Abstract
Intravenous immunoglobulin (IVIg) is used for treatment of a variety of immunological disorders and in transplantation. As one of its applications in transplantation is the reduction of donor specific antibodies in the circulation, we examined the direct effect of IVIg on essential parameters of human B cell responses in vitro. Purified human B cells, human B cell hybridomas and T cells were cultured in the presence of graded concentrations of IVIg to test its effect on their proliferative capacity. To address the effect of IVIg on immunoglobulin production, we designed a novel technique making use of quantitative polymerase chain reaction to assess IgM and IgG levels. IVIg failed to inhibit proliferation of human B cells and human B cell hybridomas. In contrast, when IVIg was added to T cell cultures, a dose-dependent reduction of the proliferative capacity was observed. IVIg did not affect the levels of IgM and IgG mRNA of activated B cells. Our data show that IVIg is not capable of directly inhibiting key B cell responses. Direct B cell inhibition by IVIg seems therefore unlikely, implying that alteration in humoral immunity by IVIg is due to indirect effects on T cells and/or interactions with circulating antibodies and complement factors.
Keywords: antibodies, humoral, IVIg, proliferation, transplantation
Introduction
Intravenous immunoglobulin (IVIg) is a preparation of pooled human plasma prepared from more than 1000 donors and was used originally as a substitution therapy for patients with immunodeficiencies [1]. IVIg is used increasingly as treatment for autoimmune and systemic inflammatory diseases, and in the field of solid organ and bone marrow transplantation. In organ transplantation settings IVIg is used, among other treatments, to reduce plasma human leucocyte antigen (HLA) antibody levels prior to transplantation [2–4] and in attempts to reverse acute humoral rejection [5].
The present study focuses on the effect of IVIg on humoral immunity. Several mechanisms have been proposed for its mode of action. First, anti-idiotypic antibodies in IVIg may interfere in the reaction of HLA-specific antibodies with their targets on HLA mismatched organs, thereby preventing humoral rejection [6]. Secondly, IVIg may bind through the Fc portion of IgG to the inhibitory FcγRIIb expressed on a variety of blood cells, including B cells, leading to the reduction of proliferation and apoptosis induction [7]. Thirdly, IVIg may inhibit complement, and thereby modulate the effector function of antibodies [8]. Fourthly, changes in monocyte Fc receptor functions can inhibit antibody-mediated immune damage [9]. Lastly, clearance of pathogenic antibodies may be accelerated by saturation of the FcRn receptor [10].
We used a robust culture system, devoid of auxiliary cells, for stimulating purified human B cells, as well as autonomously proliferating human B cell hybridomas to study the direct effect of IVIg on B cell proliferation and immunoglobulin production. The latter was assessed by a novel, polymerase chain reaction (PCR)-based technique for quantifying immunoglobulin production.
Materials and methods
Cells
Blood was collected from healthy blood bank donors with informed consent under guidelines issued by the Medical Ethics Committee of the Leiden University Medical Center (Leiden, the Netherlands). Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll Hypaque density gradient centrifugation. B cells were isolated immunomagnetically from PBMC by positive selection using Dynabeads CD19 pan B (Invitrogen, Leek, the Netherlands) and released from the beads using Detach-a-Bead CD19 (Invitrogen). This yielded > 98% pure B cells, as assessed by flow cytometric analysis. Human B cell hybridomas producing a variety of HLA monoclonal antibodies (mAbs) were established as described previously [11]. The hybridomas used were SN607D8 (HLA-A2/A28, IgG1), BVK5B10 (HLA-B8, IgM), VTM1F11 (HLA-B27/B7/B60, IgG1) and SN66E3 (HLA-A2/A28, IgM).
T cells were purified from PBMC by magnetic separation using the Pan T cell isolation kit II (Miltenyi, Bergisch-Gladbach, Germany) and magnetic affinity cell sorting (MACS) MS columns (Miltenyi). Flow cytometric analysis revealed > 85% purity.
Proliferation experiments
B cells were cultured for 7 days at 5 × 103 cells/well in 96-well round-bottomed plates (BD Falcon, Breda, the Netherlands) in culture medium consisting of Iscove's modified Dulbecco's medium (IMDM) (Gibco, Paisley, UK) supplemented with 10% fetal calf serum (FCS) (Gibco), 0·05 mM 2-mercaptoethanol (Sigma-Aldrich, Zwijndrecht, the Netherlands) and insulin–transferrin–selenium (ITS) (insulin 5 µg/ml, transferrin 5 µg/ml and selenium 5 ng/ml; Sigma-Aldrich) at 37°C and 5% CO2. B cells were stimulated with anti-CD40 mAb (1 µg/ml; R&D Systems, Minneapolis, MN, USA), 100 U/ml interleukin (IL)-2 (EuroCetus, Amsterdam, the Netherlands), 25 ng/ml IL-10 (R&D Systems), 100 ng/ml IL-21 (Invitrogen) and 2·5 µg/ml of the Toll-like receptor (TLR)-9 ligand cytosine-guanine dinucleotide oligodeoxynucleotide (CpG ODN) 2006 (Hycult Biotechnology, Uden, the Netherlands). T cells were stimulated with 1 µg/ml phytohaemagglutinin (PHA) (Remel, Lenexa, KS, USA) and cultured at 104 cells/well for 3 days in culture medium without ITS. Autonomously growing B cell hybridomas were cultured at 103 cells/well for 5 days in IMDM medium with 5% FCS, 0·05 mM 2-mercaptoethanol and 2 mM L-glycyl-L-glutamine (Sigma-Aldrich).
All cultures were performed in the absence or presence of graded concentrations of IVIg (Sanquin, Amsterdam, the Netherlands). Multiple lot numbers of IVIg were used, giving comparable results. Proliferation experiments were also performed with Gammagard (Baxter, Utrecht, the Netherlands), which gave similar results. Physical properties of the IVIg preparations are shown in Table 1. All data shown were obtained with IVIg from Sanquin. All IVIg preparations were dialysed prior to use unless stated otherwise. Dialysis was performed in 10 kDa Slide-A-Lyzer cassettes (Pierce, Rockford, IL, USA) against large volumes of IMDM. The presence of intact IgG after dialysis was confirmed by enzyme-linked immunosorbent assay (ELISA) (data not shown). Where appropriate, cell culture grade bovine serum albumin (BSA) (Calbiochem, La Jolla, CA, USA) was used as a control for the high protein concentrations and D(+) Glucose (Merck, Amsterdam, the Netherlands) was used as a control for the stabilizing agent of IVIg. Proliferation was determined by the incorporation of [3H]-tritiated thymidine ([3H]-TdR) (1 µCi/well; Amersham International, Amersham, UK), added 18 h before termination of culture.
Table 1.
Physical properties of intravenous immunoglobulin (IVIg) preparations used.
| Product | Manufacturer | Method of preparation | % IgG | Stabilizing agent |
|---|---|---|---|---|
| Immunoglobulin i.v. | Sanquin | Cohn's cold ethanol fractionation | > 95% | Glucose |
| Gammagard S/D | Baxter | Cohn–Oncley-fractionation, ion-exchange, ultrafiltration, chromatography | > 90% | Glucose, glycine, albumin, PEG |
IgG, immunoglobulin G; i.v., intravenous; PEG, polyethylene glycol.
Quantitative PCR
For IgM and IgG mRNA detection, B cells were cultured at 5 × 105 cells per well for 7 days in 24-well plates (Costar, Veenendaal, the Netherlands) and stimulated as described above in the presence of graded concentrations of IVIg. Rapamycin was used as a control for the inhibition of immunoglobulin production [12]. Cells were harvested and preserved in RNAlater solution (Qiagen, Chatsworth, CA, USA). RNA was extracted using the Rneasy® mini kit (Qiagen) following the manufacturer's instructions. RNA was treated with DNase (Qiagen) on the spin columns. RNA quantity was assessed with a spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). All samples showed A260/A280 ratios between 1·9 and 2·1. cDNA was synthesized by incubating 12·8 µl RNA solution with 7·2 µl cDNA mix containing 2′-deoxynucleosides 5′-triphosphate (dNTPs) (final concentration of 0·5 mM), 2 U reverse transcriptase–avian myeloblastosis virus (RT–AMV), 20 U rRNase inhibitor, 100 ng oligo-dT primers, 500 ng random primers and 1 × reverse transcriptase buffer (all from Promega, Leiden, the Netherlands).
Primer sets (Table 2) for quantitative polymerase chain reaction (Q-PCR) were selected using Beacon Designer Software (version 7·02; Premier Biosoft International, Palo Alto, CA, USA) and obtained from Eurogentec (Liège, Belgium). For the amplification of total IgG transcript two forward primers, one hybridizing to the IgG1 sequence and the other to IgG2, IgG3 and IgG4 sequences, were used at a 1:1 ratio. The IgG reverse primer detected all four IgG variants. PCR mixes contained 1 µM of forward and reverse primers, 3 mM MgCl2 and 1 × iQ SYBR Green supermix (Bio-Rad, Veenendaal, the Netherlands). The PCR was performed using an iCycler MyiQ (Bio-Rad). The PCR program consisted of one cycle of 10 min at 95°C, 40 cycles of 15 s at 95°C and 1 min at 60°C and was finalized with a melting curve analysis. Reactions were carried out in optical 96-well plates (Bio-Rad) covered with Microseal ‘B’ film (Bio-Rad). The signal of the stably expressed household gene 18S rRNA served as a normalization factor.
Table 2.
Sequences for primers used in quantitative polymerase chain reaction (Q-PCR).
| Transcript | Forward primer | Reverse primer | Amplicon |
|---|---|---|---|
| IgG1 | CATCTCCAAAGCCAAAGG | ATGTCGCTGGGATAGAAG | 126 bp |
| IgG2–4 | CATCTCCAAAGCCAAAGG | ATGTCGCTGGGGTAGAAG | 126 bp |
| IgM | CAGGGCACAGACGAACAC | CGGCAATCACTGGAAGAGG | 85 bp |
| 18S rRNA | AGTCCCTGCCCTTTGTACACA | GATCCGAGGGCCTCACTAAAC | 68 bp |
Ig, immunoglobulin; bp, base pairs.
Results
IVIg fails to inhibit B cell and B cell hybridoma proliferation
IVIg (at concentrations up to 35 mg/ml) failed to affect the proliferative capacity of both purified in vitro stimulated B cells from different donors (n = 5) as well as four autonomously growing B cell hybridomas (Fig. 1a and b). In contrast, non-dialysed IVIg did inhibit the proliferation of B cell hybridomas (Fig. 1c), which is in agreement with published data [13]. However, inhibition was not due to the immunoglobulin component, as is clear from the lack of proliferation inhibition with dialysed IVIg, but caused rather by the stabilizing agent glucose that is present in the IVIg solution (Fig. 1d).
Fig. 1.
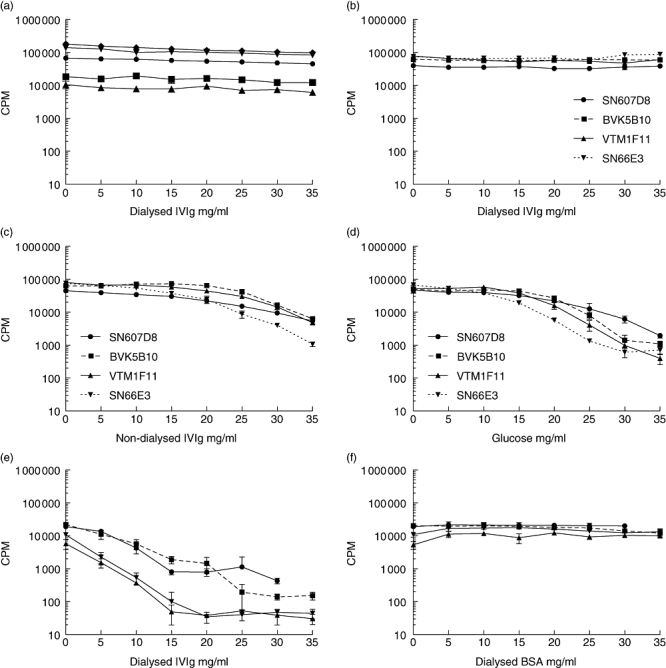
Intravenous immunoglobulin (IVIg) does not reduce the proliferative capacity of B cells and B cell hybridomas, whereas mitogen-induced T cell proliferation is inhibited. Non-dialysed IVIg does inhibit hybridoma proliferation, caused by the stabilizing agent glucose. B cells were cultured at 5 × 103 cells/well with the addition of anti-CD40 monoclonal antibody (mAb), interleukin (IL)-2, IL-10, IL-21 and cytosine-guanine dinucleotide oligodeoxynucleotide (CpG ODN) 2006 in the presence of graded concentrations of IVIg. Connecting lines represent the individuals donors (n = 5) tested (a). Autonomously growing B cell hybridomas were cultured at 103 cells/well in the presence of graded concentrations of IVIg (b), non-dialysed IVIg (c) or glucose in concentrations similar to the IVIg solution (d). Connecting lines represent individual hybridomas. Representative experiments are shown. T cells were isolated by magnetic affinity cell sorting (MACS) isolation and cultured at 104 cells per well with 1 mg/ml phytohaemagglutinin (PHA) in the presence of graded concentrations of IVIg (e) or bovine serum albumin (BSA) in concentrations equal to the IVIg solution (f). Connecting lines represent the individuals donors (n = 4) tested. Proliferation was measured by [3H]-tritiated thymidine ([3H]-TdR) incorporation; cpm: counts per minute. Depicted are mean values of triplicate measurements.
Because IVIg has been described to inhibit mitogen-induced lymphocyte proliferation [14], we tested its capacity to inhibit PHA induced T cell proliferation (n = 4). The IVIg preparation inhibited T cell proliferation dose-dependently, indicating that the IVIg preparation was functional (Fig. 1e). This inhibition was not due to high protein concentration, as shown by the inability of comparable concentrations of BSA to inhibit proliferation (Fig. 1f).
IVIg does not inhibit immunoglobulin mRNA levels
Previously, we showed that B cells cultured in the presence of anti-CD40 mAb, IL-2, IL-10, IL-21 and CpG ODN 2006 were capable of producing large quantities of both IgM and IgG [12]. This stimulation protocol induced vast elevations in mRNA levels for both IgM and IgG when compared to unstimulated B cells (Fig. 2a and b), which is in concordance with immunoglobulin levels measured in the supernatants of these B cell cultures by ELISA (data not shown).
Fig. 2.
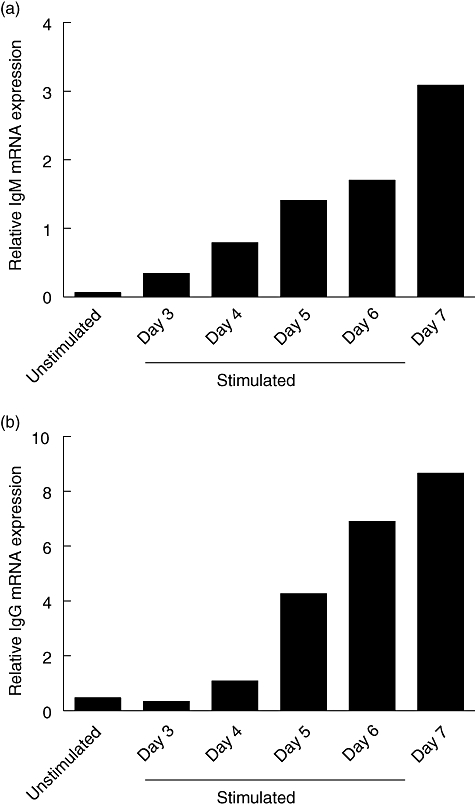
B cell activation induces immunoglobulin (Ig)M and IgG mRNA levels. B cells were activated with anti-CD40 monoclonal antibody (mAb), interleukin (IL)-2, IL-10, IL-21 and cytosine-guanine dinucleotide oligodeoxynucleotide (CpG ODN) 2006 and the cells were harvested at different time-points after activation. IgM (a) and IgG (b) mRNA levels were assessed by quantitative polymerase chain reaction. Unstimulated cells were harvested at day 0 and served as negative control. The results of one representative experiment are shown. Similar results were obtained in four independent experiments.
To test the effect of IVIg on immunoglobulin production, B cells were stimulated in the presence of IVIg at graded concentrations up to 35 mg/ml. Rapamycin was used as positive control for the reduction of immunoglobulin production. Q-PCR with primers specific for IgM and IgG revealed that IVIg did not affect the levels of IgM or IgG mRNA, whereas rapamycin reduced IgM and IgG mRNA levels dose-dependently (Fig. 3a and b).
Fig. 3.

Intravenous immunoglobulin (IVIg) does not affect the mRNA synthesis of IgM and IgG, whereas rapamycin does inhibit IgM and IgG mRNA levels. B cells were stimulated with anti-CD40 mAb, interleukin (IL)-2, IL-10, IL-21 and cytosine-guanine dinucleotide oligodeoxynucleotide (CpG ODN) 2006 and cultured in the presence of graded concentrations of IVIg (a). Control cultures were performed with graded concentrations of rapamycin. mRNA levels for IgM and IgG were quantified using quantitative polymerase chain reaction (b). The dotted lines represent the mRNA steady state level in stimulated, non-treated B cells. Data from three experiments with different donors are shown. Depicted are the percentages of IgM and IgG mRNA expression, relative to the mRNA expression of untreated controls. Statistics: paired t-test, *P < 0·05; **P < 0·01 and ***P < 0·001.
Discussion
IVIg is known to have a wide range of clinical effects. When used in vitro, IVIg reduced the reactivity of serum HLA antibodies [15,16]. It also inhibited mixed lymphocyte responses and mitogen-induced PBMC and T cell proliferation [14], as well as T cell-dependent B cell responses [17–20].
The present study shows that IVIg does not affect the proliferative capacity of polyclonally stimulated purified B cells. This lack of inhibition is not due to extremely high B cell stimulation that is no longer amenable to interference, as we have shown previously that relatively low levels of standard immunosuppressive drugs can abolish proliferation completely, as well as immunoglobulin production of B cells stimulated with the same protocol [12]. In contrast to the lack of B cell proliferation inhibition by IVIg, mitogen-induced T cell proliferation was inhibited significantly by the same IVIg preparation, indicating that the IVIg preparation was effective. This inhibition was not due to high protein levels, indicating that immunoglobulins present in the IVIg preparation were the cause of proliferation inhibition.
B cell hybridomas have been shown previously to be inhibited by a non-dialysed IVIg preparation [13]. We added non-dialysed IVIg to similar B cell hybridomas and observed a comparable inhibition. However, when we dialysed the IVIg prior to use to remove stabilizing agents such as glucose from the solution, the inhibition was abrogated. In agreement with previous studies [21], addition of glucose in concentrations similar to those in the IVIg preparation did inhibit the proliferation of B hybridomas. These data underscore the importance of dialysis of the IVIg preparation for in vitro studies.
Besides B cell proliferation, studying the effects of IVIg on immunoglobulin production is of particular interest, as anti-idiotypic antibody binding and binding to inhibitory Fc receptors are candidate mechanisms for interference with immunoglobulin production [6,7].
Unfortunately, the high IgG content of IVIg precludes the detection of newly synthesized IgG by standard ELISA techniques. De Grandmont et al. circumvented this issue by radioactive labelling of secreted immunoglobulins followed by capture with anti-IgG antibodies [22]. However, even then, competition by IVIg for capture antibody cannot be excluded. Therefore, we designed a novel approach to indirectly measure immunoglobulin production by detection of IgM and IgG mRNA levels. We found that IVIg does not affect the mRNA synthesis of both IgM and IgG by stimulated B cells. It therefore seems highly unlikely that IVIg influences immunoglobulin production directly.
This conclusion is at variance with other studies that show an inhibitory effect of IVIg on immunoglobulin production [17–20]. These studies, which describe B cells as targets for IVIg, were performed with pokeweed mitogen stimulation, which is a model of T cell-dependent antibody production by B cells [23]. However, it cannot be ruled out that in that culture system IVIg interferes with the actual stimulus, i.e. pokeweed mitogen, rather than that it truly inhibits B cell functions. In another study, inhibition of proliferation and IgE secretion of purified tonsillar B cells cultured with IL-4 and anti-CD40 was described [24]. However, these results were obtained using a non-dialysed IVIg preparation, and are probably an effect of the stabilizing agents in the IVIg preparation.
IVIg preparations differ greatly in terms of production process and Ig content. While B cell inhibitory effects were not found in two different IVIg preparations, it cannot be ruled out that other preparations will show inhibitory effects, particularly when other isotypes are present, as in pentaglobulin.
In line with our results, a series of in vivo studies show the lack of direct effects on immunoglobulin-producing cells. IVIg was unable to reduce B cell and plasma cell numbers in human splenic follicles [25]. Furthermore, IVIg failed to reduce the number of bone marrow residing long-lived plasma cells producing HLA-specific antibodies [26].
The inhibitory effect of IVIg on humoral immunity may therefore be explained by alternative mechanisms, such as inhibition of T cell help, interaction with circulating antibodies and interaction with complement factors. Inhibitory effects of IVIg on T cell priming by dendritic cells have been described [27], as well as cytokine-dependent T cell proliferation [28]. Inhibition of T cell function may lead to insufficient generation of helper T cells, which affects B cell responses. Future research on the effect of IVIg on T cell help directly, and on T cell-dependent B cell responses in co-culture systems, will provide more insight in the effect of IVIg on humoral immune responses.
Interaction of the variable regions of IVIg with variable regions of autoantibodies of multiple specificities has been described [29,30], suggesting anti-idiotypic activity of IVIg. Circulating anti-HLA antibodies may be subject to the same mechanism of suppression. In addition, interference of IVIg with complement activation [8,31] and impairment of monocyte Fc receptor functions [9] have been suggested. These mechanisms may affect the action of deleterious complement binding antibodies and/or phagocytosis, leading to protection from immune damage. Alternatively, saturation of the FcRn receptor may be involved in clearance of pathogenic antibodies [10].
Taken together, our results show that IVIg fails to act directly on immunoglobulin producing cells. The capability of IVIg to interfere with humoral immunity is therefore due more probably to effects on T cells and interactions with circulating antibodies and/or complement factors.
Acknowledgments
This work was supported by The Landsteiner Foundation for Blood Transfusion Research and the National Reference Center for Histocompatibility Testing. The authors thank Jacqueline Anholts for technical assistance in the quantitative PCR assays and Cees van Kooten and Anneke Brand for critical reading of the manuscript.
Disclosure
The authors have no financial conflicts of interest.
References
- 1.Buckley RH, Schiff RI. The use of intravenous immune globulin in immunodeficiency diseases. N Engl J Med. 1991;325:110–17. doi: 10.1056/NEJM199107113250207. [DOI] [PubMed] [Google Scholar]
- 2.Glotz D, Antoine C, Julia P, et al. Desensitization and subsequent kidney transplantation of patients using intravenous immunoglobulins (IVIg) Am J Transplant. 2002;2:758–60. doi: 10.1034/j.1600-6143.2002.20809.x. [DOI] [PubMed] [Google Scholar]
- 3.Jordan SC, Vo A, Bunnapradist S, et al. Intravenous immune globulin treatment inhibits crossmatch positivity and allows for successful transplantation of incompatible organs in living-donor and cadaver recipients. Transplantation. 2003;76:631–6. doi: 10.1097/01.TP.0000080685.31697.FC. [DOI] [PubMed] [Google Scholar]
- 4.Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359:242–51. doi: 10.1056/NEJMoa0707894. [DOI] [PubMed] [Google Scholar]
- 5.Rocha PN, Butterly DW, Greenberg A, et al. Beneficial effect of plasmapheresis and intravenous immunoglobulin on renal allograft survival of patients with acute humoral rejection. Transplantation. 2003;75:1490–5. doi: 10.1097/01.TP.0000060252.57111.AC. [DOI] [PubMed] [Google Scholar]
- 6.Jordan SC, Vo AA, Tyan D, Nast CC, Toyoda M. Current approaches to treatment of antibody-mediated rejection. Pediatr Transplant. 2005;9:408–15. doi: 10.1111/j.1399-3046.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 7.Ott VL, Fong DC, Cambier JC. Fc gamma RIIB as a potential molecular target for intravenous gamma globulin therapy. J Allergy Clin Immunol. 2001;108:S95, 8. doi: 10.1067/mai.2001.117822. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe J, Scornik JC. IVIG and HLA antibodies. Evidence for inhibition of complement activation but not for anti-idiotypic activity. Am J Transplant. 2005;5:2786–90. doi: 10.1111/j.1600-6143.2005.01056.x. [DOI] [PubMed] [Google Scholar]
- 9.Kimberly RP, Salmon JE, Bussel JB, Crow MK, Hilgartner MW. Modulation of mononuclear phagocyte function by intravenous gamma-globulin. J Immunol. 1984;132:745–50. [PubMed] [Google Scholar]
- 10.Hansen RJ, Balthasar JP. Intravenous immunoglobulin mediates an increase in anti-platelet antibody clearance via the FcRn receptor. Thromb Haemost. 2002;88:898–9. [PubMed] [Google Scholar]
- 11.Mulder A, Kardol M, Regan J, Buelow R, Claas F. Reactivity of twenty-two cytotoxic human monoclonal HLA antibodies towards soluble HLA class I in an enzyme-linked immunosorbent assay (PRA-STAT) Hum Immunol. 1997;56:106–13. doi: 10.1016/s0198-8859(97)00146-8. [DOI] [PubMed] [Google Scholar]
- 12.Heidt S, Roelen DL, Eijsink C, van Kooten C, Claas FH, Mulder A. Effects of immunosuppressive drugs on purified human B cells: evidence supporting the use of MMF and rapamycin. Transplantation. 2008;86:1292–300. doi: 10.1097/TP.0b013e3181874a36. [DOI] [PubMed] [Google Scholar]
- 13.van Schaik IN, Lundkvist I, Vermeulen M, Brand A. Polyvalent immunoglobulin for intravenous use interferes with cell proliferation in vitro. J Clin Immunol. 1992;12:325–34. doi: 10.1007/BF00920789. [DOI] [PubMed] [Google Scholar]
- 14.Klaesson S, Ringden O, Markling L, Remberger M, Lundkvist I. Immune modulatory effects of immunoglobulins on cell-mediated immune responses in vitro. Scand J Immunol. 1993;38:477–84. doi: 10.1111/j.1365-3083.1993.tb02591.x. [DOI] [PubMed] [Google Scholar]
- 15.Glotz D, Haymann JP, Sansonetti N, et al. A-specific alloantibodies by high-dose intravenous immunoglobulins (IVIg). A potential tool for transplantation of immunized patients. Transplantation. 1993;56:335–7. doi: 10.1097/00007890-199308000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Tyan DB, Li VA, Czer L, Trento A, Jordan SC. Intravenous immunoglobulin suppression of HLA alloantibody in highly sensitized transplant candidates and transplantation with a histoincompatible organ. Transplantation. 1994;57:553–62. [PubMed] [Google Scholar]
- 17.Stohl W. Cellular mechanisms in the in vitro inhibition of pokeweed mitogen-induced B cell differentiation by immunoglobulin for intravenous use. J Immunol. 1986;136:4407–13. [PubMed] [Google Scholar]
- 18.Stohl W. Modulation of the immune response by immunoglobulin for intravenous use. I. Inhibition of pokeweed mitogen-induced B cell differentiation. Clin Exp Immunol. 1985;62:200–7. [PMC free article] [PubMed] [Google Scholar]
- 19.Kondo N, Ozawa T, Mushiake K, et al. Suppression of immunoglobulin production of lymphocytes by intravenous immunoglobulin. J Clin Immunol. 1991;11:152–8. doi: 10.1007/BF00918683. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto F, Sakiyama Y, Matsumoto S. The suppressive effect of gammaglobulin preparations on in vitro pokeweed mitogen-induced immunoglobulin production. Clin Exp Immunol. 1986;65:409–15. [PMC free article] [PubMed] [Google Scholar]
- 21.Alder LB, Morgan LA, Spickett GP. Contribution of stabilizing agents present in intravenous immunoglobulin preparations to modulation of mononuclear cell proliferation in vitro. Scand J Immunol. 1996;44:585–91. doi: 10.1046/j.1365-3083.1996.d01-350.x. [DOI] [PubMed] [Google Scholar]
- 22.de Grandmont MJ, Racine C, Roy A, Lemieux R, Neron S. Intravenous immunoglobulins induce the in vitro differentiation of human B lymphocytes and the secretion of IgG. Blood. 2003;101:3065–73. doi: 10.1182/blood-2002-06-1684. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa N, Nakagawa T, Volkman DJ, Ambrus JL, Jr, Fauci AS. The role of interleukin 2 in inducing Ig production in a pokeweed mitogen-stimulated mononuclear cell system. J Immunol. 1987;138:795–801. [PubMed] [Google Scholar]
- 24.Sigman K, Ghibu F, Sommerville W, et al. Intravenous immunoglobulin inhibits IgE production in human B lymphocytes. J Allergy Clin Immunol. 1998;102:421–7. doi: 10.1016/s0091-6749(98)70130-7. [DOI] [PubMed] [Google Scholar]
- 25.Ramos EJ, Pollinger HS, Stegall MD, Gloor JM, Dogan A, Grande JP. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant. 2007;7:402–7. doi: 10.1111/j.1600-6143.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 26.Perry DK, Pollinger HS, Burns JM, et al. Two novel assays of alloantibody-secreting cells demonstrating resistance to desensitization with IVIG and rATG. Am J Transplant. 2008;8:133–43. doi: 10.1111/j.1600-6143.2007.02039.x. [DOI] [PubMed] [Google Scholar]
- 27.Tha-In T, Metselaar HJ, Tilanus HW, et al. Intravenous immunoglobulins suppress T-cell priming by modulating the bidirectional interaction between dendritic cells and natural killer cells. Blood. 2007;110:3253–62. doi: 10.1182/blood-2007-03-077057. [DOI] [PubMed] [Google Scholar]
- 28.Amran D, Renz H, Lack G, Bradley K, Gelfand EW. Suppression of cytokine-dependent human T-cell proliferation by intravenous immunoglobulin. Clin Immunol Immunopathol. 1994;73:180–6. doi: 10.1006/clin.1994.1186. [DOI] [PubMed] [Google Scholar]
- 29.Rossi F, Kazatchkine MD. Antiidiotypes against autoantibodies in pooled normal human polyspecific Ig. J Immunol. 1989;143:4104–9. [PubMed] [Google Scholar]
- 30.Dietrich G, Kazatchkine MD. Normal immunoglobulin G (IgG) for therapeutic use (intravenous Ig) contain antiidiotypic specificities against an immunodominant, disease-associated, cross-reactive idiotype of human anti-thyroglobulin autoantibodies. J Clin Invest. 1990;85:620–5. doi: 10.1172/JCI114483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basta M, Van Goor F, Luccioli S, et al. F(ab)′2-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nat Med. 2003;9:431–8. doi: 10.1038/nm836. [DOI] [PubMed] [Google Scholar]


