Abstract
Multi-nucleated giant cells (MGCs; Langhans-type cell), formed from macrophage fusion, are recognized as a hallmark histological feature in chronic inflammation. However, their precise pathological role is still poorly understood, especially for microorganism pathogens in the neonatal immune system, which are capable of surviving intracellularly in phagocytes. To conduct a partial evaluation of the monocyte function of neonates, we investigated the ability of human cord blood monocytes to form MGCs in vitro by stimulating various cytokines and comparing them with adult peripheral blood monocytes. Monocytes from cord blood and adult peripheral blood were isolated and cultured for 14 days with cytokines known to induce MGC in vitro. The fusion index in experiments with a combination of interleukin (IL)-4 and macrophage colony-stimulating factor (M-CSF) and a combination of IL-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) was significantly lower in cord blood than in adult blood monocytes (P = 0·0018 and P = 0·0141, respectively). The number of nuclei per MGC was significantly lower in cord blood than in adult blood monocytes in experiments with IL-4 alone, the combination of IL-4 and M-CSF, and the combination of IL-4 and GM-CSF (P < 0·0001). These results suggest the possibility that the susceptibility of newborns to mycobacterium infection is due partly to impaired MGC formation.
Keywords: cord blood, interleukin-4, M-CSF, monocyte, multi-nucleated giant cell (Langhans-type cell)
Introduction
Human neonates are susceptible to infections [1,2], especially by microorganism pathogens, which are capable of surviving intracellularly in phagocytes (e.g. mycobacterium [3], listeria [4], herpes simplex virus [5], cytomegalovirus and toxoplasma [6]). Neonates sometimes develop a serious systemic infection with rapid progress from these pathogens. The immune reactions to these pathogens are depressed in neonates; in particular, poor mobilization of phagocytes (neutrophils and macrophages) and decreased production of interferon (IFN)-γ by CD4+ T lymphocytes have been well established [6,7].
Multi-nucleated giant cells (MGCs; Langhans-type cells), formed from macrophage fusion, are recognized widely as a cellular feature of chronic inflammatory disorder. MGCs were first described by Langhans in tuberculoid lesions, and are found to be present in a variety of granulomatous conditions including infection by the above-listed pathogens, sarcoidosis, rheumatoid arthritis, neoplasm and the foreign body reaction by the host defence mechanism. In vitro MGCs formation was described first by Black in 1976 [8], and various cytokines including macrophage colony-stimulating factor (M-CSF), granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-4 and interferon (IFN)-γ have been found to induce MGCs formation in vitro[9–13]. In addition, several molecules have been found to be related to MGC formation [14–27]. Although the molecular mechanism of MGC formation has been gradually elucidated, the pathophysiological actions of MGC and their precise roles in immune responses are still poorly understood. Furthermore, these findings are from experiments established in animals or human adults, and there is little information on MGC in human neonates [28,29]. The granuloma structure (including MGCs) and their protective function against mycobacterial infections have been discussed in current studies [30–33]; histopathological findings in congenital tuberculosis patients have been studied and few granulomatous lesions or MGCs were shown in their lesions [34–36].
We speculated that the reason why human neonates are susceptible to the pathogens listed above is partly because of the decreased ability of MGCs formation. In this study, we investigated the ability of human cord blood monocytes to form MGCs in vitro by stimulation of the cytokines listed above. We attempted to evaluate partly the monocyte function of neonates and to compare the results with those obtained using adult peripheral monocytes.
Materials and methods
Reagents
RPMI-1640 and phosphate-buffered saline (PBS) were from Gibco (Grand Island, NY, USA). The human cytokines (IL-4, M-CSF, GM-CSF, IFN-γ and TNF-α) were from Wako Pure Chemical Industries (Osaka, Japan).
Blood samples
Adult peripheral blood was obtained from healthy volunteers, in accordance with the principles of the Declaration of Helsinki. Umbilical cord blood was obtained from healthy term neonates immediately after delivery. Parental informed consent was obtained for every donor, and the examination protocol of peripheral blood samples was in accordance with the guidelines of the Institutional Review Board of Okayama University Hospital. Both types of blood samples were collected in heparinized syringes and processed within 3 h.
Monocyte isolation and cell culture
Adult peripheral blood and cord blood mononuclear cells were isolated by Histopaque (Sigma, St Louis, MO, USA) density gradient centrifugation. The monocytes were isolated by negative selection with monoclonal antibody (mAb)-coated immunological magnetic beads and a cell sorter (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) after centrifugation. Purity, assessed by detection of reactive oxygen species with a fluorescence probe (hydroxyphenyl fluorescein) stimulated with 10 ng/ml phormol myristate acetate (PMA) and saturated with anti-CD14 mAb (IOM2; Immunotech; Marseille, France) by flow cytometer, were both more than 95%. The monocytes were cultured at 37°C and a 5% CO2 atmosphere in condition medium consisting of RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin at 1 × 106 cells/ml/well in 24-well tissue culture plates (Sumitomo Bakelite, Tokyo, Japan) with various cytokines. The cells were maintained for 14 days without changing the medium. All culturing procedures were performed under sterile conditions, and each experiment was performed with at least seven independent samples.
Cell staining and evaluation of MGCs formation
After 14 days' incubation, the culture plates were gently rinsed twice with PBS and dried well, and the cells then stained with a May – Grünwald–Giemsa stain (Merck Ltd, Darmstadt, Germany). Digital pictures at low power (10 × objectives) were taken with a microscope (Keyence, Osaka, Japan) under low voltage (LV) light for the quantification of MGC. The fusion rate of monocytes was determined by counting the number of nuclei within MGC (>3 nuclei/cell) in randomly acquired areas per total number of nuclei in the same areas (at least 500 nuclei): fusion rate (%) = (number of nuclei within MGC/total number of nuclei × 100). The number of nuclei per MGC (20 cells acquired randomly in each experiment) was also evaluated.
Collection of supernatants and analysis of cytokines
After 14 days' incubation without change of culture medium, the supernatants were collected and centrifuged at 500 g for 5 min to remove particulate debris, then stored at −30°C. IL-1β was measured by a two-site-directed ELISA, with an exclusion limit of 10 pg/ml (Pierce Biotech, Rockford, IL, USA). Tumour necrosis factor (TNF)-α was assessed by a specific ELISA, with a sensitivity of 0·5 pg/ml (Pierce Biotech). IL-4 was also measured by an ELISA with a sensitivity of 2·0 pg/ml. IFN-γ was measured by an enzyme immunoassay (EIA) with a sensitivity of 0·1 pg/ml.
Superoxide anion (O2−) production
The amount of superoxide released from isolated monocytes (1 × 106 cells/ml) was determined with a Hitachi spectrophotometer U 2000 (Tokyo, Japan) as the change in absorbance at 550 nm resulting from superoxide dismutase (SOD)-inhibitable cytochrome c reduction at 37°C [37]. The reaction was carried out for 5 min with PMA (10 ng/ml) with calcium chloride (1·5 mM) and magnesium sulphate (1·2 mM) in the presence of 1 mM NaN3, after which the reaction was stopped by addition of 0·5 mM N-ethylmalemide. The generation of superoxide was calculated by subtracting the change in absorbance in the presence of SOD (1 mM) from that in its absence, and then dividing this value by 21·1 × 103/M/cm for the molar extinction coefficient.
Statistical analysis
Data in the table and figures are shown as means ± standard deviation (s.d.) unless indicated otherwise. Statistical analysis was performed with unpaired t-test (GraphPad Prism version 5·00 for Mac OS X; GraphPad Software, San Diego, CA USA). Results with P-values less than 0·05 were considered statistically significant.
Results
IL-4 and IL-4 with M-CSF, IL-4 with GM-CSF-induced MGCs in vitro
In our culture system, many of the monocytes were degenerated, and only a small number of MGCs appeared spontaneously in the controls without cytokine (Fig. 1). These MGCs were small, generally with three to five nuclei. In the experiments to evaluate the cytokines, which were described previously as inducing MGCs, M-CSF (20–100 ng/ml) alone, GM-CSF (20 ng/ml) alone, a combination of M-CSF (20 ng/ml) and TNF-α (20 ng/ml), a combination of GM-CSF (20 ng/ml) and TNF-α (20 ng/ml) and a combination of GM-CSF (20 ng/ml) and IFN-γ (1000 U/ml) maintained cell densities and promoted monocytes adhesion, but did not affect MGC formation significantly, from both adult blood and cord blood monocytes in comparison with the controls (data not shown). IL-4 (20 ng/ml), a combination of IL-4 (20 ng/ml) and M-CSF (100 ng/ml) and a combination of IL-4 (20 ng/ml) and GM-CSF (20 ng/ml) increased the fusion index significantly both from adult blood and cord blood monocytes, respectively (Fig. 2). In addition, these MGCs differentiated sometimes into large ones with 20 or more nuclei and/or large cytoplasmic spreading. There was no distinct difference in the characteristics of MGC among the different cytokines added (IL-4 alone, the combination of IL-4 and M-CSF and the combination of IL-4 and GM-CSF) (Fig. 1).
Fig. 1.
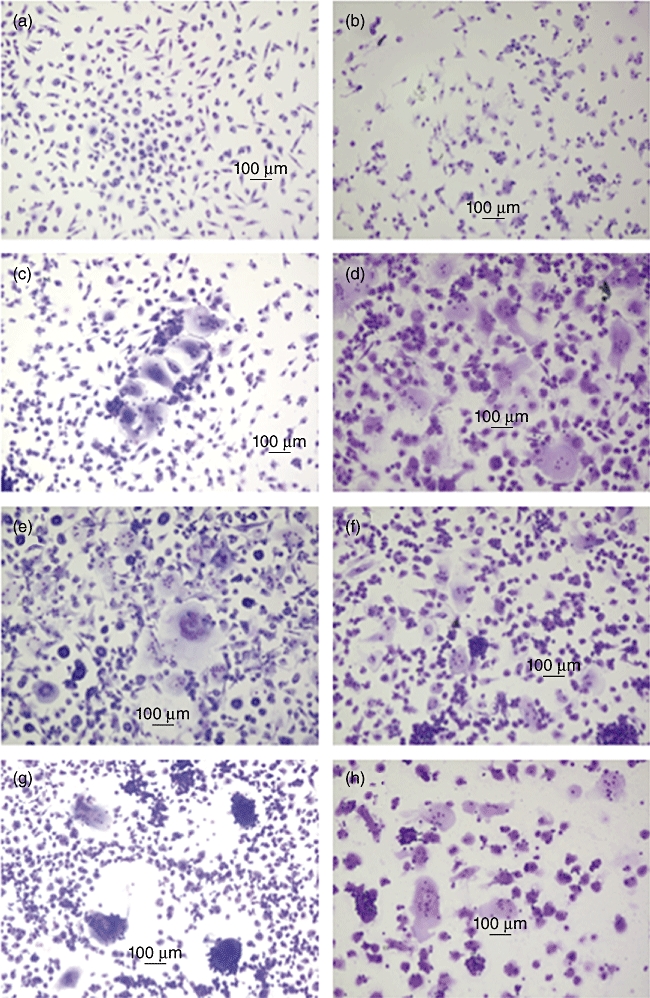
Morphology of multi-nucleated giant cells (MGCs) induced by interleukin (IL)-4, IL-4 with macrophage colony-stimulating factor (M-CSF) and IL-4 with granulocyte–macrophage colony-stimulating factor (GM-CSF) in vitro. Adult peripheral blood and cord blood monocytes were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin, at 1 × 106 cells/ml/well in 24-well tissue culture plates with or without cytokines. After 14 days' incubation without changing the medium, the cells were stained with a May–Grünwald–Giemsa stain. (a) No added cytokine: adult blood; (b) no added cytokine: cord blood; (c) IL-4 (20 ng/ml) alone: adult blood; (d) IL-4 (20 ng/ml) alone: cord blood; (e) IL-4 (20 ng/ml)+M-CSF (100 ng/ml): adult blood; (f) IL-4 (20 ng/ml)+M-CSF (100 ng/ml): cord blood; (g) IL-4 (20 ng/ml)+GM-CSF (20 ng/ml): adult blood; (h) IL-4 (20 ng/ml)+GM-CSF (20 ng/ml): cord blood.
Fig. 2.
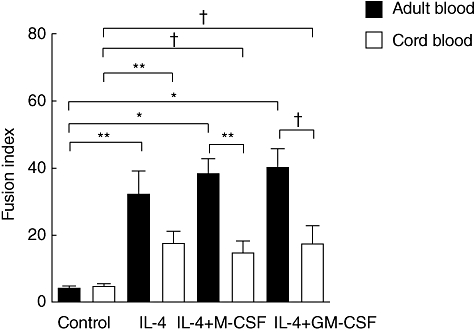
Effect of cytokines on multi-nucleated giant cell (MGC) formation; adult blood versus cord blood monocytes. Adult peripheral blood (solid bar) and cord blood (open bar) monocytes were cultured as described in the legend to Fig. 1. The results were calculated from seven independent experiments as the fusion index. Significantly different from control or between cord blood and adult blood: *P < 0·0001, **P < 0·01, †P < 0·05.
MGC formation from cord blood monocytes induced by IL-4 and IL-4 with M-CSF, and IL-4 with GM-CSF, in comparison with adult peripheral blood monocytes
As described previously, the fusion index was low and there was no difference in the index between adult blood and cord blood monocytes in the control without cytokine (Fig. 2). The fusion index in the experiment with IL-4 alone was lower in cord blood than in adult blood monocytes, but not significantly different (P = 0·0958). In contrast, the fusion index in the experiment with the combination of IL-4 and M-CSF and that with the combination of IL-4 and GM-CSF was significantly lower in cord blood than in adult blood monocytes (P = 0·0018 and P = 0·0141, respectively) (Fig. 2). These findings resulted from the number of nuclei per MGCs rather than from the prevalence of MGCs. The number of nuclei per MGC was significantly higher in adult blood than in cord blood monocytes in all experiments with IL-4 alone, the combination of IL-4 and M-CSF and the combination of IL-4 and GM-CSF (P < 0·0001) (Fig. 3). The average number of nuclei per MGCs from cord blood monocytes was about half that from adult blood monocytes.
Fig. 3.
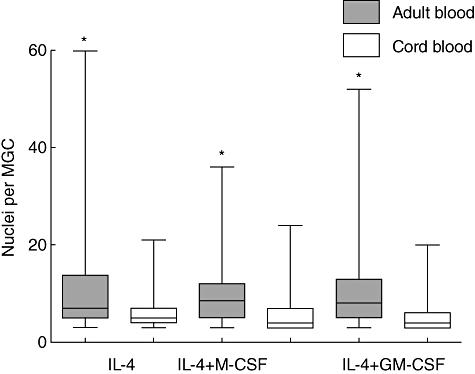
Nuclei per multi-nucleated giant cells (MGCs) induced by cytokines; adult blood versus cord blood monocytes. Adult peripheral blood (solid box) and cord blood (open box) monocytes were cultured as described in the legend to Fig. 1. The number of nuclei per MGC (20 cells acquired randomly in each experiment) was counted. Results from seven independent experiments are shown in the box-and-whisker plots. Line in the box shows median point, box shows the point from first to third quartile, whiskers show the point from smallest to largest number. Significantly different between cord blood and adult blood: *P < 0·0001.
Cytokine production from monocytes after 14-d culture with IL-4 and IL-4 with M-CSF, IL-4 with GM-CSF
The production of several cytokines was evaluated in the supernatant of culture medium for 14 days. Three individual samples were analysed in each group. In both adult blood and cord blood, IL-4 alone, the combination of IL-4 and M-CSF and the combination of IL-4 and GM-CSF did not stimulate the production of TNF-α, IFN-γ and Il-1β compared with the control (no stimulant). Differences between adult blood and cord blood in each group were not statistically significant (Table 1). Cytokine levels in the supernatant of culture medium at 5 days also showed the same results (data not shown).
Table 1.
Concentration of interferon (IFN)-γ, interleukin (IL)-1β, IL-4 and tumour necrosis factor (TNF)-α in the supernatants of adult peripheral blood and cord blood monocytes after 14 days' culture with the cytokines listed below.
| IFN-γ (IU/ml) | IL-1β (pg/ml) | IL-4 (pg/ml) | TNF-α (pg/ml) | ||
|---|---|---|---|---|---|
| Adult blood (n = 3) | Control | 0·9 ± 0·9 | 393 ± 202 | <2·0 | 49 ± 5 |
| IL-4+M-CSF | 1·0 ± 0·8 | 159 ± 180 | 3007 ± 1074 | 36 ± 20 | |
| IL-4+GM-CSF | 0·5 ± 0·2 | 147 ± 175 | 2633 ± 421 | 35 ± 13 | |
| IL-4 | 0·7 ± 0·4 | 217 ± 162 | 4740 ± 1935 | 44 ± 23 | |
| Cord blood (n = 3) | Control | 1·1 ± 1·2 | 765 ± 412 | <2·0 | 51 ± 9 |
| IL-4+M-CSF | 0·1 ± 0·1 | 583 ± 761 | 3737 ± 1004 | 28 ± 10 | |
| IL-4+GM-CSF | 0·2 ± 0·2 | 473 ± 708 | 1960 ± 1196 | 27 ± 6 | |
| IL-4 | 0·7 ± 0·6 | 479 ± 351 | 4580 ± 1403 | 40 ± 17 |
Three individual samples were analysed in each group. Differences between adult blood and cord blood in each group were not statistically significant; difference among controls and each group were also not statistically significant. M-CSF, macrophage colony-stimulating factor; GM-CSF, granulocyte–macrophage colony-stimulating factor.
Superoxide anion production by monocytes
Isolated monocytes (1 × 106 cells/ml) in PBS were stimulated with PMA. Control O2− production stimulated with PMA (10 ng/ml) ranged from 112 to 220 nmol cytochrome c reduced/106 cells for 5 min (171 ± 35 nmol; mean ± s.d. of five individual adult volunteers), and ranged from 120 to 198 nmol cytochrome c reduced/106 cells for 5 min (170 ± 30 nmol; mean ± s.d. of four individual cord blood samples).
Discussion
MGC formation from umbilical cord blood mononuclear cells has rarely been studied, and was reported first by Zur Hausen et al.[28]. They found that mononuclear cells from human umbilical cord were fused more frequently into MGCs than those from adult peripheral blood. Because of the morphological appearance of the MGCs they attempted, but failed, to prove that a viral agent induced macrophage fusion. The first comparative analysis of experimentally induced MGCs from mononuclear phagocytes isolated from human cord blood and adult peripheral blood was reported by Gerberding et al.[29]. They found that spontaneous MGC formation occurred in both cord and adult blood mononuclear cell cultures by 7 days of incubation, although significantly fewer MGCs formed in cord blood cultures. PMA treatment of adult blood mononuclear cells resulted in a significant increase in MGC formation after 7, 14 or 21 days of culture, but PMA did not increase MGC formation significantly in cord blood cultures until 14 or 21 days of culture.
In the present study, we used a pure population of monocytes from cord blood and adult peripheral blood with stimulation of various monoclonal cytokines (IL-4, M-CSF, GM-CSF, TNF-α, IFN-γ) that are known to induce the formation of MGCs in vitro. Among these cytokines IL-4 alone, a combination of IL-4 and M-CSF and a combination of IL-4 and GM-CSF could increase MGC formation significantly in both cord blood and adult peripheral blood culture. In the experiment with the combinations of IL-4 and M-CSF or IL-4 and GM-CSF, the fusion index was significantly lower in cord blood than in adult blood monocytes. In addition, we notably demonstrated that the number of nuclei per MGCs was significantly lower in cord blood than in adult blood monocytes in all experiments with IL-4 alone, the combination of IL-4 and M-CSF and the combination of IL-4 and GM-CSF. In these experiments we used a pure population of monocytes from cord blood and adult peripheral blood and evaluated the production of cytokines from cultured cells. TNF-α, IFN-γ and Il-1β were detected, but differences between adult blood and cord blood in each group were not statistically significant, so the difference in MGC formation seemed to be dependent upon the character of the monocyte itself but not the produced cytokines (autocrined monokines). Previous studies have also reported that the ability of neonatal monocytes and macrophages to produce several monokines (including TNF-α and IL-1β) is similar or reduced modestly compared with adult cells [3,38]. Additionally, neonatal monocytes/macrophages produce superoxide anion as much as adult cells.
Knowledge of the functions of MGCs is still limited, but several recent studies have revealed their pivotal functions, particularly the function played in mycobacterial infection. In studies utilizing an in-vitro model of human tuberculous granulomas, Lay et al. have shown that the high-virulence mycobacterium, Mycobacterium tuberculosis, induces large MGCs with more than 15 nuclei per cell, whereas with low-virulence mycobacterium species, M. avium and M. smegmatis induce MGCs with a low number of nuclei per cell, fewer than seven [30]. The high-virulence mycobacterium species resulted in large granulomas where the MGCs are incapable of phagocytosis but still retain a strong antigen-presentation capability, thus appearing to be devoted to the destruction of bacilli. In a report of idiopathic disseminated bacille Calmette–Guérin (BCG) infection, Emile et al. found two types of granuloma [31]. The first type (tuberculoid type) consisted of well-circumscribed and well-differentiated granulomas, with epithelioid and MGCs containing very few acid-fast rods, surrounded by lymphocytes and fibrosis and occasionally with central caseous necrosis. The second (lepromatous) type consisted of ill-defined and poorly differentiated granulomas, with few if any giant cells and lymphocytes but widespread macrophages loaded with acid-fast bacilli. There was a strong correlation between the type of granuloma and clinical outcome. The tuberculoid type was associated with patients' survival, while the lepromatous type was associated with their poor outcome and death [32,33]. Furthermore, the histopathological appearances of congenital tuberculosis in the literature are similar to the lepromatous type, and are described as being composed of central caseous necrosis with surrounding scanty epithelioid cells and lymphocytes and few or no MGCs [34–36].
These findings suggest the importance of the function of MGCs in preventing critical infection with mycobacterium. Our results showed that MGCs from cord blood monocytes had only half the number of nuclei in each cell than MGCs from adult peripheral blood. The histopathological appearances of congenital tuberculosis can be explained partly as the result of poor mobilization of phagocytes, decreased production of cytokines (especially IFN-γ) by T lymphocytes [3] and impaired formation of MGCs in the neonates.
Additional investigations are needed to confirm the decreased ability of cord blood monocytes to fuse into MGCs in other experimental conditions, and to determine what mechanism or molecule (cell surface fusion molecules) underlies the decreased ability of cord blood monocytes to fuse into MGCs. Additional research regarding the physiopathology of MGC in neonates might be helpful for the appropriate treatment of infection with microorganisms which are capable of surviving intracellularly.
Disclosure
None.
References
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and wales. Lancet. 1986;8489:1077–81. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Baker CJ, Melish ME, Hall RT, Casto DT, Vasan U, Givner LB. Interavenous immune globulin for the prevention of nosocomial infection in low-birth-weight neonates. The multicenter group for the study of immune globulin in neonates. N Engl J Med. 1992;327:213–19. doi: 10.1056/NEJM199207233270401. [DOI] [PubMed] [Google Scholar]
- 3.Smith S, Jacobs RF, Wilson CB. Immunobiology of childhood tuberculosis: a window on the ontogeny of cellular immunity. J Pediatr. 1997;131:16–26. doi: 10.1016/s0022-3476(97)70120-3. [DOI] [PubMed] [Google Scholar]
- 4.Serushago B, Macdonald C, Lee SH, Stadnyk A, Bortoiussi R. Interferon-gamma detection in cultures of newborn cells exposed to Listeria monocytogenes. J Interferon Cytokine Res. 1995;15:633–5. doi: 10.1089/jir.1995.15.633. [DOI] [PubMed] [Google Scholar]
- 5.Shore SL, Milgrom H, Wood PA, Nahmias AJ. Antibody-dependent celluar toxicity to target cells infected with herpes simplex viruses: functional adequacy in the neonate. Pediatrics. 1977;59:22–8. [PubMed] [Google Scholar]
- 6.Wilson CB. Immunologic basis for increased susceptibility of the neonate to infection. J Pediatr. 1986;108:1–12. doi: 10.1016/s0022-3476(86)80761-2. [DOI] [PubMed] [Google Scholar]
- 7.Hill HR. Biochemical, structural, and functional abnormalities of polymorphonuclear leukocytes in the neonate. Pediatr Res. 1987;22:375–82. doi: 10.1203/00006450-198710000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Black MM, Fukuyama K, Epstein WL. The induction of human multinucleated monocytes in culture. J Invest Dermatol. 1976;66:90–2. doi: 10.1111/1523-1747.ep12481419. [DOI] [PubMed] [Google Scholar]
- 9.Helming L, Gordon S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur J Immunol. 2007;37:33–42. doi: 10.1002/eji.200636788. [DOI] [PubMed] [Google Scholar]
- 10.Ikeda T, Ikeda K, Sasaki K, et al. IL-13 as well as IL-4 induces monocytes/macrophages and a monoblastic cell line (UG3) to differentiate into multinucleated giant cells in the presence of M-CSF. Biochem Biophys Res Commun. 1998;253:265–72. doi: 10.1006/bbrc.1998.9702. [DOI] [PubMed] [Google Scholar]
- 11.Lemaire HY, Lauzon W, Gendron N. M-CSF and GM-CSF promote alveolar macrophage differentiation into multinucleated giant cells with distinct phenotypes. J Leukoc Biol. 1995;60:509–18. doi: 10.1002/jlb.60.4.509. [DOI] [PubMed] [Google Scholar]
- 12.Enelow RI, Sullivan GW, Carper HT, Mandell GL. Induction of multinucleated giant cell formation from in vitro culture of human monocytes with interleukin-3 and interferon-gamma: comparison with other stimulating factors. Am J Respir Cell Mol Biol. 1992;6:57–62. doi: 10.1165/ajrcmb/6.1.57. [DOI] [PubMed] [Google Scholar]
- 13.McNally AK, Anderson JM. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Differential lymphokine regulation of macrophage fusion leads to morphological variants of multinucleated giant cells. Am J Pathol. 1995;147:1487–99. [PMC free article] [PubMed] [Google Scholar]
- 14.Maclauchlan S, Skokos EA, Meznarich N, et al. Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9. J Leukoc Biol. 2009;85:617–26. doi: 10.1189/jlb.1008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNally AK, Anderson JM. Beta1 and beta2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation. Am J Pathol. 2002;160:621–30. doi: 10.1016/s0002-9440(10)64882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han X, Sterling H, Chen Y, et al. CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J Biol Chem. 2000;275:37984–92. doi: 10.1074/jbc.M002334200. [DOI] [PubMed] [Google Scholar]
- 17.Chiozzi P, Sanz JM, Ferrari D, et al. Spontaneous cell fusion in macrophage cultures expressing high levels of the P2Z/P2X7 receptor. J Cell Biol. 1997;138:697–706. doi: 10.1083/jcb.138.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fais S, Burgio VL, Silvestri M, Capobianchi MR, Pacchiarotti A, Pallone F. Multinucleated giant cells generation induced by interferon-gamma. Changes in the expression and distribution of the intercellular adhesion molecule-1 during macrophages fusion and multinucleated giant cell formation. Lab Invest. 1994;71:737–44. [PubMed] [Google Scholar]
- 19.Yagi M, Miyamoto T, Toyama Y, Suda T. Role of DC-STAMP in cellular fusion of osteoclasts and macrophage giant cells. J Bone Miner Metab. 2006;24:355–8. doi: 10.1007/s00774-006-0697-9. [DOI] [PubMed] [Google Scholar]
- 20.Kyriakides TR, Foster MJ, Keeney GE, et al. The CC chemokine ligand, CCL2/MCP1, participates in macrophage fusion and foreign body giant cell formation. Am J Pathol. 2004;165:2157–66. doi: 10.1016/S0002-9440(10)63265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Namba K, Nishio M, Mori K, et al. Involvement of ADAM9 in multinucleated giant cell formation of blood monocytes. Cell Immunol. 2001;213:104–13. doi: 10.1006/cimm.2001.1873. [DOI] [PubMed] [Google Scholar]
- 22.Ohgimoto S, Tabata N, Suga S, et al. Molecular characterization of fusion regulatory protein-1 (FRP-1) that induces multinucleated giant cell formation of monocytes and HIV gp160-mediated cell fusion. FRP-1 and 4F2/CD98 are identical molecules. J Immunol. 1995;155:3585–92. [PubMed] [Google Scholar]
- 23.McNally AK, Macewan SR, Anderson JM. Foreign body-type multinucleated giant cell formation requires protein kinase C beta, delta, and zeta. Exp Mol Pathol. 2008;84:37–45. doi: 10.1016/j.yexmp.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orentas RJ, Reinlib L, Hildreth JE. Anti-class II MHC antibody induces multinucleated giant cell formation from peripheral blood monocytes. J Leukoc Biol. 1992;51:199–209. doi: 10.1002/jlb.51.3.199. [DOI] [PubMed] [Google Scholar]
- 25.Tabata N, Ito M, Shimokata K, et al. Expression of fusion regulatory proteins (FRPs) on human peripheral blood monocytes. Induction of homotypic cell aggregation and formation of multinucleated giant cells by anti-FRP-1 monoclonal antibodies. J Immunol. 1994;153:3256–66. [PubMed] [Google Scholar]
- 26.McNally AK, DeFife KM, Anderson JM. Interleukin-4-induced macrophage fusion is prevented by inhibitors of mannose receptor activity. Am J Pathol. 1996;149:975–85. [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg TH, Hiken JF. P2 receptors in macrophage fusion and osteoclast formation. Purinergic Signal. 2007;3:53–7. doi: 10.1007/s11302-006-9036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.zur Hausen H, de Villers EM. Syncytium formation in aged umbilical cord blood macrophage. Attempts to demonstrate an infectious etiology. Med Microbiol Immunol. 1982;170:229–40. doi: 10.1007/BF02123313. [DOI] [PubMed] [Google Scholar]
- 29.Gerberding K, Yoder MC. In vitro comparison of multinucleated giant cell formation from human umbilical cord and adult peripheral blood mononuclear phagocytes. Pediatr Res. 1993;33:19–26. doi: 10.1203/00006450-199301000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Lay G, Poquet Y, Salek-Peyron P, et al. Langhans giant cells from M. tuberculosis-induced human granulomas cannot mediate mycobacterial uptake. J Pathol. 2007;211:76–85. doi: 10.1002/path.2092. [DOI] [PubMed] [Google Scholar]
- 31.Emile JF, Patey N, Altare F, et al. Correlation of granuloma structure with clinical outcome defines two types of idiopathic disseminated BCG infection. J Pathol. 1997;181:25–30. doi: 10.1002/(SICI)1096-9896(199701)181:1<25::AID-PATH747>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 32.Saunders BM, Frank AA, Orme IM. Granuloma formation is required to contain bacillus growth and delay mortality in mice chronically infected with Mycobacterium tuberculosis. Immunology. 1999;98:324–8. doi: 10.1046/j.1365-2567.1999.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrd TF. Multinucleated giant cell formation induced by IFN-gamma/IL-3 is associated with restriction of virulent Mycobacterium tuberculosis cell to cell invasion in human monocyte monolayers. Cell Immunol. 1998;188:89–96. doi: 10.1006/cimm.1998.1352. [DOI] [PubMed] [Google Scholar]
- 34.Machin GA, Honoré LH, Fanning EA, Molesky M. Perinatally acquired neonatal tuberculosis: report of two cases. Pediatr Pathol. 1992;12:707–16. doi: 10.3109/15513819209024224. [DOI] [PubMed] [Google Scholar]
- 35.Gögüs S, Umer H, Akçören Z, Sanal O, Osmanlioglu G, Cimbiş M. Neonatal tuberculosis. Pediatr Pathol. 1993;13:299–304. doi: 10.3109/15513819309048216. [DOI] [PubMed] [Google Scholar]
- 36.Kang GH, Chi JG. Congenital tuberculosis – report of an autopsy case. J Korean Med Sci. 1990;5:59–64. doi: 10.3346/jkms.1990.5.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasui K, Yamazaki M, Miyabayashi M, Tsuno T, Komiyama A. Signal transduction pathway in human polymorphonuclear leukocytes for chemotaxis induced by a chemotactic factor. J Immunol. 1994;152:5922–28. [PubMed] [Google Scholar]
- 38.Laver J, Duncan E, Abboud M, et al. High levels of granulocyte and granulocyte macrophage colony stimulating factors in cord blood of normal full-term infants. J Pediatr. 1990;116:627–32. doi: 10.1016/s0022-3476(05)81617-8. [DOI] [PubMed] [Google Scholar]


