R., Hauri H. P. Targeting of protein ERGIC-53 to the ER/ERGIC/cis-Golgi recycling pathway. J Cell Biol. 1995 Oct;131(1):57–67. doi: 10.1083/jcb.131.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb A. J., Levitzki A. Metal-binding sites of concanavalin A and their role in the binding of alpha-methyl d-glucopyranoside. Biochem J. 1968 Oct;109(4):669–672. doi: 10.1042/bj1090669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Gaynor E. C., Hennecke S., Démollière C., Duden R., Emr S. D., Riezman H., Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic reticulum. Cell. 1994 Dec 30;79(7):1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lubas W. A., Spiro R. G. Evaluation of the role of rat liver Golgi endo-alpha-D-mannosidase in processing N-linked oligosaccharides. J Biol Chem. 1988 Mar 15;263(8):3990–3998. [PubMed] [Google Scholar]
- Lubas W. A., Spiro R. G. Golgi endo-alpha-D-mannosidase from rat liver, a novel N-linked carbohydrate unit processing enzyme. J Biol Chem. 1987 Mar 15;262(8):3775–3781. [PubMed] [Google Scholar]
- Mirkov T. E., Chrispeels M. J. Mutation of Asn128 to Asp of Phaseolus vulgaris leucoagglutinin (PHA-L) eliminates carbohydrate-binding and biological activity. Glycobiology. 1993 Dec;3(6):581–587. doi: 10.1093/glycob/3.6.581. [DOI] [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Midoux P. Uptake of neoglycoproteins via membrane lectin(s) of L1210 cells evidenced by quantitative flow cytofluorometry and drug targeting. Biol Cell. 1984;51(2):187–196. doi: 10.1111/j.1768-322x.1984.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Moore S. E., Spiro R. G. Demonstration that Golgi endo-alpha-D-mannosidase provides a glucosidase-independent pathway for the formation of complex N-linked oligosaccharides of glycoproteins. J Biol Chem. 1990 Aug 5;265(22):13104–13112. [PubMed] [Google Scholar]
- Ou W. J., Cameron P. H., Thomas D. Y., Bergeron J. J. Association of folding intermediates of glycoproteins with calnexin during protein maturation. Nature. 1993 Aug 26;364(6440):771–776. doi: 10.1038/364771a0. [DOI] [PubMed] [Google Scholar]
- Pearse B. M., Robinson M. S. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. About turn for the COPs? Cell. 1994 Dec 30;79(7):1125–1127. doi: 10.1016/0092-8674(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Peterson J. R., Ora A., Van P. N., Helenius A. Transient, lectin-like association of calreticulin with folding intermediates of cellular and viral glycoproteins. Mol Biol Cell. 1995 Sep;6(9):1173–1184. doi: 10.1091/mbc.6.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpaneau V., Midoux P., Monsigny M., Roche A. C. Characterization and isolation of an intracellular D-mannose-specific receptor from human promyelocytic HL60 cells. Carbohydr Res. 1991 Jun 25;213:95–108. doi: 10.1016/s0008-6215(00)90601-3. [DOI] [PubMed] [Google Scholar]
- Reeke G. N., Jr, Becker J. W., Edelman G. M. Changes in the three-dimensional structure of concanavalin A upon demetallization. Proc Natl Acad Sci U S A. 1978 May;75(5):2286–2290. doi: 10.1073/pnas.75.5.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche A. C., Barzilay M., Midoux P., Junqua S., Sharon N., Monsigny M. Sugar-specific endocytosis of glycoproteins by Lewis lung carcinoma cells. J Cell Biochem. 1983;22(3):131–140. doi: 10.1002/jcb.240220302. [DOI] [PubMed] [Google Scholar]
- Roth J., Berger E. G. Immunocytochemical localization of galactosyltransferase in HeLa cells: codistribution with thiamine pyrophosphatase in trans-Golgi cisternae. J Cell Biol. 1982 Apr;93(1):223–229. doi: 10.1083/jcb.93.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J. F. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990 Apr 20;61(2):197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Schindler R., Itin C., Zerial M., Lottspeich F., Hauri H. P. ERGIC-53, a membrane protein of the ER-Golgi intermediate compartment, carries an ER retention motif. Eur J Cell Biol. 1993 Jun;61(1):1–9. [PubMed] [Google Scholar]
- Schmid S. L. The mechanism of receptor-mediated endocytosis: more questions than answers. Bioessays. 1992 Sep;14(9):589–596. doi: 10.1002/bies.950140903. [DOI] [PubMed] [Google Scholar]
- Schweizer A., Fransen J. A., Bächi T., Ginsel L., Hauri H. P. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol. 1988 Nov;107(5):1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A., Fransen J. A., Matter K., Kreis T. E., Ginsel L., Hauri H. P. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur J Cell Biol. 1990 Dec;53(2):185–196. [PubMed] [Google Scholar]
- Shaanan B., Lis H., Sharon N. Structure of a legume lectin with an ordered N-linked carbohydrate in complex with lactose. Science. 1991 Nov 8;254(5033):862–866. doi: 10.1126/science.1948067. [DOI] [PubMed] [Google Scholar]
- Sharon N. Lectin-carbohydrate complexes of plants and animals: an atomic view. Trends Biochem Sci. 1993 Jun;18(6):221–226. doi: 10.1016/0968-0004(93)90193-q. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Legume lectins--a large family of homologous proteins. FASEB J. 1990 Nov;4(14):3198–3208. doi: 10.1096/fasebj.4.14.2227211. [DOI] [PubMed] [Google Scholar]
- Sousa M. C., Ferrero-Garcia M. A., Parodi A. J. Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry. 1992 Jan 14;31(1):97–105. doi: 10.1021/bi00116a015. [DOI] [PubMed] [Google Scholar]
- Sousa M., Parodi A. J. The molecular basis for the recognition of misfolded glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. EMBO J. 1995 Sep 1;14(17):4196–4203. doi: 10.1002/j.1460-2075.1995.tb00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11655–11663. [PubMed] [Google Scholar]
- Tulsiani D. R., Hubbard S. C., Robbins P. W., Touster O. alpha-D-Mannosidases of rat liver Golgi membranes. Mannosidase II is the GlcNAcMAN5-cleaving enzyme in glycoprotein biosynthesis and mannosidases Ia and IB are the enzymes converting Man9 precursors to Man5 intermediates. J Biol Chem. 1982 Apr 10;257(7):3660–3668. [PubMed] [Google Scholar]
- Tulsiani D. R., Touster O. The purification and characterization of mannosidase IA from rat liver Golgi membranes. J Biol Chem. 1988 Apr 15;263(11):5408–5417. [PubMed] [Google Scholar]
- Ware F. E., Vassilakos A., Peterson P. A., Jackson M. R., Lehrman M. A., Williams D. B. The molecular chaperone calnexin binds Glc1Man9GlcNAc2 oligosaccharide as an initial step in recognizing unfolded glycoproteins. J Biol Chem. 1995 Mar 3;270(9):4697–4704. doi: 10.1074/jbc.270.9.4697. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Tector M., Salter R. D. Calnexin recognizes carbohydrate and protein determinants of class I major histocompatibility complex molecules. J Biol Chem. 1995 Feb 24;270(8):3944–3948. doi: 10.1074/jbc.270.8.3944. [DOI] [PubMed] [Google Scholar]
- van Eijsden R. R., Hoedemaeker F. J., Díaz C. L., Lugtenberg B. J., de Pater B. S., Kijne J. W. Mutational analysis of pea lectin. Substitution of Asn125 for Asp in the monosaccharide-binding site eliminates mannose/glucose-binding activity. Plant Mol Biol. 1992 Dec;20(6):1049–1058. doi: 10.1007/BF00028892. [DOI] [PubMed] [Google Scholar]