Abstract
Coeliac disease (CD) is considered a T cell-mediated autoimmune disease, and up-regulation of T-bet and phosphorylated signal transducers and activators of transcription (pSTAT)1, key transcription factors for the development of T helper type 1 (Th1) cells, has been described in the mucosa of patients with untreated CD. Using transcription factor analysis, we investigated whether T-bet and pSTAT1 expressions are up-regulated in the peripheral blood of CD patients and correlate with disease activity. Using flow cytometry, we analysed T-bet, pSTAT1 and pSTAT3 expression in CD4+, CD8+ T cells, CD19+ B cells and monocytes from peripheral blood of 15 untreated and 15 treated CD patients and 30 controls, and longitudinally in five coeliac patients before and after dietary treatment. We evaluated using enzyme-linked immunosorbent assay (ELISA), interferon (FN)-γ, interleukin (IL)-17 and IL-10 production by peripheral blood mononuclear cell (PBMC) cultures. T-bet expression in CD4+, CD8+ T cells, CD19+ B cells and monocytes and IFN-γ production by PBMC was higher in untreated than in treated CD patients and controls. pSTAT1 expression was higher in CD4+T cells, B cells and monocytes from untreated than from treated CD patients and controls. pSTAT3 was increased only in monocytes from untreated patients compared with CD-treated patients and controls. The data obtained from the longitudinal evaluation of transcription factors confirmed these results. Flow cytometric analysis of pSTAT1 and T-bet protein expression in peripheral blood mononuclear cells could be useful and sensible markers in the follow-up of CD patients to evaluate disease activity and response to dietary treatment.
Keywords: coeliac disease, diet, STAT, T-bet, transcription factors
Introduction
Coeliac disease (CD) is a frequently occurring food gluten-sensitive enteropathy characterized by small-intestinal mucosal injury and nutrient malabsorption. It is precipitated in genetically susceptible individuals by the ingestion of wheat gluten and similar proteins of other cereals, inducing an abnormal immune response [1]. The genetic susceptibility of CD is associated with specific major histocompatibility complex (MHC) II alleles that encode human leucocyte antigen (HLA) DQ2 or HLA DQ8 heterodimers [1,2]. The immune reaction in CD involves the adaptive as well as the innate immune response and is characterized by the presence of anti-gluten and anti-transglutaminase 2 antibodies, lymphocyte infiltration and expression of multiple cytokines and other signalling proteins in the intestinal epithelial membrane and the lamina propria [3,4]. The detection of gluten-reactive CD4+ T cells producing interferon (IFN)-γ in the small intestine mucosa of CD patients suggests a T helper type 1 (Th1) polarization [4–6].
The main regulation of T cell differentiation and cytokine pathway seems to be provided by cytoplasmic and nuclear transcription factors. In coeliac disease Th1 cell polarization has been confirmed by the up-regulation of T-bet in the mucosa of untreated CD patients which returns to levels comparable with healthy controls after a gluten-free diet [6]. T-bet has been identified as a key transcription factor for the development of Th1 cells and the induction of IFN-γ production [7]. Mice deficient for T-bet do not develop Th1 cells [8], and when ectopically expressed T-bet induces Th1 cell differentiation of Th precursor cells and of polarized Th2 cells [9]. T-bet has been described in T, B and natural killer (NK) cells, antigen-presenting cells, such as monocytes, macrophages, dendritic cells (DC) and myeloid cells [10,11]. T-bet is induced during T cell activation by the IFN-γ signal transducer and activator of transcription (STAT)-1 signalling pathway [12] and once expressed, amplifies the production of IFN-γ[10]. The STAT proteins are cytoplasmic transcription factors that are activated via receptors from cytokines, growth factors and hormones [13]. In particular, IFN-γ is a potent activator of STAT-1 which, in this way, represents a regulator of T-bet expression in lymphoid and non-lymphoid cells through IFN-γ receptor/Janus kinases (JAK) signalling [10,12].
STAT3 seems to provide multiple functions in cytokine-mediated signalling in many cell types, conditioning them for differentiation, survival/apoptosis and mobility, and is involved in the generation of Th17 cells, regulation of DC and acute inflammatory response [14]. STAT3 is activated by several cytokines such as interleukin (IL)-6, leukaemia inhibitory factor (LIF), cardiotropin-1 (CT-1) and IL-10 [15] and has been considered recently, together with retinoic acid-related orphan receptor (ROR)-γ and RORα, to be an important regulatory transcription factor in Th17 differentiation [16–18].
In this report we evaluated the expression of T-bet, pSTAT1 and pSTAT3 in CD4+, CD8+ T cells, B cells and monocytes from peripheral blood of untreated and treated CD patients and controls. To assess more clearly the effect of dietary treatment, we evaluated longitudinally the changes of transcription factor expression in five coeliac patients before and after at least 1 year of dietary treatment. Furthermore, we correlated the expression of transcription factors with the production of several cytokines (IFN-γ, IL-17 and IL-10) by peripheral blood mononuclear cells (PBMC).
Materials and methods
Patients
Untreated symptomatic CD patients (diagnosed in accordance with the latest international recommendation) [19], CD patients under dietary treatment for at least 1 year and sex- and age-matched healthy subjects were included in our study. Five coeliac patients were evaluated longitudinally before and after at least 1 year of dietary treatment. All patients, both before and after dietary treatment, underwent upper gastrointestinal tract endoscopy and duodenal biopsies to evaluate the disease activity. All serum samples were analysed for anti-gliadin antibodies (AGA), anti-endomysium antibodies (EMA) and anti-tissue transglutaminase antibodies (anti-tTG). We included in our study only untreated patients with total villous atrophy and with AGA, EMA and anti-tTG antibody positivity. Dietary compliance was established on dietary history and negative AGA, EMA and anti-tTG antibodies.
The control population consisted of non-coeliac subjects, with no family history of CD or other autoimmune diseases, who were selected randomly from the same geographical area as the coeliac patients. Furthermore, five healthy subjects were evaluated at baseline and after 1 year. Blood was taken in all patients and controls after screening for infectious conditions or other inflammatory diseases (white blood cell count, C-reactive protein, fibrinogen, eritrosedimentation rate). This study was approved by the local ethics committee, and all the participants gave written informed consent before the enrolment.
Isolation of PBMC
PBMC were collected from CD patients before and after dietary treatment and from healthy subjects. PBMC were isolated from peripheral blood by density gradient centrifugation [1050 relative centrifugal force (RCF), 30 min] over a Ficoll–Hypaque density gradient (Pharmacia, Uppsala, Sweden). They were then harvested by pipetting cells from the Ficoll/serum interface and washed twice.
Culture of PBMC
PBMC (5 × 106 cells/ml) were transferred into 24-well plates in RPMI-1640 (EuroClone, West York, UK) containing 2 n-glutamine and 5% fetal calf serum (Hyclone Laboratories Inc, Logan, UT, USA). After 24 h incubation the supernatants were collected, centrifuged at 400 g for 10 min at 15°C and stored at −80°C until cytokine determination.
pSTAT1, pSTAT3 and T-bet expression by flow cytometry
For the detection of pSTAT1, pSTAT3 and T-bet expression, PBMC were analysed using a double-labelling procedure staining with an anti-CD4-phycoerythrin (PE)-Cy5, anti-CD8-PE-Cy5 and anti-CD14-PE-Cy5 (Beckman Coulter, Miami, FL, USA), followed by fixation, permeabilization and incubation with anti-pSTAT1(A-2)-PE antibody, anti-pSTAT3 (B-7)-PE antibody and anti-T-bet (4B10)-PE antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Appropriate fluorochrome-conjugated isotype-matched monoclonal antibody (mAb) (Beckman Coulter) were used as control for background staining in each flow acquisition. Each analysis was performed using at least 50 000 cells that were gated in the region of the lymphocyte–monocyte population, as determined by light-scatter properties (forward-scatter versus side-scatter). To analyse the expression of pSTAT1, pSTAT3 and T-bet in monocytes, cells were gated in both the monocyte (morphological gate) and CD14+ (immunological gate) regions. To analyse the expression of transcription factors in lymphocytes (CD4+, CD8+ T cells and CD19+ B cells), cells were gated in both the lymphocyte and CD4+/CD8+/CD19+ regions. Quadrants of dot plot were set using appropriate isotype controls for each intra- and extracellular antibody. Mean fluorescence intensity (MFI) was calculated only for positive events after subtraction of specific isotype control MFI.
Cytokine measurement
The spontaneous production of cytokines (IFN-γ, IL-17 and IL-10) was measured by enzyme-linked immunosorbent assay (ELISA) using commercial kits (R&D Systems, Minneapolis, MN, USA) following the manufacturer's instructions. Cytokine concentrations were determined from the regression line for a standard curve generated by using highly purified recombinant cytokine at various concentrations performed contemporaneously with each assay. The intra- and inter-assay coefficients of variation were 6% and 7% for IFN-γ, 4% and 7% for IL-17 and 5% and 5% for IL-10, respectively. The standard curve also served as an internal control over the sensitivity and range of each assay. Data were expressed as pg/ml. All samples were assayed in duplicate.
Statistical analysis
Differences in variables between groups were tested by analysis of variance (anova). Post-hoc tests were performed using Fisher's protected least significant difference (Fisher's PSLD). Results are expressed as mean ± standard deviation (s.d.). A P level < 0·05 was considered to be statistically significant.
Results
Patients
We included in our study 15 untreated symptomatic CD patients, 15 CD patients after at least 1 year of dietary treatment and 30 healthy subjects. There was no difference in demographic features (age and sex) among treated and untreated CD patients and controls. No demographic differences were also observed between the five coeliac patients and five healthy controls evaluated longitudinally. Demographic characteristics of CD patients and controls are summarized in Table 1.
Table 1.
Demographic features of patients and controls.
| CD patients |
|||
|---|---|---|---|
| New diagnosis | Long-term gluten-free diet | Healthy subjects | |
| Number | 15 | 15 | 30 |
| Age (years) | 37·56 ± 13·56 | 38·57 ± 12·87 | 36·53 ± 17·14 |
| Sex (female : male) | 8/7 | 9/6 | 16/14 |
| Gluten-free diet duration (years) | n.a. | 2·42 ± 0·93 | n.a. |
CD, coeliac disease; n.a., not available.
T-bet, pSTAT1 and pSTAT3 expression in circulating T cells, B cells and monocytes
We observed higher T-bet expression in CD4+, CD8+ T cells, monocytes and CD19+ B cells from untreated than from treated CD patients (P = 0·0013, P = 0·0021, P = 0·0003 and P = 0·0002, respectively; Fig. 1a) and healthy subjects (P = 0·0031, P = 0·0008, P = 0·0035 and P = 0·0029, respectively; Fig. 1a). No significant difference in T-bet expression was observed between treated CD patients and controls both in CD4+, CD8+ T cells, CD19+ B cells and monocytes (Fig. 1a). pSTAT1 was significantly higher in CD4+ T cells, monocytes and CD19+ B cells from untreated than from treated CD patients (P = 0·0022, P < 0·0001 and P = 0·0011, respectively; Fig. 1b) and healthy subjects (P = 0·0043, P = 0·0008 and P = 0·0032, respectively; Fig. 1b). pSTAT1 expression in CD8+ T cells was higher in untreated than in treated CD patients and controls without reaching statistical significance (P = 0·0812 and P = 0·0961, respectively). We found no significant difference in pSTAT1 expression between treated CD patients and controls both in CD4+, CD8+ T cells, CD19+ B cells and monocytes (Fig. 1b).
Fig. 1.
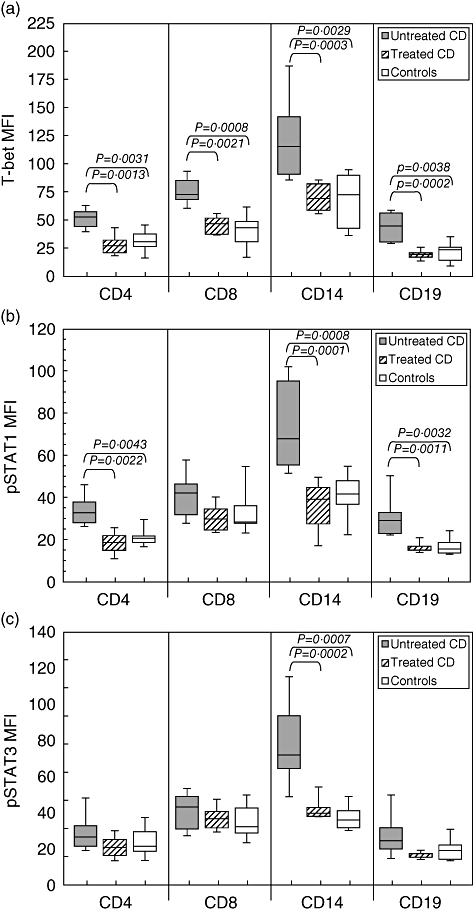
Mean expression of T-bet, phosphorylated signal transducers and activators of transcription (pSTAT)1 and pSTAT3 in T, B cells and monocytes from coeliac disease (CD) patients before and after dietary treatment and from healthy subjects. (a) Mean T-bet expression, assessed by flow cytometric analysis as intensity, was significantly higher in CD4+, CD8+ T cells, CD19+ B cells and in monocytes from untreated than from treated CD patients and controls. (b) Mean pSTAT1 expression was significantly higher in CD4+ T cells, CD19+ B cells and monocytes from untreated than from treated CD patients and controls. (c) Mean pSTAT3 expression was significantly higher in monocytes from untreated than from treated CD patients and controls. Box plots express the first (Q1) and third (Q3) quartiles within a given data set by the upper and lower horizontal lines in a rectangular box, in which there is a horizontal line showing the median. The whiskers extend upwards and downwards to the highest or lowest observation within the upper (Q3+1·5 × interquartile range) and lower (Q1-1·5 × interquartile range) limits. P-values indicate statistical significances (< 0·05) between the different groups. MFI: mean fluorescence intensity.
We found higher expression of pSTAT3 only in monocytes from untreated than from treated CD patients and controls (P < 0·0001 and P = 0·0002, respectively; Fig. 1c), whereas no significant difference was observed in CD4+, CD8+ T cells and CD19+ B cells. No significant difference in pSTAT3 expression was observed between treated CD patients and controls both in CD4+, CD8+ T cells, CD19+ B cells and monocytes (Fig. 1c). Representative two-parameter plots of T-bet+, pSTAT1+ and pSTAT3+ in T cells, B cells and monocytes from one untreated (d) and one treated CD patient (e) are shown in Fig. 2.
Fig. 2.
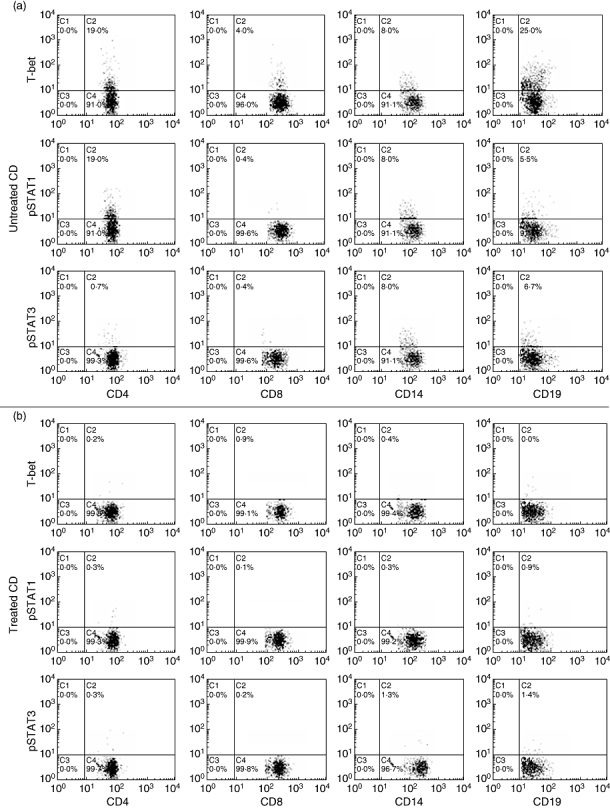
Representative two-parameter plots showing only cells gated on the T and B lymphocytes (CD4, CD8 and CD19 staining) and monocytes (CD14 staining) from one untreated and one treated coeliac disease (CD) patient. The y-axis of each histogram represents specific fluorescence of T-bet-phycoerythrin (PE), phosphorylated signal transducers and activators of transcription (pSTAT)1-PE, pSTAT3-PE; the x-axis represents specific fluorescence of extracellular CD4-PE-Cy5, CD8-PE-Cy5 (T lymphocytes), CD19-PE-Cy5 (B lymphocytes), CD14-PE-Cy5 (monocytes) on four-decade logarithmic scales. These representative two-parameter plots are obtained from one untreated (a) and one treated (b) CD patient. Quadrants were set using appropriate isotype controls for each intra- and extracellular antibody.
The data obtained from the longitudinal evaluation of transcription factors in five CD patients confirmed these results. In particular, we observed a significant reduction of T-bet expression in CD4+, CD8+ T cells, monocytes and CD19+ B cells in CD patients after dietary treatment (P = 0·0094, P = 0·0239, P = 0·0073 and P = 0·0003, respectively; Fig. 3). pSTAT1 expression was reduced significantly after dietary treatment in CD4+ T cells, monocytes and CD19+ B cells (P = 0·0221, P = 0·0048 and P = 0·016, respectively; Fig. 3), whereas pSTAT3 was reduced significantly only in monocytes (P = 0·0089; Fig. 3). No significant differences in transcription factor expression were observed in lymphomonocyte subpopulations from five healthy subjects evaluated at baseline and after 1 year (Fig. 3a). Representative two-parameter plots of T-bet+, pSTAT1+ and pSTAT3+ in T cells, monocytes and B cells from the same patient before and after dietary treatment are shown in Fig. 3b.
Fig. 3.
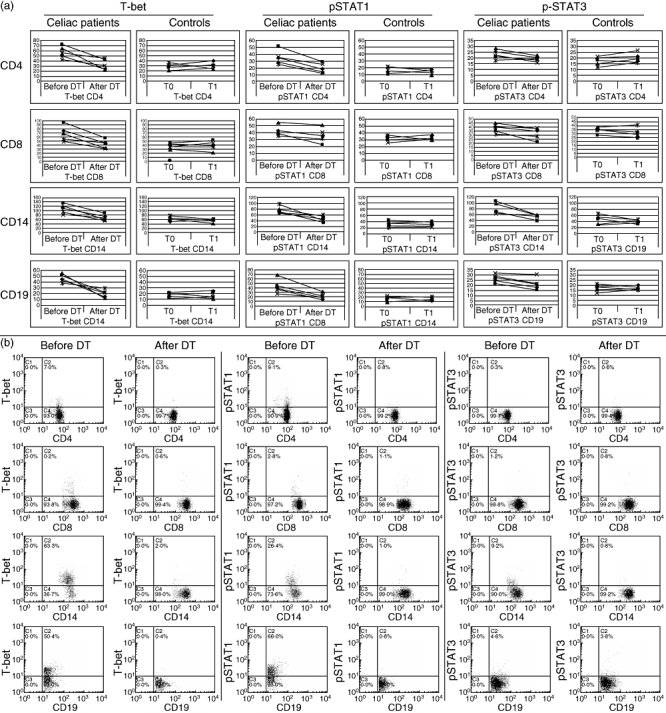
Longitudinal expression of T-bet, phosphorylated signal transducers and activators of transcription (pSTAT)1 and pSTAT3 in CD4+, CD8+ T cells, CD14+ monocytes and CD19+ B cells from five coeliac patients before and after dietary treatment. (a) Mean T-bet, pSTAT1, pSTAT3 expression in CD4+, CD8+ T cells, monocytes and B cells from five coeliac patients who we studied longitudinally before and at least after 1 year of dietary treatment. (b) The y-axis of each histogram represents specific fluorescence of T-bet-phycoerythrin (PE), pSTAT1-PE, pSTAT3-PE; the x-axis represents specific fluorescence of extracellular CD4-PE-Cy5, CD8-PE-Cy5 (T lymphocytes), CD19-PE-Cy5 (B lymphocytes), CD14-PE-Cy5 (monocytes) on four-decade logarithmic scales. These representative two-parameter plots are obtained from the same patient before and after dietary treatment. Quadrants were set using appropriate isotype controls for each intra- and extracellular antibody. DT: dietary treatment
IFN-γ, IL-17 and IL-10 production by PBMC from treated and untreated CD patients and controls
We observed higher levels of IFN-γ in supernatants of PBMC from CD patients before dietary treatment than from controls (P = 0·0004, Fig. 4a). Levels of IFN-γ production were higher in untreated than in treated CD patients (P = 0·0058, Fig. 4a). After dietary treatment, CD patients showed higher levels of IFN-γ than controls without reaching statistical significance (P = 0·1021, Fig. 4a).
Fig. 4.
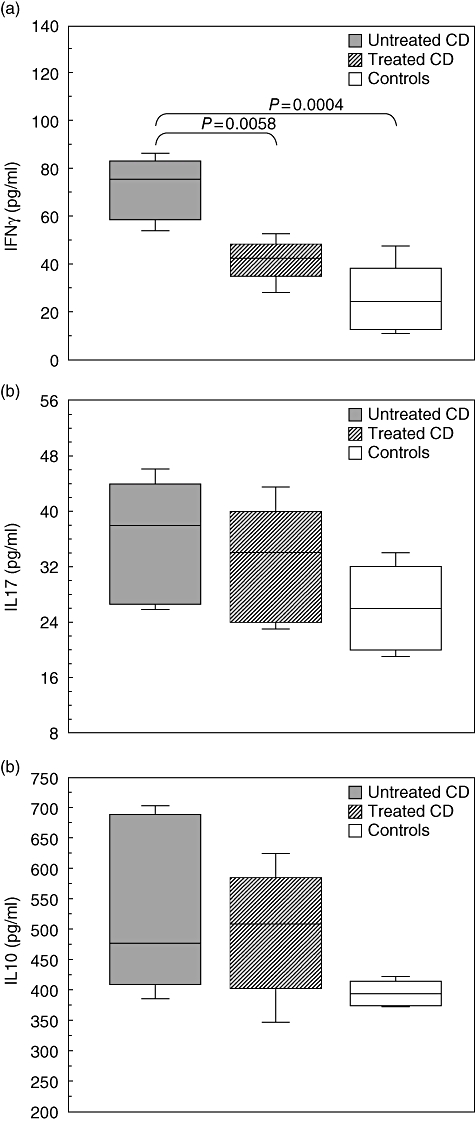
Levels of cytokine production by peripheral blood mononuclear cells (PBMC) from coeliac disease (CD) patients before and after dietary treatment and from healthy subjects. (a) Spontaneous interferon (IFN)-γ production was significantly higher in supernatant of PBMC from untreated than from treated CD patients and controls. (b,c) No differences were observed in interleukin (IL)-17 and IL-10 productions by PBMC among treated and untreated CD patients and healthy subjects. Box plots express the first (Q1) and third (Q3) quartiles within a given data set by the upper and lower horizontal lines in a rectangular box, in which there is a horizontal line showing the median. The whiskers extend upwards and downwards to the highest or lowest observation within the upper (Q3+1·5 × interquartile range) and lower (Q1-1·5 × interquartile range) limits. P-values indicate statistical significances (< 0·05) between the different groups.
No significant difference in IL-17 and IL-10 spontaneous production was observed in the supernatants of PBMC cultures among CD patients before and after dietary treatment and controls (Fig. 4b,c).
Discussion
CD is considered a T cell-mediated autoimmune disease and the involvement of Th1 cells in its pathogenesis have been suggested [4–6]. T-bet plays a role in controlling mucosal Th1 responses in the gastrointestinal tract [20]. In CD, the upper bowel lesions are characterized by a marked infiltration of the mucosa with Th1 cells secreting IFN-γ and expressing T-bet [6]. In CD specimens nearly all intraepithelial CD8+ cells and many CD4+ cells in the lamina propria expressed T-bet [21]. In murine models, gastrointestinal and systemic T cell responses seem to be linked intimately, as oral administration of antigen is followed by proliferation of antigen-specific T cells in gut and systemic lymphoid tissue [22]. To date there are no data concerning the expression of T-bet in circulating lymphomononuclear cells from CD patients. Some previous studies indicate that the Th1/Th2 balance in peripheral blood of CD is shifted towards Th1 cytokines [23–25] and circulating gliadin-specific CD4+[26] and CD8+ T cells producing IFN-γ are present in CD patients [27].
In our study untreated CD patients showed higher T-bet expression in peripheral blood CD4+, CD8+ T cells and monocytes than controls. The increased expression of T-bet was associated with an increased production of IFN-γ by PBMC suggesting the involvement of type 1 immunity in the pathogenesis of the disease. T-bet expression was also higher in circulating B cells of untreated CD patients than in controls. In addition to T cells, T-bet plays a critical role in B cell effector function, especially type 1-related responses [28]. T-bet is expressed at very low levels in naive B cells [29], but B cells in the presence of polarized Th1 cells and antigens can develop into high rate IFN-γ-producing B effector 1 (Be1) cells that express high levels of T-bet and are capable of promoting the differentiation of naive T cells into Th1 effectors [28,29]. In untreated CD patients the cognate interactions that occurs between T-bet expressing and IFN-γ-producing B and T cells may result in an amplification of type 1 immune responses.
To support the data of a Th1 polarization in PBMC from untreated CD patients we also analysed the expression of pSTAT1. Previous studies showed that STAT1 protein is over-expressed in mononuclear cells of the lamina propria and epithelial cells of CD biopsies [30], and that the activation of STAT1 by IFN-γ promotes T-bet expression in CD mucosa [6]. In this study untreated CD patients showed an up-regulation of pSTAT1 in CD4+ and CD8+ T cells, B cells and monocytes, confirming a role of this transcription factor in regulating the extensive activation of the innate and adaptive immune response in coeliac disease. Several studies report the expression of pSTAT3 in mucosal biopsy specimens from untreated CD patients but not in the normal mucosa of control subjects [30,31]. STAT3 is activated by the binding of a variety of cytokines such as IL-6 and IL-10 to their receptors, and has a variety of seemingly contradictory roles due to recruitment of distinct sets of target genes in different cell types. IL-6-mediated STAT3 activation favours monocytic differentiation [32], while IL-10-mediated STAT3 signalling can inhibit the maturation of DC from monocytes [33]. In monocytes STAT3 activation induces IL-10 production [34,35] and promotes their migration [36], while a delayed active repression of IL-10 gene expression is critically dependent on STAT1[37] which seems to favour monocytes migration arrest [36]. IL-6-mediated STAT3 activation can also induce IL-10 production in Th1, Th2 and Th17 T cells to temper inflammatory response [38,39]. We found an increased expression of both pSTAT1 and pSTAT3 only in circulating monocytes from untreated CD patients, which was not associated with increased production of IL-10 by PBMC. Taken together, these data suggest that STAT3-induced production of IL-10 by monocytes and their migration from or to inflammatory sites might be inhibited partially in untreated CD patients by the high expression of pSTAT1 in the same cells favouring the inflammatory process. Consistent with this, a recent report showed that among CD patients the early onset of the disease and the presence of severe intestinal lesions were associated significantly with the -1082 A/A IL-10 genotype, which corresponds to a low producer phenotype [40].
STAT3 is also involved in the differentiation of Th17 cells [14]. IL-17-producing cells have been found in mucosal samples and PBMC from other inflammatory bowel diseases [41], but little is known about this T cell subpopulation in CD. A recent study reported an increased mRNA expression of IL-17 in intestinal biopsies from CD patients with active disease [42]. In our study, untreated CD patients showed neither an increased pSTAT3 expression in CD4+, CD8+ T cells nor an increased IL-17 production by PBMC. An explanation for this discrepancy could be that IL-17-producing cells are present mainly in the inflamed tissues but not in the periphery, as suggested by recent studies on other autoimmune diseases [43,44].
In agreement with other authors who found comparable levels of T-bet mRNA in CD biopsies from treated CD patients and controls [6], our group of CD patients examined at least 1 year after dietary treatment showed similar T-bet levels in circulating CD4+, CD8+ T cells, B cells and monocytes to controls. pSTAT1 and pSTAT3 expression in lymphomonocytes and the production of IFN-γ, IL-10 and IL-17 by PBMC were also similar in the two groups.
In conclusion, we showed that in active CD, T-bet and pSTAT1 expression is increased not only in intestinal mucosa, as reported previously, but also in peripheral blood both in CD4+ and CD8+ T cells, B cells and monocytes. Furthermore, pSTAT1 and T-bet expression in PBMC returns to normal levels after gluten withdrawn from the diet. Further studies are necessary to evaluate the sensitivity of transcription factor compared to autoantibody analysis. In our opinion, flow cytometric analysis of pSTAT1 and T-bet proteins in PBMC could be useful in the follow-up of CD patients to evaluate disease activity and response to dietary treatment.
Disclosure
None.
References
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.Roschmann E, Wienker TF, Gerok W, Volk BA. T-cell receptor variable genes and genetics susceptibility to celiac disease: an association and linkage study. Gastroenterology. 1993;105:1790–96. doi: 10.1016/0016-5085(93)91077-u. [DOI] [PubMed] [Google Scholar]
- 3.Alaedini A, Green PH. Narrative review: celiac disease: understanding a complex autoimmune disorder. Ann Intern Med. 2005;142:289–98. doi: 10.7326/0003-4819-142-4-200502150-00011. [DOI] [PubMed] [Google Scholar]
- 4.Sollid LM, Jabri B. Is celiac disease an autoimmune disorder? Curr Opin Immunol. 2005;17:595–600. doi: 10.1016/j.coi.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Salvati VM, MacDonald TT, Bajaj-Elliott M, et al. Interleukin 18 and associated markers of T helper cell type 1 activity in coeliac disease. Gut. 2002;50:186–90. doi: 10.1136/gut.50.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteleone G, Del Vecchio Blanco G, Palmieri G, et al. Regulation of the T helper cell type 1 transcription factor T-bet in coeliac disease mucosa. Gut. 2004;53:1090–95. doi: 10.1136/gut.2003.030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 8.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effect of T-bet in TH1 commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–42. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 9.Lametschwandtner G, Biedermann T, Schwärzler C, et al. Sustained T-bet expression confers polarized human TH2 cells with TH1-like cytokine production and migratory capacities. J Allergy Clin Immunol. 2004;113:987–94. doi: 10.1016/j.jaci.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Lighvani AA, Frucht DM, Jankovic D, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci USA. 2001;98:15137–42. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C, Glimcher LH. T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells. Proc Natl Acad Sci USA. 2003;100:7749–54. doi: 10.1073/pnas.1332767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afkarian M, Sedy JR, Yang J, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–57. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 13.Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- 14.Stepkowski SM, Chen W, Ross JA, Nagy ZS, Kirken RA. STAT3: an important regulator of multiple cytokine functions. Transplantation. 2008;85:1372–77. doi: 10.1097/TP.0b013e3181739d25. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz J, Dahmen H, Grimm C, et al. The cytoplasmic tyrosine motifs in full-length glycoprotein 130 have different roles in IL-6 signal transduction. J Immunol. 2000;164:848–54. doi: 10.4049/jimmunol.164.2.848. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Mathur AN, Chang HC, Zisoulis DG, et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–7. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 18.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagnoff MF, American Gastroenterology Association Institute American Gastroenterology Association Institute medical position statement on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1977–80. doi: 10.1053/j.gastro.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weigmann B, Neurath MF. T-bet and mucosal Th1 responses in the gastrointestinal tract. Gut. 2002;51:301–3. doi: 10.1136/gut.51.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jöhrens K, Anagnostopoulos I, Stein H. T-bet expression patterns in coeliac disease, cryptic and overt enteropathy-type T-cell lymphoma. Histopathology. 2005;47:368–74. doi: 10.1111/j.1365-2559.2005.02237.x. [DOI] [PubMed] [Google Scholar]
- 22.Gütgemann I, Fahrer AM, Altman JD, Davis MM, Chien YH. Induction of rapid T cell activation and tolerance by systemic presentation of an orally administered antigen. Immunity. 1998;8:667–73. doi: 10.1016/s1074-7613(00)80571-3. [DOI] [PubMed] [Google Scholar]
- 23.O'Keeffe J, Mills K, Jackson J, Feighery C. T cell proliferation, MHC class II restriction and cytokine products of gliadin-stimulated peripheral blood mononuclear cells (PBMC) Clin Exp Immunol. 1999;117:269–76. doi: 10.1046/j.1365-2249.1999.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizrachi A, Broide E, Buchs A, et al. Lack of correlation between disease activity and decreased stimulated secretion of IL-10 in lymphocytes from patients with celiac disease. Scand J Gastroenterol. 2002;37:924–30. doi: 10.1080/003655202760230883. [DOI] [PubMed] [Google Scholar]
- 25.Anderson RP, van Heel DA, Tye-Din JA, et al. T cells in peripheral blood after gluten challenge in coeliac disease. Gut. 2005;54:1217–23. doi: 10.1136/gut.2004.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Horin S, Green PH, Bank I, Chess L, Goldstein I. Characterizing the circulating, gliadin-specific CD4+ memory T cells in patients with celiac disease: linkage between memory function, gut homing and Th1 polarization. J Leukoc Biol. 2006;79:676–85. doi: 10.1189/jlb.0705414. [DOI] [PubMed] [Google Scholar]
- 27.Gianfrani C, Troncone R, Mugione P, et al. Celiac disease association with CD8+ T cell responses: identification of a novel gliadin-derived HLA-A2-restricted epitome. J Immunol. 2003;170:2719–26. doi: 10.4049/jimmunol.170.5.2719. [DOI] [PubMed] [Google Scholar]
- 28.Peng SL. The T-box transcription factor T-bet in immunity and autoimmunity. Cell Mol Immunol. 2006;3:87–95. [PubMed] [Google Scholar]
- 29.Harris DP, Goodrich S, Gerth AJ, Peng SL, Lund FE. Regulation of INF-gamma production by B effector 1 cells: essential roles for T-bet and the IFN-gamma receptor. J Immunol. 2005;174:6781–90. doi: 10.4049/jimmunol.174.11.6781. [DOI] [PubMed] [Google Scholar]
- 30.Mazzarella G, MacDonald TT, Salvati VM, et al. Constitutive activation of the signal transducer and activator of transcription pathway in celiac disease lesions. Am J Pathol. 2003;162:1845–55. doi: 10.1016/S0002-9440(10)64319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musso A, Dentelli P, Carlino A, et al. Signal transducers and activators of transcription 3 signaling pathway: an essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm Bowel Dis. 2005;11:91–8. doi: 10.1097/00054725-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Mangan JK, Rane SG, Kang AD, Amanullah A, Wong BC, Reddy EP. Mechanisms associated with IL-6-induced up-regulation of Jak3 and its role in monocytic differentiation. Blood. 2004;103:4093–101. doi: 10.1182/blood-2003-06-2165. [DOI] [PubMed] [Google Scholar]
- 33.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–18. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 34.Levy DE, Lee C. What does STAT3 do? J Clin Invest. 2002;109:1143–48. doi: 10.1172/JCI15650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staples KJ, Smallie T, Williams LM, et al. IL-10 induces IL-10 in primary human monocyte-derived macrophages via the transcription factor Stat3. J Immunol. 2007;178:4779–85. doi: 10.4049/jimmunol.178.8.4779. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, Hu X, Boumsell L, Ivashkiv LB. IFN-gamma and STAT1 arrest monocyte migration and modulate RAC/CDC42 pathways. J Immunol. 2008;180:8057–65. doi: 10.4049/jimmunol.180.12.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanDeusen JB, Shah MH, Becknell B, et al. STAT-1-mediated repression of monocyte interleukin-10 gene expression in vivo. Eur J Immunol. 2006;36:623–30. doi: 10.1002/eji.200535241. [DOI] [PubMed] [Google Scholar]
- 38.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–71. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 39.O'Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–28. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 40.Barisani D, Ceroni S, Meneveri R, Cesana BM, Bardella MT. IL-10 polymorphisms are associated with early-onset celiac disease and severe mucosal damage in patients of Caucasian origin. Genet Med. 2006;8:169–74. doi: 10.1097/01.gim.0000204464.87540.39. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi T, Okamoto S, Hisamatsu T, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut. 2008;57:1682–89. doi: 10.1136/gut.2007.135053. [DOI] [PubMed] [Google Scholar]
- 42.Castellanos-Rubio A, Santin I, Irastorza I, Castaño L, Carlos Vitoria J, Ramon Bilbao J. TH17 (and TH1) signatures of intestinal biopsies of CD patients in response to gliadin. Autoimmunity. 2009;42:69–73. doi: 10.1080/08916930802350789. [DOI] [PubMed] [Google Scholar]
- 43.Evans HG, Gullick NJ, Kelly S, et al. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc Natl Acad Sci USA. 2009;106:6232–37. doi: 10.1073/pnas.0808144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pène J, Chevalier S, Preisser L, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol. 2008;180:7423–30. doi: 10.4049/jimmunol.180.11.7423. [DOI] [PubMed] [Google Scholar]


