Abstract
Patients relapsing from multiple sclerosis (MS) are treated with high-dose, short-term intravenous injection of glucocorticoid (GC), although its mechanism of action remains only partly understood. We evaluated the ex vivo and in vitro effects of GC on regulatory T cell (Treg) function in 14 relapsing–remitting MS (RR-MS) patients in acute phase and 20 healthy controls (HC). Treg function was enhanced significantly after 5 days of GC treatment. Furthermore, there was a trend towards increasing proportions of CD4+CD25+forkhead box P3+ T cells and interleukin-10 secretion with GC treatment when compared with HC. In conclusion, GC treatment restores the impaired Treg function in patients with RR-MS in its acute phase.
Keywords: FoxP3, glucocorticoid, IL-10, multiple sclerosis, regulatory T cells
Introduction
Multiple sclerosis (MS) is an inflammatory, demyelinating disease of the central nervous system (CNS), believed to be initiated and mediated by autoreactive T cells directed against myelin antigens [1]. The development of autoimmune disease implies a breakdown of the mechanisms that control self-reactive lymphocytes [2,3]. Recent data suggest that such control mechanisms entail a subset of regulatory T cells (Tregs) enriched in the naturally activated CD4+ T cells and that express the CD25 molecule constitutively [3]. Tregs are pivotal agents in the regulation of tolerance by dampening harmful autoimmune T cells in the periphery. As a consequence, loss of Treg function appears to be a fundamental factor in MS [2].
Over the past few years, quantitative and qualitative analyses of Treg cells have been performed in MS in the attempt to shed light on the pathological impairments associated with these conditions [4–7]. However, some studies on MS and healthy controls (HC) have shown that the frequency of CD4+CD25hi Treg cells does not differ between patients with MS and HC [7,8]. Additionally, several groups have shown that CD4+CD25hi Treg cells are functionally impaired in patients with MS [9,10]. Data suggest that the dysfunction of suppressor function of certain Treg cells is associated with MS [10]. Therapeutic strategies that could augment the number and the activity of Treg cells are thus being considered for treatment of these pathologies [2,11]. Notably, the available immunomodulatory agents, such as interferon (IFN)-β, exert their beneficial effects partially via Tregs[12].
It has been demonstrated clearly that a brief course of high-dose glucocorticoid (GC) treatment has a short-term beneficial effect on functional recovery in patients with MS relapse [13,14], while the exact mechanism of action of GCs in MS is unclear. Only an increased frequency of CD4+CD25+ Tregs in experimental autoimmune encephalomyelitis (EAE) treated with high-dose intravenous GC was observed, although it has not yet been demonstrated conclusively whether GC treatment would influence Tregs in humans with MS. To investigate the modification by GC on CD4+ CD25+ Tregs in humans with MS, we therefore compared these cells in the peripheral blood of relapsing–remitting MS patients with either acute (AMS) or stable (SMS) disease. We also measured the production of interleukin (IL)-10 by Tregs before and after GC treatment. Moreover, we looked for a correlation between variation of transcription factor expression and patients' clinical outcome.
Materials and methods
Subjects
Forty-seven patients with MS diagnosed by clinical and laboratory parameters were included in the study. There were 15 consecutive relapsing–remitting MS (RR-MS) patients, defined according to McDonald's criteria [15], in the acute phase of disease. All patients in the acute phase underwent clinical examination before and after GC therapy with high-dose (1 g/day) methylprednisolone intravenous pulse therapy for 5 days. Patients were considered to be in relapse when they showed an episode of new neurological disturbance lasting at least 24 h and magnetic resonance imaging (MRI) activity. Disability degree was assessed using the Expanded Disability Status Scale (EDSS). This study was approved by the ethics committee of Third Military Medical University, and all the participants gave written informed consent before enrolment.
Isolation of peripheral blood mononuclear cells (PBMC) and CD4+ CD25+ Tregs
PBMC were isolated from fresh heparinized blood by density gradient centrifugation using Ficoll-Hypaque (Histopaque; Sigma, St Louis, MO, USA) and suspended at the concentrations indicated in RPMI-1640 supplemented with 10% fetal calf serum (FCS). For isolation of CD4+CD25+ and CD4+CD25− T cells, PBMC were separated further using a Treg isolation kit (Miltenyi Biotec, Gladbach, Germany) according to the manufacturer's instructions. The purity of enriched cells for CD4+CD25+ (>90%) and CD4+CD25− T cells (>90%) was determined by flow cytometry with antibodies against CD3, CD4 and CD25.
Phenotype analysis and fluorescence activated cell sorter (FACS) analysis of T cell subsets from peripheral blood
PBMCs were isolated from peripheral blood as before, and CD4+ T cells were purified by magnetic separation as described above. Purified CD4+ T cells were stained with a mixture of antibodies consisting of anti-CD4-peridinin chlorophyll (PerCP)-cy5.5 (BD, Franklin Lakes, NJ, USA) and anti-CD25-phycoerythrin (PE) (BD) for 30 min at 4°C, washed in phosphate-buffered saline (PBS)/2% bovine serum albumin (BSA), filtered, and sorted on a BD FACSCalibur. Gates were set so that purified CD4+CD25hi cells represented the brightest 2% of total CD4 population; both CD4+CD25hi and CD4+CD25− populations were collected from the CD3+ fraction. Cells were analysed using a FACSCalibur with CellQuest software (BD).
Forkhead box P3 (FoxP3) and IL-10 expression were analysed by FACS according to the protocol described by Berhanu et al.[16]. In brief, fresh PBMCs were washed in PBS, fixed in 1 ml of PBS with 1% paraformaldehyde and 0·05% Tween-20, and kept overnight at 4°C. Cells were treated twice with 0·5 ml of DNase at 100 Kunitz/ml according to the manufacturer's instructions (Sigma-Aldrich, St. Louis, MO, USA). Cells were incubated with mouse anti-human FoxP3-APC (eBioscience, San Diego, CA, USA) or mouse anti-human IL-10-Alexa Fluor 647 (eBioscience) for 1 h at room temperature and washed with FACS buffer (PBS 3%, FCS 0·5%, Tween-20 0·05% azide). Cell-surface staining was performed using anti-CD4-PerCP-cy5.5 (BD) and anti-CD25-PE (BD) for 20 min at room temperature.
Suppression assay
To assess the functional activity of Treg cells, CD4+CD25− cells were isolated and labelled with 5 µM of carboxyl fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR, USA). We resuspended 1 × 105 autologous mitomycin-C-treated (25 µg/ml) PBMCs plus 2 × 104 CD4+CD25− cells in the absence or presence of 2 × 104 CD4+CD25hi Treg cells in 200 µl RPMI-1640 medium and stimulated with plate-bound anti-CD3/CD28 antibodies (10 µg/ml; eBioscience). Cultures were set up as duplicates in U-bottomed wells and incubated at 37°C in a humidified atmosphere with 5% CO2. After 5 days the cells were harvested, and the CFSE signal of gated lymphocytes was analysed by flow cytometry.
Statistical analysis
Statistical analyses were performed using spss 14·0 for Windows. Results are expressed as mean values ± standard error of the mean (s.e.m.) unless indicated otherwise in the figure legends. For comparisons of Treg parameters between patients and controls, Student's t-tests were applied. Correlations between parameters were analysed using Pearson's correlation tests. Relationships between variables were evaluated further by multiple regression statistical analyses. Differences were considered significant when P < 0·05.
Results and discussion
Frequencies of CD4+CD25hi T cells are similar in MS patients and healthy controls
One major caveat with Tregs remains the lack of a specific cell surface marker, which hampers the purification of these cells. To date, the most specific marker of Tregs is transcription factor FoxP3, which cannot be helpful for the isolation of these cells because of its exclusive intracellular expression. Cell surface markers for Tregs have been described, such as the expression of CD25, glucocorticoid-induced tumour necrosis factor (TNF) receptor family-related protein (GITR), cytotoxic T lymphocyte antigen (CTLA)-4 molecules or the down-regulation of CD127 (IL-7 receptor). However, high expression of CD25 is considered widely as a main marker of Tregs, allowing the provision of a highly enriched population of Tregs. Therefore, we used a stringent gating approach as detailed in the Methods. Frequencies of CD4+CD25hi T cells in the peripheral blood of MS patients and normal individuals were compared by means of flow cytometry. Mean numbers of CD4+CD25hi T cells were comparable in RR-MS patients (3·90% ± 0·31%), secondary-progressive MS (SP-MS) patients (4·01% ± 0·35%) and HC (4·19% ± 0·48%, Fig. 1a).
Fig. 1.
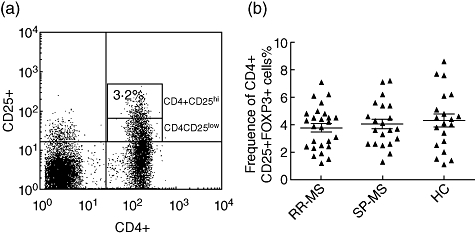
Frequencies of CD4+CD25hi T cells in the peripheral blood of healthy controls (HC) and relapsing–remitting multiple sclerosis (RR-MS) and secondary-progressive MS (SP-MS) patients. (a) A representative plot of healthy control is shown. Peripheral blood mononuclear cell (PBMC) preparations were stained for the expression of surface CD4 and CD25. Data are presented as percentage of CD4+CD25hi T cells in total CD4+ T cells. (b) The frequency of CD4+CD25hi T cells was measured by flow cytometry in patients with RR-MS (n = 26), SP-MS (n = 21) and healthy controls (n = 20). These data represent three independent experiments.
Impaired functioning of CD4+CD25hi regulatory T cells in patients with MS correlate with its FoxP3 expression
More recent studies have shown that FoxP3 is not only a key intracellular marker, but is also a crucial developmental and functional factor for CD4+CD25+ Tregs. Huan et al. found that patients with MS have lower levels of FoxP3 expression than do healthy individuals, suggesting an involvement of diminished FoxP3 expression in impaired Treg-cell immunoregulation in MS. Venken et al. found an impairment of Treg-cell function accompanied by decreased FoxP3 expression in patients with RR-MS, but the FoxP3 level and suppressive function were normalized during secondary progressive MS. We found that the frequency of CD4+CD25+FoxP3+ Treg was depressed significantly in the RR-MS patients (2·19 ± 0·23%) compared to SP-MS patients or healthy controls (3·22 ± 0·32% and 3·43 ± 0·34%, respectively, Fig. 2a).
Fig. 2.
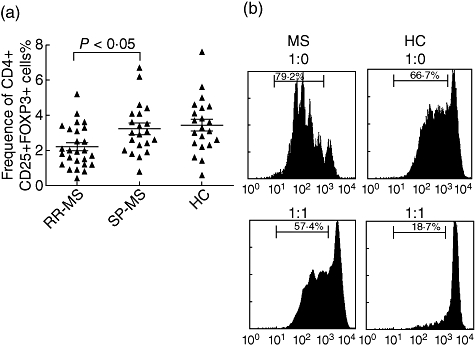
Frequencies and function of CD4+CD25+forkhead box P3 (FoxP3)+ T cells in the peripheral blood of healthy controls (HC), relapsing–remitting multiple sclerosis (RR-MS) and secondary-progressive MS (SP-MS) patients. (a) The frequency of CD4+CD25+FoxP3+ T cells was measured by flow cytometry in patients with RR-MS, SP-MS and HC. Data are presented as percentage of CD4+CD25hi T cells in total CD4+ T cells and were performed in three independent experiments. (b) Proliferation of CD4+CD25− T cell subsets from HC and co-cultures with regulatory T cells (Tregs) from HC and MS in response to plate-bound anti-CD3/CD28 antibodies. Bead-sorted CD4+CD25− T cells (2 × 104 cell/well) with carboxyl fluorescein diacetate succinimidyl ester (labelled) (5 µM) and CD4+CD25+ T cells (2 × 104 cell/well) isolated from HC, RR-MS and SP-MS patients were stimulated with 10 µg/ml anti-CD3 and anti-CD28 antibody in the presence of 105 irradiated feeders/well. After 5 days of culture, proliferation was measured by means of CFSE-labelled fluorescence activated cell sorter (FACS). CFSE histograms from one representative HC and RR-MS patient are shown.
To determine the effect of CD4+CD25+ Tregs on responder cells and to investigate the mechanism underlying this effect, CD4+CD25− T cells from healthy controls were co-cultured with CD4+CD25hi Tregs under stimulation with plate-bound anti-CD3/CD28 antibodies. Previous studies have indicated that the suppressive capacity of the total population of CD25hi regulatory T cells was decreased in RR-MS patients, whereas SP-MS patients showed a normal Treg function. To determine whether the CD4+CD25hi T cells of RR-MS and SP-MS in our study were functional Treg cells, we used an in vitro cellular co-culture system. When activated with plate-bound anti-CD3/CD28 antibodies, CD4+CD25− T cells responded with robust proliferation and the Tregs from HC inhibited these T cells proliferations significantly (Fig. 2b, right column). Tregs from SP-MS also inhibited significantly CD4+CD25− T cell proliferation in a dose-dependent manner (data not shown), while Tregs from RR-MS showed impaired suppression capacity compared to those from HC (n = 15, P < 0·05; Fig. 2b, left column).
Glucocorticoid treatment up-regulates FoxP3 expression and IL-10 secretion of Tregs
The 26 RR-MS patients were subdivided subsequently in patients with either stable (SMS; n = 12) or acute (AMS; n = 14) disease based on clinical parameters and on the absence or presence of enhancing lesions, as determined by brain and spinal cord MRI with gadolinium. Fourteen RR-MS patients in relapse included in our study were treated with intravenous methylprednisolone 1 g/day for 5 days. Glucocorticoids are highly effective in dampening down inflammation in most individuals. In order to investigate the suppressive capacity of intravenous GC treatment on circulating CD4+CD25hi T cells, we tested suppression of CFSE-labelled responder cells co-cultured with Treg before and after the GC treatment. CFSE-labelled CD4+CD25− T cells proliferated strongly in vitro after activation with plate-bound monoclonal antibody to CD3 and CD28, with 66·7% of CFSE-labelled naive T cells dividing. However, the proliferation of naive CD4+CD25− T cells was inhibited significantly when CD4+CD25hi Treg cells sorted from RR-MS patients with GC treatment. In these recipients, 61·1% of the naive T cells failed to divide. These data indicate that glucocorticoid treatment restore the impaired suppressive function of Tregs in patients with RR-MS (Fig. 3a).
Fig. 3.

Functional and frequency analysis of regulatory T cells of relapsing–remitting multiple sclerosis (RR-MS) before and after glucocorticoid (GC) treatment. (a) Suppression of carboxyl fluorescein diacetate succinimidyl ester (CFSE)-labelled responder cells from healthy controls (HC) by bead-sorted CD4+CD25+ T cells from HC and MS patients. CFSE histograms from one representative HC and RR-MS patient are shown. (b) Frequencies of CD4+CD25+forkhead box P3 (FoxP3)+ T cells in the peripheral blood of HC, RR-MS and secondary-progressive MS (SP-MS) patients. (c) Frequencies of regulatory T cells with interleukin (IL)-10 secretion.
We observed a significant increase of the percentage of CD4+CD25+FoxP3+ T cells after 5 days (2·63 ± 0·94–5·2 ± 0·8%, P < 0·05) of treatment (Fig. 3b). Additionally, we found that spontaneous IL-10 production by PBMCs was significantly higher in relapsing RRMS patients after 5 days of GC treatment than before treatment (Fig. 3c). The mechanisms that underlie Treg-cell dysfunction are currently poorly understood. The capacity of naturally occurring Treg cells to inhibit the proliferation of naive T cells in vitro requires cell–cell contact; however, in vivo, these cells can also function through induction of inhibitory cytokines, such as IL-10. We did not find any significant modification of GITR spontaneous production by PBMCs from RRMS patients in relapse before and after GC treatment (data not shown). Furthermore, we found a correlation between the decrease of EDSS after 5 days of GC treatment and a statistically significance increase of FoxP3 expression.
Conclusion
Emerging evidence suggests that Treg cells, particularly CD4+CD25+ Treg cells, are key mediators of peripheral tolerance. Treg lymphocytes modulate immune responses against autologous antigens, thus playing a pivotal role in preventing autoimmune diseases. Our data showed that quantitatively circulating CD4+CD25hi T cell populations did not differ remarkably in patients affected with MS compared to HC. However, there was a reduced number of peripheral blood CD4+CD25+FoxP3+ T cells and lower FoxP3 expression in RR-MS patients than in SP-MS patients or HC, which was correlated with the suppressive capacity of Tregs in these patients. Our study also revealed that GC-treated RR-MS patients showed restored functional and frequency of FoxP3+ Tregs, which is in accordance with a previous study [17]. This observation is strengthened further by the follow-up of MS patients before and during GC treatment where there were significant changes in the IL-10 secretion of Treg cells.
The present study investigated whether glucocorticoids affect the activity of Treg cells on the basis of FoxP3 and cytokine expression. These findings have demonstrated that glucocorticoid treatment is not only immunosuppressive and anti-inflammatory, but also promoted increasing frequency of Treg cells.
Acknowledgments
We thank Dr Xiaoyun Shang (Institute of Immunology PLA, Third Military Medical University, PR China) for helpful advice and comments on preparation of the manuscript.
Disclosure
None.
References
- 1.Markovic-Plese S, McFarland HF. Immunopathogenesis of the multiple sclerosis lesion. Curr Neurol Neurosci Rep. 2001;1:257–62. doi: 10.1007/s11910-001-0028-4. [DOI] [PubMed] [Google Scholar]
- 2.Zozulya AL, Wiendl H. The role of regulatory T cells in multiple sclerosis. Nat Clin Pract Neurol. 2008;4:384–98. doi: 10.1038/ncpneuro0832. [DOI] [PubMed] [Google Scholar]
- 3.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–19. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 4.Liu GZ, Gomes AC, Fang LB, Gao XG, Hjelmstrom P. Decreased 4-1BB expression on CD4+CD25(high) regulatory T cells in peripheral blood of patients with multiple sclerosis. Clin Exp Immunol. 2008;154:22–9. doi: 10.1111/j.1365-2249.2008.03730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haas J, Fritzsching B, Trubswetter P, et al. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol. 2007;179:1322–30. doi: 10.4049/jimmunol.179.2.1322. [DOI] [PubMed] [Google Scholar]
- 6.Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp Immunol. 2007;147:412–18. doi: 10.1111/j.1365-2249.2006.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venken K, Hellings N, Hensen K, et al. Secondary progressive in contrast to relapsing-remitting multiple sclerosis patients show a normal CD4+CD25+ regulatory T-cell function and FOXP3 expression. J Neurosci Res. 2006;83:1432–46. doi: 10.1002/jnr.20852. [DOI] [PubMed] [Google Scholar]
- 8.Fritzsching B, Korporal M, Haas J, Krammer PH, Suri-Payer E, Wildemann B. Similar sensitivity of regulatory T cells towards CD95L-mediated apoptosis in patients with multiple sclerosis and healthy individuals. J Neurol Sci. 2006;251:91–7. doi: 10.1016/j.jns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas J, Hug A, Viehover A, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–52. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 11.Yamamura T, Takahashi K, Araki M. Multiple sclerosis and regulatory cells. Nippon Rinsho. 2005;63(Suppl. 5):422–6. [PubMed] [Google Scholar]
- 12.de Andres C, Aristimuno C, de Las Heras V, et al. Interferon beta-1a therapy enhances CD4+ regulatory T-cell function: an ex vivo and in vitro longitudinal study in relapsing–remitting multiple sclerosis. J Neuroimmunol. 2007;182:204–11. doi: 10.1016/j.jneuroim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Frisullo G, Nociti V, Iorio R, et al. Glucocorticoid treatment reduces T-bet and pSTAT1 expression in mononuclear cells from relapsing remitting multiple sclerosis patients. Clin Immunol. 2007;124:284–93. doi: 10.1016/j.clim.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Whitaker JN, Layton BA, Herman PK, Kachelhofer RD, Burgard S, Bartolucci AA. Correlation of myelin basic protein-like material in cerebrospinal fluid of multiple sclerosis patients with their response to glucocorticoid treatment. Ann Neurol. 1993;33:10–17. doi: 10.1002/ana.410330104. [DOI] [PubMed] [Google Scholar]
- 15.Wiendl H, Kieseier BC, Gold R, Hohlfeld R, Bendszus M, Hartung HP. Revision of McDonald's new diagnostic criteria for multiple sclerosis. Nervenarzt. 2006;77:7–45. doi: 10.1007/s00115-006-2127-6. [DOI] [PubMed] [Google Scholar]
- 16.Berhanu A, Huang J, Watkins SC, Okada H, Storkus WJ. Treatment-enhanced CD4+Foxp3+ glucocorticoid-induced TNF receptor family related high regulatory tumor-infiltrating T cells limit the effectiveness of cytokine-based immunotherapy. J Immunol. 2007;178:3400–8. doi: 10.4049/jimmunol.178.6.3400. [DOI] [PubMed] [Google Scholar]
- 17.Navarro J, Aristimuno C, Sanchez-Ramon S, et al. Circulating dendritic cells subsets and regulatory T-cells at multiple sclerosis relapse: differential short-term changes on corticosteroids therapy. J Neuroimmunol. 2006;176:153–61. doi: 10.1016/j.jneuroim.2006.03.022. [DOI] [PubMed] [Google Scholar]


