Abstract
This study is a prospective, randomized, double-blind study to compare the efficacy and safety of 10 mg/kg infliximab with those of 3 mg/kg infliximab treatment in methotrexate-refractory rheumatoid arthritis patients. After the patients received 3 mg/kg infliximab infusion at weeks 0, 2, and 6, they were randomly assigned to be administered 3, 6 or 10 mg/kg infliximab every 8 weeks from week 14 to 46. Mean American College of Rheumatology improvement (ACR-N) at week 54, the primary endpoint, was 51.3% and 58.3% for the 3 mg/kg and 10 mg/kg groups, respectively, with a statistically significant difference. Treatment with 10 mg/kg was found to be remarkably beneficial in patients who had not responded to three infusions with 3 mg/kg at week 10. The median changes in the modified Sharp score were 0.0 in the two groups. There were no significant differences in the incidences of adverse events between the groups. In patients who achieved better clinical response or greater inhibition of progression of joint damage, trough serum infliximab level was significantly higher than in patients who did not. The magnitudes of both efficacies were correlated with the trough serum infliximab level (ClinicalTrials.gov number: NCT00691028).
Keywords: Clinical trial, Infliximab, Rheumatoid arthritis, Serum level, Tumor necrosis factor (TNF) antagonist
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory disease with the potential to cause substantial joint damage and disability [1]. Tumor necrosis factor-alpha (TNF-alpha) plays a central role in the pathogenesis of RA, as demonstrated by the clinical benefit of anti-TNF alpha therapy, and infliximab (anti-human TNF-alpha monoclonal antibody) therapy has been a great advance in the treatment of RA patients [2–7]. The pivotal multinational clinical study, the Anti-TNF Trial in Rheumatoid Arthritis with Concomitant Therapy (ATTRACT), showed that repeated treatment with 3 or 10 mg/kg infliximab was more effective than methotrexate (MTX) alone in reducing the clinical symptoms of RA, inhibiting the progression of joint damage, and improving physical function [3, 8]. However, the main purpose of the ATTRACT study was to evaluate the usefulness of the concomitant treatment of infliximab and MTX in comparison with MTX monotherapy, and there was not enough evidence to show an advantage for therapy with 10 mg/kg over 3 mg/kg. In this prospective, randomized, double-blind study (the RISING study: impact on radiographic and clinical response to infliximab therapy concomitant with methotrexate in patients with rheumatoid arthritis by trough serum level in a dose-escalating study), we examined the usefulness of infliximab at the maximum dose (10 mg/kg) compared with the minimum dose (3 mg/kg) as a control. In addition, we investigated the association between the trough serum infliximab level and the magnitude of clinical response or inhibition of progression of joint damage.
Patients and methods
Patients
Eligible patients were those aged between 18 and 75 years who met the 1987 revised criteria of the American College of Rheumatology (ACR) for the classification of RA [9]. Patients were eligible if they had active RA despite treatment with MTX for more than 12 weeks. Active RA was defined in this study by the presence of six or more swollen joints, six or more tender joints, and an erythrocyte sedimentation rate (ESR) of at least 28 mm/h, or a serum C-reactive protein (CRP) concentration of at least 2.0 mg/dl.
Patients were excluded if they had: dysfunction with Steinbrocker functional class 4 [10]; other connective tissue disease with joint symptoms except Sjögren’s syndrome; a history of infliximab therapy; experience of therapy with other biological agents within 4 months before registration; been treated with glucocorticoid injections or immunosuppressive agents such as lefulnomide and tacrolimus. Other exclusion criteria were: a history of serious or opportunistic infection within 6 months before registration; active tuberculosis; hepatitis B virus, hepatitis C virus or human immunodeficiency virus (HIV) carriers; and those with chronic infectious diseases.
Study protocol
The RISING study was a prospective, multicenter, double-blind, paralleled, comparative study conducted at 88 medical institutes in Japan. The study protocol was approved by the local institutional review board (IRB) of each study institution, and was carried out in accordance with the Helsinki Declaration and Good Clinical Practice. Patients gave their written informed consent prior to registration for this study. This study was registered with http://www.clinicaltrials.gov (NCT00691028).
In the open-label study from weeks 0 to 14, all patients enrolled in this study received 3 mg/kg infliximab at weeks 0, 2, and 6. At week 10, patients were randomly assigned to three treatment groups (3, 6 or 10 mg/kg) using a dynamic assignment conducted so that the clinical efficacy in ACR20 and ACR50 responses [11] at week 10 was similar among the three groups. Then, infliximab at doses of 3, 6 or 10 mg/kg was administered every 8 weeks from week 14 to 46 in a double-blind fashion, and the efficacies were evaluated at week 54. Adverse events were evaluated until week 54. In patients in whom administration was discontinued, adverse events were assessed until 12 weeks after final administration.
Over the entire study period, disease modifying antirheumatic drugs (other than leflunomide, tacrolimus, cyclosporine, and azathioprine), nonsteroidal anti-inflammatory drugs, oral glucocorticoids (prednisolone ≤10 mg/day), and folic acid preparations were permitted at the stable dose from at least 4 weeks before registration. The dose of MTX must have been stable (6 mg/week or more: the approved maximum dose of MTX for RA in Japan is 8 mg/week) for more than 4 weeks just before registration and over the entire study period.
Endpoints
The primary endpoint for clinical response was mean percentage American College of Rheumatology improvement (ACR-N) [4, 12, 13] in 3 and 10 mg/kg groups from baseline to week 54. ACR responses (ACR20, ACR50, and ACR70), disease activity score in 28 joints (DAS28) change [14], and European League against Rheumatism (EULAR) response [15] were also evaluated at week 54. We also subanalyzed the clinical response at week 54 in the patients with EULAR no response to three infusions (at week 0, 2 and 6) with 3 mg/kg at week 10.
Radiographic progression of joint damage was quantified as the change from baseline to week 54 in the total modified Sharp score (TSS) with a range of 0–390 [16, 17]. Two readers scored the radiographs independently without knowledge of treatment assignment, clinical response or the order of the radiographs. Radiographic progression of disease was defined as damage from baseline in TSS that was larger than the smallest detectable difference (SDD) [18]. The SDD in this study was 4.1. The progression of joint damage was categorized in TSS as follows: progressed (>4.1), no change (≥−4.1 and ≤4.1), and improved (<−4.1).
Improving physical function at week 54 was evaluated by the change in the health assessment questionnaire (HAQ) score [19] and the percentage of patients who achieved an improvement of HAQ score exceeding 0.22 units, a value which may be clinically significant [20]. The trough serum infliximab level at week 54 was measured by enzyme-linked immunosorbent assay (ELISA), using a monoclonal antibody against infliximab obtained from Centocor Ortho Biotech Inc., as previously described [2]. The lowest level of infliximab that could be reliably detected was 0.1 µg/ml. The serum trough level was measured in Mitsubishi Tanabe Pharma Corporation, Osaka, Japan, and the coefficient of variation or relative error values of intra- and interassay was within 20% or within ±25%, respectively.
The associations between the clinical response or the progression of joint damage and the trough serum infliximab level at week 54 were investigated in patients for whom the trough serum levels and DAS28 or TSS were obtained at week 54.
Statistical analysis
Since the aim of the RISING study is to compare the usefulness of the 10 mg/kg infliximab treatment with that of the 3 mg/kg in MTX-refractory RA patients, the sample size of the study was determined by the predicted values of ACR-N in 3 and 10 mg/kg groups in the ATTRACT study. A size of 100 patients per group gave 90% power to detect a difference in the primary endpoint (ACR-N) between the 3 and 10 mg/kg groups by use of the two-sided t test at α = 0.05 with detection power of 1 − β = 0.90.
Because the jumped dose escalation from 3 to 10 mg/kg was thought not to be realistic in clinical practice, we also investigated the efficacy and safety of 6 mg/kg treatment as an intermediate dose.
Efficacy was analyzed in the full analysis set. The efficacy other than the joint damage was assessed using the last observation carried forward approach.
Covariance analysis was performed, using the treatment groups as factors and ACR-N at week 10 as a covariant, to compare the parameters for evaluating differences in clinical responses between the 3 and 10 mg/kg groups. As a subanalysis, the results were compared between the 3 and 6 mg/kg groups, as well as between the 6 and 10 mg/kg groups. The HAQ scores were compared by covariance analysis using the treatment groups as factors and HAQ score at week 0 as a covariant. To compare the TSS changes among the treatment groups, we employed Van Elteren’s test. Spearman’s rank correlation coefficient was used in assessing the clinical response at week 54 in the patients with EULAR no response at week 10, and the association between the efficacy and the trough serum infliximab level. The incidences of adverse events were compared using the chi-squared test.
Results
Patient background
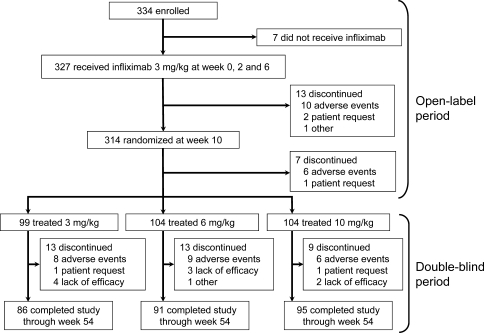
In the RISING study, 334 patients were enrolled. Of these, 327 received infliximab therapy at 3 mg/kg during the open-label period (Fig. 1). At week 10, 314 patients were randomized. The most common reason for discontinuation from week 0 to 14 was adverse events. A total of 307 patients were assigned to one of the 3, 6 or 10 mg/kg groups in the double-blind period starting from week 14. There were no significant differences in the backgrounds of each group such as age, dose of MTX, disease activity, progression of joint damage or physical function (Table 1).
Fig. 1.
Randomization, reason for discontinuing treatment, and number of patients completing the study. All patients received concomitant methotrexate
Table 1.
Baseline demographics and clinical characteristics of patients enrolled in the double-blind study
| 3 mg/kg (n = 99) |
6 mg/kg (n = 104) |
10 mg/kg (n = 104) |
|
|---|---|---|---|
| Age, mean (SD) (years) | 49.7 (11.7) | 48.8 (11.8) | 50.4 (12.5) |
| Body weight, mean (SD) (kg) | 57.3 (11.2) | 54.1 (9.1) | 54.7 (10.1) |
| Women, no. (%) | 78 (78.8) | 86 (82.7) | 89 (85.6) |
| Comorbidity, no. (%) | 81 (81.8) | 80 (76.9) | 78 (75.0) |
| Steinbrocker grade, no. (%) | |||
| I | 8 (8.1) | 8 (7.7) | 14 (13.5) |
| II | 39 (39.4) | 44 (42.3) | 27 (26.0) |
| III | 30 (30.3) | 31 (29.8) | 37 (35.6) |
| IV | 22 (22.2) | 21 (20.2) | 26 (25.0) |
| Steinbrocker class, no. (%) | |||
| 1 | 15 (15.2) | 26 (25.0) | 15 (14.4) |
| 2 | 77 (77.8) | 68 (65.4) | 80 (76.9) |
| 3 | 7 (7.1) | 10 (9.6) | 9 (8.7) |
| Duration of disease, mean (SD), (years) | 8.3 (7.8) | 7.2 (7.1) | 8.4 (7.7) |
| Duration of disease <3 years, no (%) | 26 (26.3) | 38 (36.5) | 32 (30.8) |
| Weekly MTX dose, mean (SD) (mg/week) | 7.8 (1.6) | 7.9 (1.9) | 7.7 (1.7) |
| Oral glucocorticoid, no. (%) | 66 (66.7) | 73 (70.2) | 71 (68.3) |
| Tender joint count, mean (SD) | 18.6 (11.3) | 18.0 (10.5) | 17.5 (10.9) |
| Swollen joint count, mean (SD) | 14.2 (6.1) | 13.1 (8.4) | 13.7 (7.3) |
| CRP level, mean (SD) (mg/dl) | 3.0 (2.4) | 3.0 (2.7) | 3.0 (2.3) |
| HAQ score, mean (SD) | 1.18 (0.64) | 1.18 (0.65) | 1.21 (0.68) |
| DAS28, mean (SD) | 6.2 (1.0) | 6.2 (1.0) | 6.2 (0.8) |
| Total Sharp score, median (IQR) | 28.0 (9.0, 77.5)a | 32.2 (12.0, 62.4)b | 38.3 (11.0, 73.8) |
| Total Sharp score, mean (SD) | 49.6 (53.7)a | 47.4 (52.3)b | 51.9 (47.1) |
Tender joint count: sixty-eight joints were assessed. Swollen joint count: sixty-six joints were assessed. HAQ score: scores can range from 0 (no difficulty) to 3 (unable to perform this activity). Total Sharp score: scores can range from 0 to 390 (erosion score: 0–230, and joint space narrowing score: 0–160), with high scores indicating more joint damage
CRP C-reactive protein, HAQ Health Assessment Questionnaire; DAS28 disease activity score in 28 joints; IQR interquartile range
an = 98
bn = 103
Two hundred seventy-two patients (88.6%) of the 307 patients completed this study. The main reason for discontinuation was adverse events, and there was no significant difference among all treatment groups.
Clinical response and improvement in physical function
ACR-N at week 54 in the 10 mg/kg group, the primary endpoint, was significantly higher (p = 0.024) than that in the 3 mg/kg group (Table 2). The ACR20, ACR50, and ACR70 responses at week 54 were 75.8%, 60.6%, and 37.4% in the 3 mg/kg group, 78.8%, 58.7%, and 42.3% in the 6 mg/kg group, and 82.7%, 66.3%, and 43.3% in the 10 mg/kg group, respectively, with no significant difference. There were significant differences in the reduction in DAS28 change and EULAR responses between the 3 and 10 mg/kg groups. No significant difference was observed in the proportions of patients achieving remission (DAS28 < 2.6) between the two groups.
Table 2.
Clinical efficacy of high-dose infliximab therapy in RA patients from baseline to week 54
| 3 mg/kg (n = 99) |
6 mg/kg (n = 104) |
10 mg/kg (n = 104) |
|
|---|---|---|---|
| Reducing signs and symptoms | |||
| ACR-N, mean (SD), % | 51.3 (32.1) | 53.8 (34.4) | 58.3 (31.3)* |
| Reduction in DAS28, mean (SD) | 2.30 (1.56) | 2.57 (1.69) | 2.80 (1.58)** |
| EULAR response, no. (%) | |||
| Moderate or good response | 78 (78.8) | 87 (83.7) | 94 (90.4)** |
| Good response | 37 (37.4) | 52 (50.0)* | 52 (50.0)* |
| DAS28 remission (DAS28 < 2.6), no. (%) | 25 (25.3) | 34 (32.7) | 34 (32.7) |
| Improving physical function | |||
| Improvement in HAQ score, mean (SD) | 0.48 (0.70) | 0.56 (0.64) | 0.59 (0.63) |
| Rates of clinically meaningful improvement, no. (%) | 69 (69.7) | 75 (72.1) | 76 (73.1) |
Clinically meaningful improvement was defined as an improvement in HAQ score >0.22
ACR-N numeric ACR response
*p < 0.05 versus 3 mg/kg group, **p < 0.01 versus 3 mg/kg group
Improvement in the HAQ score and the rate of patients with >0.22 units improvement were more marked in the 10 mg/kg groups than in the 3 mg/kg group, although there was no significant difference.
In the 6 mg/kg group, clinical responses and the improvement in physical function were intermediate between the 3 and 10 mg/kg groups.
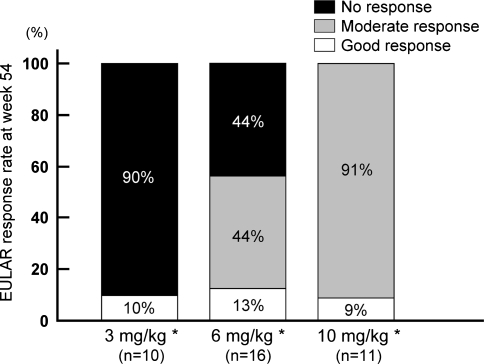
Figure 2 showed the EULAR responses at week 54 in patients with EULAR no responses to three infusions with 3 mg/kg at week 10 (n = 37). The rate of responders (good or moderate response) at week 54 for 3 mg/kg was only 10%, while it was 56% and 100% for 6 and 10 mg/kg, respectively, with significant differences (p < 0.001, overall).
Fig. 2.
Clinical response at week 54 in each group according to EULAR response criteria in nonresponders at week 10 to three infusions with 3 mg/kg. *p < 0.001, overall
Radiographic progression
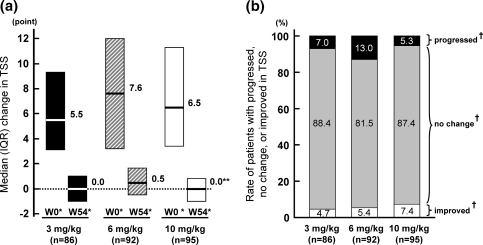
The median changes of TSS at week 54 were 0.0 in the 3 and 10 mg/kg groups (Fig. 3); the progression of joint damage was inhibited in most of the patients. There were no significant differences between the two groups. In the 6 mg/kg group, the median change in TSS was 0.5, significantly different to that at 10 mg/kg group. This was possibly associated with the finding that the most rapid yearly progression of joint damage was in the 6 mg/kg group. The percentages of patients with no progression of joint damage (improved or no change) in the 3, 6, and 10 mg/kg groups were 93.0%, 87.0%, and 94.7%, respectively. There was no significant difference among these groups.
Fig. 3.
Progression of joint damage in each group according to total modified Sharp score (TSS) at week 54: median (IQR) change score in TSS (a), and the rate of patients with progression, no change or improved in TSS (b). †Radiographic progression was categorized in TSS as follows: progressed (>4.1), no change (≥−4.1 and ≤4.1), and improved (<−4.1). W0*: Estimated yearly progression of TSS before infliximab therapy. W54*: Progression of TSS from baseline to week 54. **p = 0.022 versus 6 mg/kg group
Association between trough serum infliximab level and clinical response or radiographic progression
To explore the usefulness of higher doses of infliximab, the relationship between trough serum infliximab level and the magnitude of response was evaluated. The median (interquartile range, IQR) trough serum levels at week 54 in the 3, 6, and 10 mg/kg groups were 0.4 (<0.1, 1.5), 2.3 (0.3, 4.7), and 5.5 (1.5, 9.0) µg/ml, respectively, showing dose dependency.
As shown in Table 3, a significant association was observed between clinical response and trough serum infliximab levels at week 54. Better EULAR response was obtained in patients with higher trough serum infliximab levels (p < 0.0001). Furthermore, patients achieving remission also had significantly higher trough serum levels than patients without remission (p < 0.0001).
Table 3.
Serum trough level of infliximab in patients who showed efficacy of infliximab
| Trough serum infliximab level, median (IQR), µg/ml | p value (overall) | |
|---|---|---|
| EULAR response | ||
| No response (n = 31) | <0.1 (<0.1, 0.3) | |
| Moderate response (n = 106) | 1.1 (<0.1, 3.6) | <0.0001 |
| Good response (n = 134) | 3.0 (1.5, 7.2) | |
| DAS28 remission (DAS28 < 2.6) | ||
| No remission (n = 182) | 1.0 (<0.1, 3.7) | <0.0001 |
| Remission (n = 89) | 3.1 (1.5, 7.1) | |
| Radiographic progression | ||
| Progressed (n = 23) | 0.5 (<0.1, 2.1) | |
| No change (n = 231) | 2.0 (0.1, 5.4) | 0.0022 |
| Improved (n = 16) | 3.8 (1.6, 6.7) | |
Blood samples were obtained at week 54. Serum infliximab level were quantified by ELISA
Radiographic progression was categorized by total modified Sharp score as follows: progressed (>4.1), no change (≥−4.1 and ≤4.1), and improved (<−4.1)
Significant differences were observed among trough serum infliximab levels at week 54 in patients classified as progressed, no change or improved in joint damage (p = 0.0022). Overall, the proportion of patients showing a good response increased with increasing trough serum infliximab level.
On the other hand, we classified the patients into four groups based on the trough serum level at week 54 (<0.1, ≥0.1 and <1.0, ≥1.0 and <10, and ≥10.0 µg/ml), and examined the EULAR response and the TSS change in each group (Table 4). Median (IQR) estimated yearly progression of TSS before infliximab therapy in each group (<0.1, ≥0.1 and <1.0, ≥0.1 and <1.0, and ≥10.0 µg/ml) was 5.7 (3.6, 10.0), 6.7 (2.1, 13.0), 7.2 (3.4, 12.0), and 4.8 (2.6, 7.9), and there was no significant difference among these groups. The proportion of patients assessed as having no EULAR response decreased with increasing trough serum level, and there were no patients who showed no response when the trough serum level was 10.0 µg/ml or more. Overall, the proportion of patients showing good response increased with increasing trough serum level. There was a significant correlation between trough serum level and DAS28 remission as well as EULAR response (p < 0.0001).
Table 4.
Magnitude of efficacy in relation to different trough serum infliximab levels
| Trough serum infliximab level | p value (overall) | ||||
|---|---|---|---|---|---|
| <0.1 µg/ml | ≥0.1 and <1.0 µg/ml | ≥1.0 and <10 µg/ml | ≥10 µg/ml | ||
| EULAR response | |||||
| Total (n = 271) | 66 (100) | 40 (100) | 143 (100) | 22 (100) | |
| No response (n = 31) | 21 (31.8) | 8 (20.0) | 2 (1.4) | 0 (0.0) | |
| Moderate response (n = 106) | 31 (47.0) | 20 (50.0) | 50 (35.0) | 5 (22.7) | <0.0001 |
| Good response (n = 134) | 14 (21.2) | 12 (30.0) | 91 (63.6) | 17 (77.3) | |
| DAS28 remission | |||||
| Total (n = 271) | 66 (100) | 40 (100) | 143 (100) | 22 (100) | |
| No remission (n = 182) | 58 (87.9) | 34 (85.0) | 80 (55.9) | 10 (45.5) | <0.0001 |
| Remission (n = 89) | 8 (12.1) | 6 (15.0) | 63 (44.1) | 12 (54.5) | |
| Radiographic progression | |||||
| Total (n = 270) | 65 (100) | 40 (100) | 143 (100) | 22 (100) | |
| Progressed (n = 23) | 9 (13.8) | 4 (10.0) | 10 (7.0) | 0 (0.0) | |
| No change (n = 231) | 56 (86.2) | 33 (82.5) | 122 (85.3) | 20 (90.9) | 0.0043 |
| Improved (n = 16) | 0 (0.0) | 3 (7.5) | 11 (7.7) | 2 (9.1) | |
Values are the number (%) of patients
Blood samples were obtained at week 54. Serum infliximab levels were quantified by ELISA. Patients were grouped according to four different ranges of trough serum infliximab level as shown
Radiographic progression was categorized in change of total modified Sharp score as follows: progressed (>4.1), no change (≥−4.1 and ≤4.1), and improved (<−4.1)
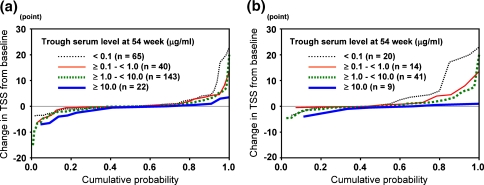
Progression of joint damage was most frequently observed in patients with <0.1 µg/ml trough serum level, and none of these patients showed improvement. In contrast, there was no case with progression of joint damage in patients with >10.0 µg/ml trough serum level. There was also a negative correlation between progression of joint damage and trough serum level (p = 0.0043). The change of TSS as a cumulative probability plot showed that inhibition of progression of joint damage was more accurately predicted by an increase in trough serum level (Fig. 4a). This tendency was more remarkable in early RA patients whose duration of disease was less than 3 years (Fig. 4b) [4, 13, 21]. In patients with early RA and with <0.1 µg/ml trough serum level, the percentage of the progressed category was 35.0%.
Fig. 4.
Cumulative probability plot of the total modified Sharp score (TSS) in relation to trough serum infliximab level at week 54 in all patients (a, n = 270), and in early RA patients (b, n = 84). Patients were grouped according to four different ranges of trough serum infliximab levels as shown
Safety profile
There was no significant difference in the incidence of adverse events or serious adverse events among the groups (Table 5). The incidences of adverse events leading to discontinuation or serious infections also showed no significant difference. The main adverse events classified using the system organ classes (SOCs) were: laboratory tests (70.2–73.7%), infections and infestations (46.2–53.8%), and skin and subcutaneous tissue disorders (21.2–32.3%). The types of adverse events in the groups administered 6 and 10 mg/kg were similar to those in the 3 mg/kg group (data not shown). No patient died over the entire study period.
Table 5.
Incidence of adverse events (AEs)
| All periods (0–54 weeks) | Open-label period (0–14 weeks) | Double-blind period (14–54 weeks) | |||
|---|---|---|---|---|---|
| 3 mg/kg | 6 mg/kg | 10 mg/kg | |||
| (n = 327) | (n = 327) | (n = 99) | (n = 104) | (n = 104) | |
| Any AEs | 319 (97.6) | 242 (74.0) | 97 (98.0) | 97 (93.3) | 101 (97.1) |
| Serious AEsa | 38 (11.6) | 17 (5.2) | 7 (7.1) | 5 (4.8) | 9 (8.7) |
| AEs leading to discontinuation of study agents | 39 (11.9) | 19 (5.8) | 7 (7.1) | 9 (8.7) | 5 (4.8) |
| Infections | 230 (70.3) | 124 (37.9) | 56 (56.6) | 57 (54.8) | 67 (64.4) |
| Serious infections | 17 (5.2) | 7 (2.1) | 3 (3.0) | 2 (1.9) | 5 (4.8) |
| Infections leading to discontinuation of study agents | 12 (3.7) | 6 (1.8) | 3 (3.0) | 3 (2.9) | 0 (0.0) |
| Infusion reactionsb | 92 (28.1) | 41 (12.5) | 17 (17.2) | 25 (24.0) | 23 (22.1) |
| Serious infusion reactions | 1 (0.3) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Values are the number (%) of patients
aAny AEs that resulted in any life-threatening events, inpatient hospitalizations, prolongation of existing hospitalization or significant disability/incapacity
bAny AEs that occurred during an infusion or within 2 h after completion of an infusion
Discussion
Some pivotal clinical studies, such as the ATTRACT study, have demonstrated that infliximab treatment brought about a reduction in signs and symptoms, inhibition of the progression of joint damage, and improvement of physical function in patients with RA. It was reported that this treatment was very effective in Japanese RA patients [7, 22, 23]. However, it has been noted that infliximab is not sufficiently efficacious in some patients because the approved dosage is only 3 mg/kg every 8 weeks in Japan. The RISING study was performed to investigate the efficacy and safety of treatment of RA with infliximab, comparing 10 mg/kg with 3 mg/kg.
The ACR-N, the primary end point of this study, was 58.3% in the 10 mg/kg group and 51.3% in the 3 mg/kg group, representing a significant difference. In addition, as regards change in DAS28 and EULAR response criteria, significantly higher responses were observed in the 10 mg/kg group compared with the 3 mg/kg group. These results are the first evidence of better clinical responses at 10 mg/kg in comparison with 3 mg/kg in a double-blind study. This was possibly because we employed the ACR-N and DAS28 change, which indicate the magnitude of improvement, and the EULAR response, which reflects actual disease activity, whereas the ACR responses, which represent categorical criteria for improvement, were used in the ATTRACT study. In addition, when we focused on nonresponders at 10 weeks, EULAR response rates at week 54 were significantly increased in the high-dose groups compared with those in 3 mg/kg group.
The ATTRACT trial and the Safety Trial for Rheumatoid Arthritis with Remicade (infliximab) Therapy (START) have already demonstrated a significant association between clinical response and trough serum infliximab level [24, 25]. In the RISING study, a significant correlation was observed between serum trough level and EULAR response or DAS28 remission. The median trough serum level at week 54 in EULAR nonresponders was <0.1 µg/ml, and that in EULAR responders (good or moderate response) was 1.1 µg/ml, which was consistent with that reported in the ATTRACT and START studies. This suggests that a trough serum level of 1.0 µg/ml is the threshold level for clinical response, as reported previously.
Infliximab treatment inhibited the progression of joint damage in most of patients regardless of infliximab dose in the RISING study. It is interesting to note that infliximab inhibited progression of joint damage even in patients receiving low dose of MTX. A significant correlation between progression or improvement of joint damage and trough serum infliximab level, which had not been investigated sufficiently so far, was also shown, raising the possibility that joint damage might progress in patients with a low trough serum level. This correlation was more remarkable in early RA patients in whom joint destruction progresses rapidly. The factors that influenced the trough serum infliximab level were thought to be the serum clearance of infliximab as well as production of anti-infliximab antibodies [26]. This study showed the significant association between serum trough level and clinical responses or radiographic progression. It appears that anti-infliximab antibodies may be one of the important factors that influence the efficacies of infliximab therapy.
In the RISING study, the safety profile of the high-dose groups was similar to that of the 3 mg/kg group, and no significant difference was found in the incidence of adverse events or serious adverse events including infections. However, the START study showed that the incidence of serious infections in the 10 mg/kg group increased in the 22-week period after the start of treatment [5]. It was reported that bacterial pneumonia (a major serious infection) was commonly observed in the early phase of the treatment (until week 14) in the Japanese post-marketing surveillance (PMS) of 5,000 patients [27]. It should be noted that the three induction infusions at 0, 2, and 6 weeks in the RISING study used a 3 mg/kg dose for all three dosing groups, whereas in the previous reports the same dose was used throughout the entire study period, including the induction phase. The different dose in the induction phases of these studies might account for the different results regarding serious adverse events. It is very important to examine the association between trough serum infliximab level and safety. However, the time of occurrence of adverse events varied in every patient, and we did not measure the serum level at week 54 in patients who discontinued infliximab treatment because of adverse events. Therefore, we were unable to investigate the association between them. If there is a significant association between infliximab serum level and safety, the efficacy should be higher in the discontinued patients than in the patients who continued with infliximab therapy because the efficacy is dependent on the infliximab level. However, the ACR-N in the discontinued patients before discontinuation was lower than that of the continued patients at week 54. This means that the serum infliximab level of the former patients was lower than that of the latter. Furthermore, no significant difference of the rate of adverse events or infections was observed between the 3, 6, and 10 mg/kg groups in this study. These results may indicate the possibility that serum trough infliximab level does not influence the safety of infliximab therapy. Further investigation on this matter is considered to be necessary.
The RISING study showed that infliximab treatment with 3 mg/kg resulted in a good clinical response in many patients, while treatment with 10 mg/kg demonstrated a statistically significant advantage compared with 3 mg/kg in the efficacy outcomes. What is noted in this study was that infliximab treatment with 10 mg/kg was found beneficial in those patients who had not responded to three infusions of infliximab with 3 mg/kg at week 10. It is clinically relevant to escalate the dose of infliximab when patients do not respond to 3 mg/kg.
It was also suggested that higher trough serum infliximab levels by dose escalation of infliximab provided better clinical responses and greater inhibition of progression of joint damage.
Acknowledgments
This study was funded by Mitsubishi Tanabe Pharma Corporation, Osaka, Japan.
The authors wish to thank all the investigators participated in the RISING study:
F. Hirano, K. Taneichi, T. Atsumi, K. Ohnishi, H. Takahashi, Hokkaido; N. Chiba, Iwate; T. Sasaki, Miyagi; K. Chiba, T. Kanno, Fukushima; S. Ohta, T. Sumida, Ibaraki; T. Yoshio, K. Kurasawa, Tochigi; Y. Handa, T. Mimura, Saitama; R. Matsumura, N. Nakagawa, N. Watanabe, Chiba; K. Saigo, M. Takei, T. Suguro, T. Kasama, M. Hirakata, Y. Kuga, A. Nakajima, K. Yamaji, K. Yamamoto, E. Saito, H. Yamagata, M. Tateishi, S. Sawada, N. Kamatani, H. Yamanaka, M. Nakajima, Tokyo; S. Toma, H. Endo, Kanagawa; T. Kuroda, Niigata; S, Honjo, Toyama; I. Koni, M. Kawano, S. Nakazaki, Ishikawa; K. Sugimoto, Fukui; K. Kanzaki, Yamanashi; T. Kanamono, A. Suzuki, Nagano; T. Ohashi, Y. Kusaka, Gifu; H. Ohashi, Y. Izui, N. Ogawa, T. Miyamoto, Shizuoka; N. Ishiguro, Y. Eto, S. Yoshida, Aichi; S. Tamaki, Mie; T. Fujii, M. Tanaka, Kyoto; M. Inaba, T. Koike, Y. Shimaoka, T. Takeuchi, A. Ogata, Y. Saeki, S. Osawa, K. Shi, Osaka; S. Kumagai, T. Nishiyama, H. Sano, T. Matsubara, Hyogo; Y. Murakawa, Shimane; M. Yamamura, K. Ezawa, Okayama; Y. Yamanishi, S. Yamana, Hiroshima; H. Tanaka, Yamaguchi; K. Tani, Tokushima; M. Tokuda, H. Dobashi, M. Inoo, Kagawa; S. Nakata, K. Takasugi, Ehime; I. Furugou, Y. Tanaka, K. Saito, T. Horiuchi, T. Shuto, Y. Nakashima, H. Miyahara, M. Kondo, Fukuoka; K. Eguchi, Y. Ueki, Nagasaki; M. Tsukano, Kumamoto; Y. Fujikawa, Oita; T. Matsuda, Kagoshima; Y. Shiohira, Okinawa.
Conflict of interest statement All of the authors have received consulting fees from Mitsubishi Tanabe Pharma Corporation, Osaka, Japan.
Footnotes
K. Inoue: deceased.
References
- 1.Wolfe F, Hawley DJ. The longterm outcomes of rheumatoid arthritis: work disability: a prospective 18 year study of 823 patients. J Rheumatol. 1998;25:2108–17. [PubMed]
- 2.Maini RN, Breedveld FC, Kalden JR, Smolen J, Davis D, MacFarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–63. [DOI] [PubMed]
- 3.Lipsky PE, van der Heijde DM, St Clair EW, Furst DE, Breedveld FC, Kalden JR, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med. 2000;343:1594–602. [DOI] [PubMed]
- 4.St Clair EW, van der Heijde DM, Smolen JS, Maini RN, Bathon JM, Emery P, et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 2004;50:3432–43. [DOI] [PubMed]
- 5.Westhovens R, Yocum D, Han J, Berman A, Strusberg I, Geusens P, et al. The safety of infliximab, combined with background treatments, among patients with rheumatoid arthritis and various comorbidities: a large, randomized, placebo-controlled trial. Arthritis Rheum. 2006;54:1075–86. [DOI] [PubMed]
- 6.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381–90. [DOI] [PubMed]
- 7.Abe T, Takeuchi T, Miyasaka N, Hashimoto H, Kondo H, Ichikawa Y, et al. A multicenter, double-blind, randomized, placebo controlled trial of infliximab combined with low dose methotrexate in Japanese patients with rheumatoid arthritis. J Rheumatol. 2006;33:37–44. [PubMed]
- 8.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50:1051–65. [DOI] [PubMed]
- 9.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. [DOI] [PubMed]
- 10.Steinbrocker O, Traeger CH, Batterman RC. Therapeutic criteria in rheumatoid arthritis. JAMA. 1949;140:659–62. [DOI] [PubMed]
- 11.Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–35. [DOI] [PubMed]
- 12.Schiff M, Weaver A, Keystone E, Moreland L, Spencer-Green G. Comparison of ACR response, numeric ACR and ACR AUC as measures of clinical improvement in RA clinical trials. Arthritis Rheum. 1999;42(Suppl):S81.
- 13.Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, Keystone EC, et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med. 2000;343:1586–93. [DOI] [PubMed]
- 14.Prevoo ML, van’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. [DOI] [PubMed]
- 15.van Riel PL, van Gestel AM, van de Putte LB. Development and validation of response criteria in rheumatoid arthritis: steps towards an international consensus on prognostic markers. Br J Rheumatol. 1996;35(Suppl 2):4–7. [DOI] [PubMed]
- 16.Sharp JT, Lidsky MD, Collins LC, Moreland J. Methods of scoring the progression of radiologic changes in rheumatoid arthritis: correlation of radiologic, clinical and laboratory abnormalities. Arthritis Rheum. 1971;14:706–20. [DOI] [PubMed]
- 17.van der Heijde DMFM, van Leeuwen MA, van Riel PLCM, Koster AM, van ‘t Hof MA, van Rijswijk MH, et al. Biannual radiographic assessments of hands and feet in the three-year prospective followup of patients with early rheumatoid arthritis. Arthritis Rheum. 1992;35:26–34. [DOI] [PubMed]
- 18.Lassere M, Boers M, van der Heijde D, Boonen A, Edmonds J, Saudan A, et al. Smallest detectable difference in radiological progression. J Rheumatol. 1999;26:731–9. [PubMed]
- 19.Fries JF, Spitz PW, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45. [DOI] [PubMed]
- 20.Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient’s perspective. J Rheumatol. 1993;20:557–60. [PubMed]
- 21.Breedveld FC, Emery P, Keystone E, Patel K, Furst DE, Kalden JR, et al. Infliximab in active early rheumatoid arthritis. Ann Rheum Dis. 2004;63:149–55. [DOI] [PMC free article] [PubMed]
- 22.Tanaka Y, Takeuchi T, Inoue E, Saito K, Sekiguchi N, Sato E, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan: one-year clinical outcomes (RECONFIRM-2). Mod Rheumatol. 2008;18:146–52. [DOI] [PMC free article] [PubMed]
- 23.Takeuchi T, Yamanaka H, Inoue E, Nagasawa H, Nawata M, Ikari K, et al. Retrospective clinical study on the notable efficacy and related factors of infliximab therapy in a rheumatoid arthritis management group in Japan: one-year outcome of joint destruction (RECONFIRM-2J). Mod Rheumatol. 2008;18:447–54. [DOI] [PubMed]
- 24.St Clair EW, Wagner CL, Fasanmade AA, Wang B, Schaible T, Kavanaugh A, et al. The relationship of serum infliximab concentrations to clinical improvement in rheumatoid arthritis: results from ATTRACT, a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:1451–9. [DOI] [PubMed]
- 25.Rahman MU, Strusberg I, Geusens P, Berman A, Yocum D, Baker D, et al. Double-blinded infliximab dose escalation in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:1233–8. [DOI] [PMC free article] [PubMed]
- 26.Svenson M, Geborek P, Saxne T, Bendtzen K. Monitoring patients treated with anti-TNF-α biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodies. Rheumatology. 2007;46:1828–34. [DOI] [PubMed]
- 27.Takeuchi T, Tatsuki Y, Nogami Y, Ishiguro N, Tanaka Y, Yamanaka H, et al. Postmarketing surveillance of the safety profile of infliximab in 5000 Japanese patients with rheumatoid arthritis. Ann Rheum Dis. 2008;67:189–94. [DOI] [PubMed]