Abstract
Vitamin D is now known to be of physiological importance outside of bone health and calcium homeostasis, and there is mounting evidence that it plays a beneficial role in the prevention and/or treatment of a wide range of diseases. In this brief review the known effects of vitamin D on immune function are described in relation to respiratory health. Vitamin D appears capable of inhibiting pulmonary inflammatory responses while enhancing innate defence mechanisms against respiratory pathogens. Population-based studies showing an association between circulating vitamin D levels and lung function provide strong justification for randomized controlled clinical trials of vitamin D supplementation in patients with respiratory diseases to assess both efficacy and optimal dosage.
Keywords: inflammation, immune function, lung, vitamin D
Introduction
1,25-dihydroxyvitamin D3[1,25(OH)2D3] is the biologically active form of vitamin D, which is produced predominantly from precursors within the skin through the action of ultraviolet B (UVB) radiation on 7-dehydrocholesterol [1], hence its nickname ‘the sunshine vitamin’. To a lesser extent, vitamin D can be sourced in the diet from foods such as fortified dairy products and cereals, oily fish and fish liver oils [1]. Synthesized or dietary vitamin D, from the skin and the gut, respectively, are hydroxylated in the liver to form 25-hydroxyvitamin D [25(OH)D] through the action of cytochrome P450 enzymes, as shown in Fig. 1[2]. 25(OH)D is the major circulating form of vitamin D and serum levels are measured as being indicative of an individual's ‘vitamin D status’. Serum 25(OH)D concentrations of 10 ng/ml (25 nmol/l) have long been considered the cut-off for defining the lower limit of adequacy (in terms of preventing rickets), but there is a growing consensus that a serum concentration of > 30 ng/l (> 75 nmol/l) is more appropriate to define physiologically optimal concentrations, associated with many other health benefits [3,4]. In the absence of adequate sun exposure, at least 800–1000 IU (20–25 µg) vitamin D per day may be needed to achieve this [5].
Fig. 1.
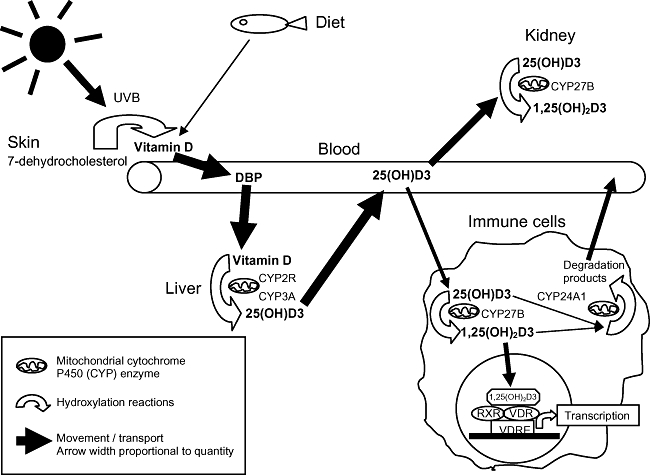
Simplified version of vitamin D metabolism. Vitamin D, produced mainly from ultraviolet B (UVB) action on the skin, but also available from the diet, binds vitamin D binding protein (DBP) and is transported to the liver where different mitochondrial cytochrome P450 enzymes (CYP) hydroxylate it to form 25(OH)D. This is transported by DBP predominantly to the kidneys, where CYP enzymes hydroxylate it to the active form, 1,25(OH)2D3. However, immune cells also have the CYP enzymes required to activate 1,25(OH)2D3. Being lipophilic, 1,25(OH)2D3 is able to traverse the cell membrane and act within the cell by binding to vitamin D receptors (VDR) in the nucleus. VDR are ligand-activated transcription factors that interact with vitamin D response elements (VDRE) on vitamin D-regulated genes either as homodimers or heterodimers with the retinoid X receptors (RXR). Vitamin D is also regulated by CYP enzymes which inactivate calcitriol into water-soluble derivatives. Vitamin D binding to the VDR enhances CYP24A1 and down-regulates CYP27B1, which is an important negative feedback mechanism regulating vitamin D levels. For more detail refer to [7].
In northern latitudes, between November and March, there are insufficient UVB photons reaching the earth's surface to enable vitamin D synthesis and there is a noticeable decrease in individuals' vitamin D status in these regions during winter. In a study of middle-aged adults in the United Kingdom, 40% had serum 25(OH)D concentrations above 30 ng/l in the summer months but this fell to less than 13% in the winter, while 15% had insufficient levels (< 10 ng/l) during this season [6]. Many other factors can influence the amount of vitamin D that can be synthesized cutaneously from sunlight: darker skin pigmentation, the use of sunscreen, wearing of clothing that completely covers the skin or spending the majority of time indoors all limit the amount of UV light that penetrates the skin. Ageing is associated with decreased concentrations of 7-dehydrocholesterol in the skin, thereby reducing the capacity to synthesize vitamin D, and the use of glucocorticoids are another common cause of vitamin D deficiency [5].
Vitamin D regulates more than 200 genes including genes for cellular proliferation differentiation and apoptosis [7]. Study of the physiological importance of vitamin D is a field which is expanding rapidly, with deficiencies of vitamin D giving an insight into many new roles. In particular, vitamin D deficiency is now associated with an increased risk of certain cancers and a number of autoimmune and infectious diseases [5]. In addition, a recent meta-analysis of randomized controlled trials concluded that use of vitamin D supplements is associated with a decrease in total mortality rates [8]. Specifically in respiratory health, vitamin D deficiency has been shown to increase the risk of upper respiratory tract infections and tuberculosis and to decrease the forced expiratory volume in 1 s (FEV1) in asthma and wheezing diseases [5]. Interest in this area has grown further following the results of a large population survey in the United States, which observed a dose–response relationship between vitamin D status and FEV1[9].
Vitamin D as an immunomodulator
Conventionally, vitamin D is known for its actions in bone mineralization and calcium homeostasis. However, there is now extensive evidence supporting its actions in immunity and inflammation. The discovery of the expression of nuclear vitamin D receptors (VDR) and hydroxylase enzymes by immune cells has led to a surge of research into the potential role of vitamin D in maintaining immune homeostasis and preventing the development of autoimmune processes.
Antigen-presenting cells, essential for the initiation and maintenance of cell-mediated immune responses, can be inhibited directly by vitamin D. The expression of major histocompatibility complex (MHC) class II and co-stimulatory receptors is inhibited, as is the differentiation of monocytes to dendritic cells [10]. Inflammatory cytokine expression [e.g. interleukin (IL)-1α, IL-1β, tumour necrosis factor (TNF)-α] is also inhibited and the vitamin D-induced inhibition of IL-12 release by dendritic cells has a profound effect on T lymphocyte differentiation [11]. IL-12 stimulates the development of T helper type 1 (Th1) lymphocytes and inhibits the development of Th2 lymphocytes. Vitamin D is associated with a dose-dependent reduction in transcription of Th1 cytokines such as IL-2, granulocyte–macrophage colony-stimulating factor (GM–CSF) and interferon (IFN)-γ, and increased expression of the Th2 cytokines IL-4, -5 and -10 [1]. Conversely, vitamin D insufficiency deregulates the balance between types 1 and 2 responses, leading to overexpression of Th1 cytokines. Vitamin D also has potent anti-proliferative effects on T cells, principally T helper cells, and suppresses B cell antibody production both directly and indirectly in vitro[12,13].
Inhibition of IL-12 expression by 1,25(OH)2D3 has been shown to be achieved by interfering with the nuclear factor kappa B (NF-κB) pathway, a key transcription factor for the induction of inflammatory cytokines. Activation and binding of NF-κB to the NF-κB binding site within the promoter of the p40 subunit of IL-12 are down-regulated by 1,25(OH)2D3[11]. Paradoxically, however, 1,25(OH)2D3 can also activate NF-κB by stimulating inhibitor kappa B (IκB) phosphorylation/degradation [14]. A possible explanation for these contradictory findings is the recent observation that 1,25(OH)2D3 can exert a biphasic regulation of NF-κB, combining an early suppressive effect followed by a prolonged reactivation of NF-κB [15].
Perhaps one of the most important modulatory actions of 1,25(OH)2D3 is its effect on regulatory T cells (Tregs) which prevent the activation of peripheral autoreactive T cells. In the absence of 1,25(OH)2D3 the numbers and functions of Tregs are reduced, potentially contributing to the development of autoimmune diseases, such as multiple sclerosis and type 1 diabetes, where low vitamin D status is associated with an increased risk of developing these disorders [16,17]. The role of vitamin D in autoimmune diseases has been reviewed recently [18].
In addition to immune cells, respiratory epithelial cells can also constitutively convert inactive 25(OH)D to 1,25(OH)2D3, enabling high local concentrations of active vitamin D to increase the expression of vitamin D-regulated genes with important innate immune functions [19].
Vitamin D and respiratory infections
Tuberculosis
Exposure to sunlight has been known for more than 100 years to help with the treatment of tuberculosis [5], although the first indicator of vitamin D having anti-microbial activity against Mycobacterium tuberculosis was from studies in the 1980s, where adding vitamin D to monocytes and macrophages infected with M. tuberculosis showed that the bacterial load was reduced [20].
More than 60 years ago clinical studies were carried out administering oral vitamin D as a treatment for mycobacterial infections with high success rates [13]. However, following the dawn of antibiotic therapy this seems to have been largely overlooked, but it is very likely that improving the vitamin D status of these patients would be beneficial. A recent meta-analysis found that low serum 25(OH)D levels are associated with higher risk of active tuberculosis [21], and several studies have associated low serum 25(OH)D levels with increased susceptibility to tuberculosis and disease progression [20]. In addition, a recent study of young Finnish men serving on a military base observed an association between low vitamin D status and days of absence from duty due to physician-diagnosed acute respiratory tract infections [3].
Tuberculosis patients administered vitamin D or placebo following the sixth week of standard tuberculosis treatment had higher sputum conversion and radiological improvement (100%) compared to a placebo group (76·7%) in the same study [22]. Addition of 1,25(OH)2D3 to primary human macrophages infected with virulent M. tuberculosis reduced the number of viable bacilli [23]. Adding a single oral dose (2·5 mg) of vitamin D to the treatment regimen of patients with tuberculosis enhanced significantly the ability of the participants' whole blood to restrict growth of mycobacteria in vitro without affecting antigen-stimulated IFN-γ responses [24].
The immune system is able to detect invading pathogens such as M. tuberculosis via pathogen-associated molecular patterns (PAMPs); structural proteins expressed by the pathogen which are detected by Toll-like receptors (TLRs) in the host. PAMPs shed from M. tuberculosis interact with the TLR2/1 dimer on macrophages, resulting in the up-regulation of both CYP27b1 and VDR [7,25]. It has been shown recently that IL-15 is responsible for the induction of CYP27b1, leading to bioconversion of 25(OH)D to 1,25(OH)2D3, VDR activation and induction of cathelicidin [26]. The cathelicidin gene encodes an anti-microbial peptide, LL-37, and this gene, in humans (but not in mice), contains a vitamin D response element. Therefore binding of vitamin D leads to LL-37-mediated killing of M. tuberculosis[13]. The cathelicidin gene has been found to be expressed in respiratory epithelial cells [19] and vitamin D induction of cathelicidin has been shown in a number of cell lines including bronchial epithelial cells [12].
Influenza and the ‘common cold’
Influenza A virus causes severe epidemics of respiratory illness in humans and is transmitted through airborne droplets and by direct contact. It is characterized by acute neutrophil infiltration and narrowing of the bronchioles [27]. Controversy remains concerning whether there is a direct link between the seasonality of influenza and vitamin D deficiency, which is also observed more commonly in winter [28].
Influenza infection involves both innate and adaptive arms of the immune system. Although vitamin D can inhibit proinflammatory cytokine release by macrophages, its ability to up-regulate the expression of anti-microbial peptides is relevant, as these peptides can also exhibit anti-viral activity. In addition, viral infection increases activation of vitamin D and increases cathelicidin production further [19]. Not only do immune cells secrete these anti-microbial peptides, but epithelial cells present in the upper and lower airways can also secrete them as a host defence mechanism against infection [28].
Upper respiratory tract infections (URTI), or ‘common colds’, are the most widespread of infectious diseases, with more than 200 viruses contributing to the clinical symptoms. Early epidemiological studies found a strong association between rickets and RTI [29], and a recent large cross-sectional study of the US population reported that vitamin D status is associated inversely with recent URTI and that the association may be stronger in those with respiratory diseases, such as asthma [30]. Randomized controlled trials are needed to examine the direct effect of vitamin D supplementation and to establish the optimal serum levels of 25(OH)D to aid prevention of RTI.
Cystic fibrosis
Cystic fibrosis is a hereditary disease which not only causes mucus hypersecretion within the lungs and resulting airway obstruction and inflammation, but also affects other systems including pancreatic secretions. Due to the increase in mucus, patients are prone to frequent infections and reduced pancreatic secretions result in patients having problems with malabsorption of fat-soluble vitamins such as vitamin D.
Several studies have shown that patients with cystic fibrosis have reduced circulating levels of 25(OH)D despite supplementation [31], suggesting that either higher than normal levels of supplementation are needed to increase serum concentrations or that there is insufficient conversion of the dietary vitamin D to 25(OH)D. It has been reported recently that portable tanning devices can improve the vitamin D status of patients with cystic fibrosis (CF) during the winter months [32] but, of course, caution is required to avoid overexposure to UVB radiation.
Vitamin D binding protein (DBP), also known as group-specific component (Gc), is an α-macroglobulin belonging to the serum albumin superfamily. This is a multi-functional protein involved in bone resorption but it can also activate macrophages. A healthy range of DBP in the circulation is defined as 300–600 mg/l, and low concentrations have been reported in acute respiratory distress syndrome and in the sera of patients with CF [33,34]. There is also a higher proportion of low bone density and osteoporosis within this population group.
In the previously mentioned study showing that cathelicidin can be induced by vitamin D in primary bronchial epithelial cells, cells from patients with CF also showed increased cathelicidin expression [35], suggesting that vitamin D can augment anti-bacterial activity in airway epithelia in cystic fibrosis. This may provide a novel therapy for prevention and treatment of airways infections in this disorder.
Vitamin D and respiratory inflammation
Chronic obstructive pulmonary disease (COPD)
COPD is characterized by narrowing of the airways. It is an umbrella term for two main chronic inflammatory pathologies; chronic bronchitis, where the bronchioles narrow due to excess mucus production, and emphysema in the alveoli caused by tissue destruction. COPD is characterized by a neutrophil and macrophage-induced inflammation developing over many years, is predicted to be the third leading cause of death worldwide by 2020, and more than 90% of all cases are associated with smoking [36].
We have observed recently that the mean vitamin D status of patients with COPD in Norfolk, UK, is lower than that of the general population in winter and only one of 24 patients had plasma levels of 25(OH)D above 20 ng/l (unpublished). We are currently exploring the potentially beneficial effects of 1,25(OH)2D3 on the function of airway epithelial cells. There is an imbalance in matrix metalloproteinase (MMP) activity in sputum cells from COPD patients, including an increase in MMP-9 levels at times of acute exacerbations of COPD (acute deterioration in lung function) [37]. TNF-α increases MMP-9 [38] from alveolar macrophages from patients with COPD, while IL-10 reduces the ratio of MMP-9 to the MMP inhibitor, tissue inhibitor of metalloproteinase (TIMP)-1 [39]. Airway remodelling in COPD has been linked to the equilibrium of MMP-9 and TIMP-1 and because vitamin D can inhibit TNF-α and enhance IL-10 in immune cells from healthy individuals, we are exploring whether vitamin D acts in the same manner in cells from patients with COPD, and whether it exerts similar effects in airway epithelial cells.
As acute exacerbations in COPD are often triggered by viral or bacterial infections, the ability of vitamin D to enhance cathelicidin expression, as detailed above, might reduce pathogen load and the frequency of these exacerbations. Of note, exacerbations of COPD peak in winter, when serum 25(OH)D levels are at their lowest.
An important systemic consequence of COPD is muscle weakness, and this is associated with an increased risk of mortality. Vitamin D plays a role in influencing skeletal muscle function, with deficiency resulting in muscle weakness, and VDRs are present in this tissue [40]. It has been reported recently that polymorphisms in the VDR can influence muscle weakness in both healthy individuals and patients with COPD [41], suggesting that the VDR has a significant influence on one of the important complications of this disease. There is therefore broader potential for vitamin D to improve the quality of life of patients with this disease.
Asthma
Asthma and atopic diseases are characterized by inflammatory responses initiated and sustained by inappropriate Th lymphocyte responses of the Th2 phenotype. Direct evidence for a role of vitamin D in asthma comes from studies showing that VDR variants are a risk factor for asthma [42]. As stated above, a large cross-sectional study has shown that vitamin D intakes and serum levels are associated with lung function in adults [9] and similar findings have been reported in adolescents [43]. An inverse association between maternal intakes of vitamin D during pregnancy and early childhood wheezing has been reported in studies from the United States [44] and the United Kingdom [45]. In the latter study the association was independent of maternal smoking status, maternal intakes of vitamin E, zinc, calcium and vitamin D intake by the children. These studies raised the possibility that vitamin D may have alternate modulatory influences on immune function relevant to asthma depending on the time of exposure to it. As stated above, vitamin D is associated with skewing the immune response to a Th2 phenotype, with an increase in IL-4 expression. In contrast, others have observed that in human cord blood T cells vitamin D inhibits both IL-12-generated IFN-γ and IL-4 secretion by Th2 cells [46]. Therefore, the response to vitamin D exposure of naive T cells in the fetus or neonate may be quite different to that of mature T cells.
However, the possibility remains that excessive vitamin D intake (i.e. at levels higher than just to correct a deficiency) may potentiate Th2 responses in adult asthmatic patients and clinical trials are required to address this concern. On the other hand, the potential for vitamin D to increase pulmonary defence against respiratory infections may, in the same way as in COPD, reduce the triggering of asthma exacerbations caused by RTI [47]. This is supported by the previously mentioned particularly strong negative association between vitamin D status and URTI in individuals with asthma in the Third National Health and Nutrition Examination Survey (NHANES III) study [30].
Glucocorticoids are the most effective anti-inflammatory treatments available for many immune diseases, including asthma. However, glucocorticoid resistance or insensitivity in some patients with asthma represents an important barrier to effective treatment and accounts for significant health-care costs [48]. Recently, some evidence has emerged that administration of vitamin D to glucocorticoid-resistant asthmatic patients can enhance subsequent responsiveness to dexamethasone by restoring the defective IL-10 response to glucocorticoids by CD4+ T cells in these individuals [49]. This finding provides encouragement to undertake trials of vitamin D in overcoming glucocorticoid resistance in both asthma and a number of other inflammatory diseases [48].
In addition, in asthma there is a degree of airway remodelling with an increase in smooth muscle cell numbers. MMP-9 is the most relevant enzyme in airway remodelling and is expressed highly in patients with severe irreversible narrowing of the airways. In addition ‘a disintegrin and metalloproteinase-33’ (ADAM33) has been identified as a novel asthma susceptibility gene by genome-wide screening, and is now known to play an important role in airway remodelling. Its level of expression is associated with asthma development and severity and it declines with therapeutic interventions [42]. In vitro studies have shown that 1,25(OH)2D3 has a direct anti-proliferative effect on human airway smooth muscle cells and can inhibit the expression of both MMP-9 and ADAM33 [42], suggesting a further beneficial role for vitamin D in the prevention and treatment of asthma.
Future directions
A recent meta-analysis found that vitamin D supplementation reduces total mortality [8]. Global levels of vitamin D deficiency have been underestimated widely and there is a growing consensus that serum levels of vitamin D should be maintained above 30 ng/l (75 nmol/l). With low vitamin D status being associated with so many diseases it would seem that dietary supplementation would be a cost-effective measure to improve the general health of the population, particularly in the elderly, and in individuals who receive insufficient exposure to direct sunlight.
The future may also involve more personalized therapy, with individuals' vitamin D status measured and then supplemented accordingly, to avoid toxic hypercalcaemic effects of high-dose ‘blind’ supplementation. To allow higher doses to be given safely there has also been a great deal of pharmacological research into developing analogues of vitamin D which retain the therapeutically important properties of 1,25(OH)2D3 but with lower calcaemic activity. Additionally, ways may be found to activate CYP enzymes in specific tissues, to enhance local production of 1,25(OH)2D3, thereby inducing beneficial effects locally at the required sites.
Finally, the population-based studies showing associations between vitamin D status and lung function provide strong justification for randomized controlled clinical trials of vitamin D supplementation in patients with respiratory diseases to assess both efficacy and optimal dosage.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Etten EV, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Ebert R, Schütze N, Adamski J, Jakob F. Vitamin D signaling is modulated on multiple levels in health and disease. Mol Cell Endocrinol. 2006;248:149–59. doi: 10.1016/j.mce.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 3.Laaksi I, Ruohola J-P, Tuohimaa P, et al. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr. 2007;86:714–17. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 4.Baeke F, Ev E, Gysemans C, Overbergh L, Mathieu C. Vitamin D signaling in immune-mediated disorders: evolving insights and therapeutic opportunities. Mol Aspects Med. 2008;29:376–87. doi: 10.1016/j.mam.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–6S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 6.Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007;85:860–8. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–7. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 9.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the Third National Health and Nutrition Examination Survey. Chest. 2005;128:3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 10.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:6800–5. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Ambroxio DCM, Cocciolo MG, Mazzeo D, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–62. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams JS, Chen H, Chun R, et al. Substrate and enzyme trafficking as a means of regulating 1,25-dihydroxyvitamin D synthesis and action: the human innate immune response. J Bone Miner Res. 2007;22(Suppl. 2):V20–4. doi: 10.1359/jbmr.07s214. [DOI] [PubMed] [Google Scholar]
- 14.Berry DM, Clark CS, Meckling-Gill KA. 1,25-Dihydroxyvitamin D3 stimulates phosphorylation of IkB and synergizes with TPA to induce nuclear translocation of NF-kB during monocytic differentiation of NB4 leukemia cells. Exp Cell Res. 2002;272:176–84. doi: 10.1006/excr.2001.5410. [DOI] [PubMed] [Google Scholar]
- 15.Tse AK-W, Wan C-K, Shen X-L, et al. 1,25-Dihydroxyvitamin D3 induces biphasic NF-kB responses during HL-60 leukemia cells differentiation through protein induction and PI3K/Akt-dependent phosphorylation/degradation of IkB. Exp Cell Res. 2007;313:1722–34. doi: 10.1016/j.yexcr.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Embry AF, Snowdon LR, Vieth R. Vitamin D and seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2000;48:271–2. [PubMed] [Google Scholar]
- 17.Hyppönen E, Läärä E, Reunanen A, Järvelin M-R, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 18.Szodoray P, Nakken B, Gaal J, et al. The complex role of vitamin D in autoimmune diseases. Scand J Immunol. 2008;68:261–9. doi: 10.1111/j.1365-3083.2008.02127.x. [DOI] [PubMed] [Google Scholar]
- 19.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–9. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu PT, Modlin RL. Human macrophage host defense against Mycobacterium tuberculosis. Curr Opin Immunol. 2008;20:371–6. doi: 10.1016/j.coi.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37:113–19. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 22.Nursyam EWAZ, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 23.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 24.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to Mycobacteria. Am J Respir Crit Care Med. 2007;176:208–13. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 25.Ralph AP, Kelly PM, Anstey NM. L-arginine and vitamin D: novel adjunctive immunotherapies in tuberculosis. Trends Microbiol. 2008;16:336–44. doi: 10.1016/j.tim.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Krutzik SR, Hewison M, Liu PT, et al. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–20. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Reeth K. Cytokines in the pathogenesis of influenza. Vet Microbiol. 2000;74:109–16. doi: 10.1016/s0378-1135(00)00171-1. [DOI] [PubMed] [Google Scholar]
- 28.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najada AS, Habashneh MS, Khader M. The frequency of nutritional rickets among hospitalized infants and its relation to respiratory diseases. J Trop Pediatr. 2004;50:364–8. doi: 10.1093/tropej/50.6.364. [DOI] [PubMed] [Google Scholar]
- 30.Ginde AA, Mansbach JM, Camargo CA., Jr. Association between serum 25-dydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–90. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephenson A, Brotherwood M, Robert R, Atenafu E, Corey M, Tullis E. Cholecalciferol significantly increases 25-hydroxyvitamin D concentrations in adults with cystic fibrosis. Am J Clin Nutr. 2007;85:1307–11. doi: 10.1093/ajcn/85.5.1307. [DOI] [PubMed] [Google Scholar]
- 32.Chandra P, Wolfenden LL, Ziegler TR, et al. Treatment of vitamin D deficiency with UV light in patients with malabsorption syndromes: a case series. Photodermatol Photoimmunol Photomed. 2007;23:179–85. doi: 10.1111/j.1600-0781.2007.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speeckaert M, Huang G, Delanghe JR, Taes YEC. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clin Chim Acta. 2006;372:33–42. doi: 10.1016/j.cca.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Speeckaert MM, Wehlou C, Vandewalle S, Taes YE, Robberecht E, Delanghe JR. Vitamin D binding protein, a new nutritional marker in cystic fibrosis patients. Clin Chem Lab Med. 2008;46:365–70. doi: 10.1515/CCLM.2008.084. [DOI] [PubMed] [Google Scholar]
- 35.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D3. J Cyst Fibros. 2007;6:403–10. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuebler KK, Buchsel PC, Balkstra CR. Differentiating chronic obstructive pulmonary disease from asthma. J Am Acad Nurse Pract. 2008;20:445–54. doi: 10.1111/j.1745-7599.2008.00332.x. [DOI] [PubMed] [Google Scholar]
- 37.Mercer PF, Shute JK, Bhowmik A, Donaldson GC, Wedzicha JA, Warner JA. MMP-9, TIMP-1 and inflammatory cells in sputum from COPD patients during exacerbation. Respir Res. 2005;6:151. doi: 10.1186/1465-9921-6-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Zhang Z, Xu Y, Xiong S, Ni W, Chen S. TNF-alpha up-regulates matrix metalloproteinase-9 expression and activity in alveolar macrophages from patients with chronic obstructive pulmonary disease. J Huazhong Univ Sci Technolog Med Sci. 2006;26:647–50. doi: 10.1007/s11596-006-0604-6. [DOI] [PubMed] [Google Scholar]
- 39.Lim SAM, Roche N, Oliver BG, Mattos W, Barnes PJ, Fan Chung K. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers regulation by interleukin-10. Am J Respir Crit Care Med. 2000;162:1355–60. doi: 10.1164/ajrccm.162.4.9910097. [DOI] [PubMed] [Google Scholar]
- 40.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13:187–94. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- 41.Hopkinson NS, Li KW, Kehoe A, et al. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr. 2008;87:385–90. doi: 10.1093/ajcn/87.2.385. [DOI] [PubMed] [Google Scholar]
- 42.Song Y, Qi H, Wu C. Effect of 1,25-(OH)2D3 (a vitamin D analogue) on passively sensitized human airway smooth muscle cells. Respirology. 2007;12:486–94. doi: 10.1111/j.1440-1843.2007.01099.x. [DOI] [PubMed] [Google Scholar]
- 43.Burns JSDD, Speizer FE. Low levels of dietary vitamin D intake and pulmonary function in adolescents. Proc Am Thoracic Soc. 2006;3:A536. abstract. [Google Scholar]
- 44.Camargo CA, Rifas-Shiman SL, Litonjua AA, et al. Prospective study of maternal intake of vitamin D during pregnancy and risk of wheezing illnesses in children at age 2 years. J Allergy Clin Immunol. 2006;117:721–2. [Google Scholar]
- 45.Devereux G, Litonjua AA, Turner SW, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–9. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 46.Pichler J, Gerstmayr M, Szepfalusi Z, Urbanek R, Peterlik M, Willheim M. 1α,25(OH)2D3 inhibits not only Th1 but also Th2 differentiation in human cord blood T cells. Pediatr Res. 2002;52:12–18. doi: 10.1203/00006450-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–17. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 49.Xystrakis E, Boswell S, Peek E, et al. Reversing the defective induction of IL-10 secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–55. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]


