Abstract
Alcoholic liver cirrhosis (ALC) is characterized by increased circulating levels of immunoglobulins (Igs). ALC patients undergo bacterial translocation evidenced by the presence of bacterial DNA in peripheral blood. Bacterial pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), peptidoglycan (PGN) and unmethylated cytosine-guanine dinucleotide (CpG) DNA are ligands of Toll-like receptor (TLR)-4, TLR-2 and TLR-9, respectively. Although TLR activation results generally in the secretion of proinflammatory cytokines, activation of B cells through TLR-7 or TLR-9 is involved in their maturation and Ig synthesis. The aim of the present study was to assess Ig synthesis by ALC B cells under PAMP activation in order to evaluate the possible involvement of TLR pathways in the increased Ig levels, and especially the hyper-IgA observed in ALC. CpG, in combination with interleukin (IL)-10 or IL-21, enhanced IgA, IgG and IgM synthesis by healthy donor (HD) PBMCs, but had only a weak effect on ALC PBMCs. Relative CpG-induced IgA production by purified ALC B cells was less important when compared to HD B cells, in accordance with the lower TLR-9 expression on ALC B cells compared to HD B cells, but the absolute IgA production by CpG-activated B cells was enhanced significantly for ALC when compared to HD, in agreement with their intrinsic ability to produce spontaneously more IgA than HD. LPS and PGN had no direct activity on B cells, whereas R848 also enhanced Ig synthesis, as reported recently. Taken together, these results suggest that TLR priming of B cells could account for the hyperimmunoglobulinaemia observed in ALC patients.
Keywords: alcoholic liver cirrhosis, immunoglobulins, Toll-like receptors
Introduction
In cirrhosis, intestinal bacterial overgrowth and translocation of viable or non-viable bacterial products in the absence of visceral injury are widespread [1,2]. These products may lead to activation of monocytes and lymphocytes and production of proinflammatory cytokines [3].
Toll-like receptors (TLR) belong to a family of at least 10 members, playing key roles in innate immunity to microbial pathogens via recognition of conserved pathogen-associated molecular patterns (PAMPs). In most of the cases, TLR ligation triggers the induction of cell signalling through MyD88 and nuclear factor kappa B (NF-κB) [4,5]. Activation of NF-κB leads to the secretion of proinflammatory cytokines [6]. Lipopolysaccharide (LPS), also known as endotoxin, a PAMP from the Gram-negative bacterial wall, is sensed via circulating LPS binding protein through the complex comprising CD14, MD-2 and TLR-4 [7]. PAMPs from the Gram-positive bacterial wall, such as peptidoglycan (PGN), stimulate TLR-2 [8], whereas bacterial DNA sequences with unmethylated cytosine-guanine dinucleotide (CpG) motifs are recognized by TLR-9 [9]. TLR-7 and TLR-8 recognize single-stranded RNA, and the synthetic imidazoquinoline R848. Among the 10 different human TLRs, both TLR-4 and TLR-2 are expressed on the plasma membrane, whereas TLR-3, -7, -8 and -9 have an intracellular localization [10]. In humans, TLR-2, TLR-4 and TLR-8 are expressed strongly by monocytes/macrophages, but expressed poorly by B cells. In contrast, TLR-7 and TLR-9 are expressed mainly by B lymphocytes and plasmacytoid dendritic cells [11–13].
Besides the secretion of proinflammatory cytokines induced by TLR triggering of monocytes/macrophages and plasmacytoid dendritic cells, stimulation of B cells by TLR ligands can lead to polyclonal activation and immunoglobulin (Ig) production. In humans, TLR-9-mediated B cell stimulation by CpG induces the production of IgG and IgM [14] and inhibits the isotypic switch towards IgG1 and IgE induced by interleukin (IL)-4 and anti-CD40 monoclonal antibodies (mAb) [15,16]. In the presence of IL-10, CpG induces IgG1, IgG2 and IgG3 synthesis [16]. Furthermore, CD19+ mucosal B cells were demonstrated to express TLR-9 and to secrete increased levels of IgA upon stimulation with CpG [17]. In primary biliary cirrhosis, a disease characterized by elevated levels of IgM, bacterial CpG enhances IgM production by CD27+ memory B cells [18]. Interestingly, the presence of bacterial DNA has been evidenced in ascites and plasma from cirrhotic patients with non-infected ascitic fluid, and reported as a poor prognosis indicator [19]. It has been reported recently that TLR-7-mediated B cell activation also induces IgG, IgA and IgM production [13,20,21]. In mice, TLR-4 expressed by B lymphocytes contributes to IgM and IgG production, whereas CD40 signalling is required for isotype switching [22]. However, the presence of normal levels of IgA in the serum of myeloid differentiation factor 88 (MyD88) knock-out mice suggests that TLR signalling might be dispensable for mouse IgA production [22].
In alcoholic liver cirrhosis (ALC) patients, high blood levels of LPS binding protein, soluble CD14, tumour necrosis factor (TNF)-α and IL-6 have been evidenced [23]. It is suggested that these elevated circulating levels of proinflammatory cytokines contribute to the hyperactivation of immune cells, although no significant correlation between circulating endotoxin and cytokines have been found [2,24–27].
The expression level of TLR-2, but not TLR-4, at the monocyte surface is up-regulated significantly in ALC patients and correlated with serum TNF-α and soluble TNF receptor levels [28]. Besides the increase of circulating cytokines and the modulation of TLR expression, ALC is accompanied by hypergammaglobulinaemia, due in particular to IgA and IgA-immune complexes [29,30]. The mechanisms leading to the increase of IgA levels in ALC are not understood fully. We have reported previously a defective clearance of IgA and IgA-immune complexes through altered monocytes Fcα receptor expression and subsequent defective Fcα receptor-triggered endocytosis [31]. However, this impaired Fcα receptor function can only partially explain the hyper-IgA observed in alcoholic cirrhosis. Prior to the discovery of TLR, it was hypothesized that the increase of Ig synthesis in ALC could be associated with bacterial stimulation [32]. In the present study, we compared the ability of B cells from patients with ALC and healthy donors (HD) to express TLR-2, -4 and -9, and to produce immunoglobulins in vitro under cytokines and PAMPs activation. The aim of this study was to investigate the possible involvement of B cell activation resulting from TLR stimulation by PAMPs in the hypergammaglobulinaemia observed in ALC.
Material and methods
Patients
The 17 patients studied had alcoholic liver disease as confirmed by liver biopsy. All patients were studied prospectively as they were hospitalized for either ascites paracentesis or for follow-up of their cirrhosis (Child Pugh A, n = 4; Child Pugh B, n = 13). Patients with large oesophageal varices, recent bleeding, infection, two or more criteria of systemic inflammatory response syndrome or intake of antibiotics in the preceding 2 weeks, including norfloxacin as primary or secondary prophylaxis of spontaneous bacterial peritonitis, were not included. Patients with hepatocellular carcinoma, portal thrombosis, transjugular intrahepatic portosystemic shunt, alcoholic hepatitis were also not included. According to recommendations, patients were considered to have alcohol-related cirrhosis as alcohol intake had been in excess of 80 g/day in men and 30 g/day in women. These patients were hospitalized and studied after at least 2 weeks without alcohol ingestion. Testing for viral, metabolic and immune aetiologies was negative. Patients did not receive any specific therapy, such as non-selective beta-blockers, corticosteroids or other immunosuppressive treatments in the preceding 6 months.
Fifteen healthy donors (HD) with no history of liver disease, alcohol intake less than 20 g/day and normal liver function tests served as controls. Informed written consent was obtained from each patient and normal volunteer. Spleen cells were obtained from splenectomy performed for abdominal trauma. Informed consent was obtained after surgery. This study was approved by the Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale de la Région Poitou-Charentes.
Cell culture and reagents
Spleen mononuclear cells (SMCs) from normal subjects were obtained after teasing tissue gently through a 100-µm cell strainer. SMCs and PBMCs were isolated by Ficoll-Hypaque (Biochrom AG, Berlin, Germany) centrifugation. CD19+ B lymphocytes were isolated from SMCs or PBMCs using a preparative magnetic cell sorter (VarioMacs; Miltenyi Biotec, Paris, France) according to the experimental procedure recommended by the manufacturer. CD19 was expressed on > 98% of the selected cells as assessed by fluorescence activated cell sorter analysis using a FACSCanto II (BD Biosciences, San Jose, CA, USA). All cultures were carried out in RPMI-1640 supplemented with Glutamax-I (Invitrogen Life Technologies, Cergy Pontoise, France), 10% heat-inactivated fetal calf serum (Sigma-Aldrich, Saint-Quentin Fallavier, France), 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen Life Technologies). For measurement of Ig production, 106 PBMCs or SMCs, or 2 × 105 CD19+ B lymphocytes were cultured in 1 ml of medium in the presence or absence of 2 µg/ml anti-CD40 mAb 89 (kind gift from Dr G. Aversa, DNAX Research Institute, Palo Alto, CA, USA), 10 ng/ml IL-4 (R&D Systems, Lille, France), 20 ng/ml IL-10 (kind gift fron Dr F. Brière, Schering-Plough, Dardilly, France), 20 ng/ml IL-21 (kind gift from Dr H. Yssel, INSERM U454, Montpellier, France), 1 µg/ml Escherichia coli LPS (Sigma-Aldrich), 2 µg/ml Staphylococcus aureus peptidoglycan (Sigma-Aldrich), 2 µM phosphorothioate CpG oligodeoxynucleotide 2006 (5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′) (Hycult Biotechnology, Uden, the Netherlands) or 1 µg/ml R848 (imidazoquinoline; Invivogen, San Diego, CA, USA). After 12 days of culture, supernatants were collected for Ig measurements.
Immunoglobulin measurements
IgG, IgA, IgM and IgE were measured by enzyme-linked immunosorbent assay (ELISA) in 12-day culture supernatants, as described previously [36,37]. Briefly, IgA, IgM, IgG and IgE were captured using goat anti-human IgA, IgM or IgG polyclonal antibody (SouthernBiotech, Birmingham, AL, USA) or rabbit anti-human IgE polyclonal antibody (Dako, Trappes, France), respectively, and revealed using horseradish peroxidase-conjugated goat anti-human IgA (Jackson Immunoresearch, Newmarket, UK), anti-human IgM (Calbiochem, Darmstadt, Germany) or anti-human IgG (Invitrogen Life Technologies) polyclonal antibody, or mouse anti-human IgE mAb (SouthernBiotech) followed by horseradish peroxidase-conjugated rabbit anti-mouse IgG polyclonal antibody (Jackson Immunoresearch). All assays were performed in duplicate in 96-well, flat-bottomed Maxisorp microtitre plates (Nunc, Rochester, NY, USA). Serum IgG, IgA and IgM levels were determined by immunonephelometry (Dade Behring, Eschborn, Germany).
Flow cytometry
PBMCs or CD19+ B cells were stained with monoclonal allophycocyanin-labelled mouse anti-human CD19 antibody or allophycocyanin-labelled mouse IgG1, κ (BD Biosciences). For intracellular TLR-9 expression cells were fixed and permeabilized using the BD Cytofix/Cytoperm kit (BD Biosciences) and stained with phycoerythrin-labelled monoclonal anti-human TLR-9 antibody (Imgenex, San Diego, CA, USA) or phycoerythrin-labelled mouse IgG1, κ as a control (Biolegend, San Diego, CA, USA) according to the manufacturer's recommendations. Data were acquired with a FACSCanto II cytometer and analysed with FACSDiva software (BD Biosciences).
Real-time reverse transcription–polymerase chain reaction (RT–PCR) analysis
Total cellular RNA from PBMCs was extracted using TRIzol reagent (Invitrogen Life Technologies) and treated with DNase I (0.05 U/µl; Roche, Bâle, Switzerland). Four micrograms of total RNA were reverse-transcribed using ImProm-II Reverse Transcriptase (Promega, Charbonnières, France) and 160 ng/µl pd(N)6random hexamer (GE Healthcare, Chalfont St Giles, UK), according to the manufacturer's instructions (Promega). Quantitative real-time PCR was conducted using the LightCycler-Fast-Start DNA MasterPLUS SYBR Green I kit (Roche). The reaction components were 1× FastStart DNA Master SYBR Green I and 0.5 µM of the reported forward and reverse primers for TLR-2, TLR-4, TLR-9 [38] and hydroxymethylbilane synthase (HMBS) [39] as a housekeeping gene. After cDNA fluorescent quantification using propidium iodide, 750, 75 and 7·5 ng of cDNA were added as a PCR template in the LightCycler glass capillaries. The cycling conditions comprised a 10-min polymerase activation at 95°C and 45 cycles at 95°C for 10 s, 60°C for 5 s and 72°C for 18 s with a single fluorescence measurement. Melting curve analysis, obtained by increasing the temperature from 60°C to 95°C with a heating rate of 0·1°C/s and a continuous fluorescence measurement, revealed a single, narrow peak of suspected fusion temperature. A mathematical model was used to determine the relative quantification of target genes compared with the HMBS reference gene [40].
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). Significance was determined using the Mann–Whitney U-test when HD were compared to ALC patients, or Student's paired t-test when different culture conditions were compared in the same cohort, except for IgE production, for which Wilcoxon's matched pairs t-test was used. For experiments implicating spleen cells, significance was determined using one-way analysis of variance (anova) followed by Newman–Keuls post-test.
Results
Decreased TLR-9 expression in B lymphocytes from ALC patients
TLR-2, TLR-4 and TLR-9 gene expression levels were analysed in PBMCs from HD and ALC patients by quantitative real-time RT–PCR. TLR-2 and TLR-4 mRNA relative expression levels were increased slightly (data not shown), albeit not significantly, in ALC PBMCs. In contrast, a significant decrease in TLR-9 mRNA relative expression level was observed in ALC PBMCs when compared with HD PBMCs (Fig. 1a). The decreased expression of TLR-9 was confirmed at the protein level in CD19+ B cells from ALC patients by flow cytometry (Fig. 1b and c). Indeed, within the CD19+ B cell population, the percentage of TLR-9+ cells decreased from 98·2% ± 1·7% for HD (n = 5) to 71·9% ± 26% for ALC patients (n = 7; P < 0·05). In addition, the mean fluorescence intensity of TLR-9 labelling within the CD19+/TLR9+ population was also weaker for ALC patients [2183 relative fluorescence units (RFU) ± 1747] than for HD (6232 RFU ± 2620, P < 0·05).
Fig. 1.
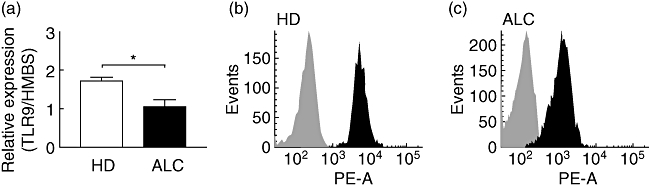
Toll-like receptor (TLR)-9 mRNA expression in peripheral blood mononuclear cells (PBMCs) (a), and intracellular TLR-9 labelling in CD19+ B cells from healthy donors (HD) (b) and alcoholic liver cirrhosis (ALC) patients (c). (a) TLR mRNA expression is normalized using hydroxymethylbilane synthase (HMBS) mRNA expression level. Each bar represents mean ± standard error of the mean (n = 5 for ALC, n = 4 for HD). *P < 0·05 according to Mann–Whitney U-test. (b) Intracellular labelling with anti-TLR-9 antibody (black histogram) or with isotypic control antibody (light grey histogram) were obtained after gating CD19+ B cells from HD (b) and ALC patients (c). One representative of n = 7 ALC patients and n = 5 HD.
Immunoglobulin serum levels and immunoglobulin spontaneous secretion by cultured PBMCs
We investigated the spontaneous secretions of IgA, IgG, IgM and IgE in the supernatants of cultured PBMCs. ALC patients' PBMCs secreted significantly higher levels of IgA and IgE than HD PBMCs (Fig. 2a and d). The spontaneous secretion of IgA by both HD and ALC PBMCs were correlated significantly with the serum levels of IgA (data not shown). IgG and IgM levels were increased, albeit not significantly, in the supernatants from ALC PBMCs when compared with HD PBMCs (Fig. 2b and c). As expected, serum IgA, IgG and IgM were increased significantly in ALC patients compared with HD (Table 1). No significant differences were indicated between the circulating number of leucocytes and lymphocytes in ALC patients compared to HD, whereas the percentage of CD19+ B lymphocytes was decreased significantly in ALC patients (Table 1).
Fig. 2.
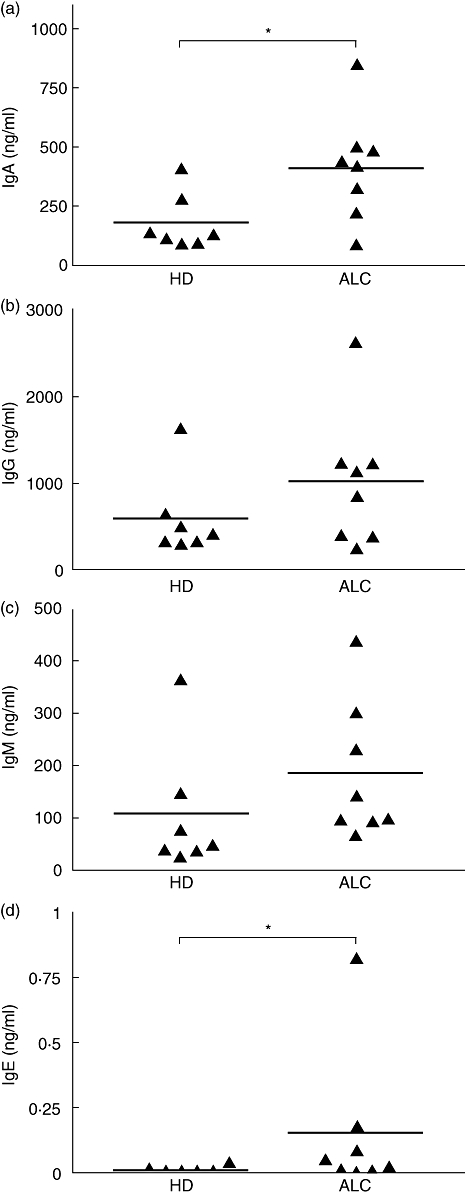
Immunoglobulin (Ig)A (a), IgG (b), IgM (c) and IgE (d) spontaneous secretions by peripheral blood mononuclear cells (PBMCs) from alcoholic liver cirrhosis (ALC) patients and healthy donors (HD). Igs were measured in 12-day culture supernatants by specific enzyme-linked immunosorbent assay; n = 8 for ALC, n = 7 for HD. *P < 0·05 according to the Mann–Whitney U-test.
Table 1.
Circulating cell numeration and serum immunoglobulin (Ig)A, G and M levels in cirrhotic patients (ALC) and healthy donors.
| Leucocytes (cells/mm3) | Lymphocytes (cells/mm3) | CD19+ cells (%) | Serum IgA (g/l) | Serum IgG (g/l) | Serum IgM (g/l) | |
|---|---|---|---|---|---|---|
| Healthy donors | 7350 ± 897 | 1905 ± 322 | 10·6 ± 1·4 | 1·9 ± 0·2 | 10 ± 0·8 | 0·7 ± 0·2 |
| n = 15 | (5400–9200) | (1190–2650) | (5·8–23·1) | (0·7–2·8) | (6·4–12) | (0·4–1·6) |
| ALC patients | 6300 ± 585 | 1500 ± 224 | 6·6 ± 1·1 | 5·9 ± 0·7 | 16·2 ± 1·7 | 1·8 ± 0·3 |
| n = 17 | (2800–10600) | (320–3290) | (1·5–14·7) | (2·5–12·1) | (4·9–28) | (0·4–4·4) |
| NS | NS | P < 0·05 | P < 0·001 | P < 0·05 | P < 0·01 |
Leucocytes and lymphocytes numerations were performed for the normal management of the donors. The proportion of CD19+ B cells was studied by immunostaining and flow cytometry analysis. Serum IgA, G and M were assayed by immunonephelometry. Results are expressed as mean ± standard error of the mean and range.
CpG enhances IgA production by HD PBMCs, an effect that is attenuated in PBMCs from ALC patients
In order to evaluate the effect of TLR stimulation on IgA production, PBMCs were cultured with or without LPS, PGN, CpG, anti-CD40 mAb and/or IL-4, IL-10 or IL-21, a selected set of cytokines reported to be involved in different Ig isotype synthesis. In HD PBMCs, the addition of LPS to IL-10 enhanced IgA production significantly, and this phenomenon was strengthened by the addition of anti-CD40 mAb (Fig. 3a). In ALC PBMCs stimulated by anti-CD40 mAb, the addition of LPS to IL-10 enhanced IgA production (Fig. 3b). In combination with IL-10, PGN increased IgA production by both HD and ALC PBMCs, with or without CD40 stimulation (Fig. 3c and d). CpG enhanced IgA synthesis by HD PBMCs stimulated by IL-10 or IL-21 in the absence or presence of anti-CD40 mAb (Fig. 3e). In contrast, CpG, IL-10 or IL-21 alone had no significant effect (Fig. 3e). In the absence of CpG, IgA synthesis by ALC PBMCs was higher than that of HD PBMCs in all the culture conditions tested. Further enhancement by CpG was only observed in combination with IL-10 in the presence of anti-CD40 mAb (Fig. 3f).
Fig. 3.
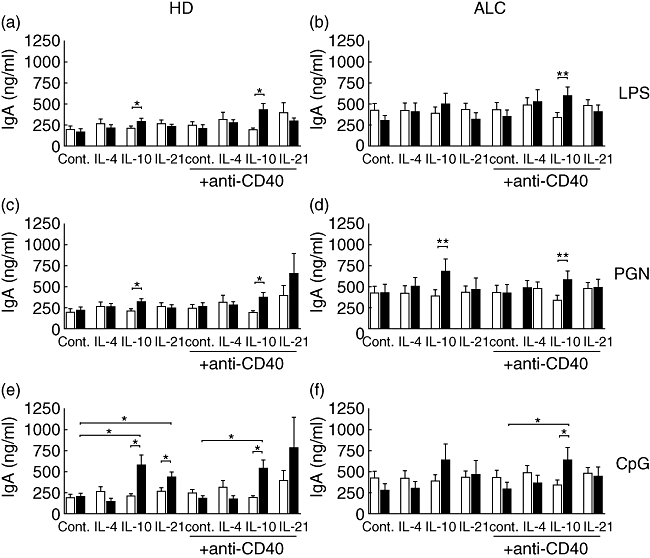
Effects of lipopolysaccharide (LPS) (a,b), peptidoglycan (PGN) (c,d) and cytosine-guanine dinucleotide (CpG) (e,f) on immunoglobulin (Ig)A synthesis by peripheral blood mononuclear cells (PBMCs) from alcoholic liver cirrhosis (ALC) patients (b,d,f) and healthy donors (HD) (a,c,e). White bars: culture medium; black bars: 1 µg/ml LPS (a,b), 2 µg/ml PGN (c,d), 2 µM CpG (e,f). IgA was measured by enzyme-linked immunosorbent assay in 12-day culture supernatants. Each bar represents mean ± standard error of the mean (n = 8 for ALC, n = 7 for HD). *P < 0·05, **P < 0·01 according to Student's paired t-test.
Role of accessory cells in the enhancement of IgA secretion by PAMPs
In order to determine further if the effect of PAMPs on IgA synthesis resulted from direct activation of B cells or a stimulation of accessory cells, we tested whether TLR stimulation of total SMCs or purified spleen CD19+ B cells from control subjects affected their Ig synthesis. As shown in Fig. 4a, PGN enhanced IgA secretion by total SMCs cultured in the presence of anti-CD40 mAb combined with IL-10 or IL-21. In the absence of CD40 stimulation a lower, but similar effect was observed only when PGN was combined with IL-21. LPS increased IgA secretion significantly by total SMCs only when combined with anti-CD40 mAb and IL-21. Neither PGN nor LPS enhanced IgA secretion by CD19+ B cells (Fig. 4b). In contrast, CpG enhanced IgA production strongly by both total SMCs and CD19+ B cells in almost all the culture conditions tested (Fig. 4). The powerful enhancing effects of CpG were observed in combination with IL-10 or IL-21. Similar secretion profiles were observed for IgM and IgG (data not shown). Taken together, these data show that LPS and PGN enhance IgA, IgG and IgM secretion indirectly by stimulating accessory cells, while the effects of CpG are mediated at least partially by direct activation of B cells.
Fig. 4.
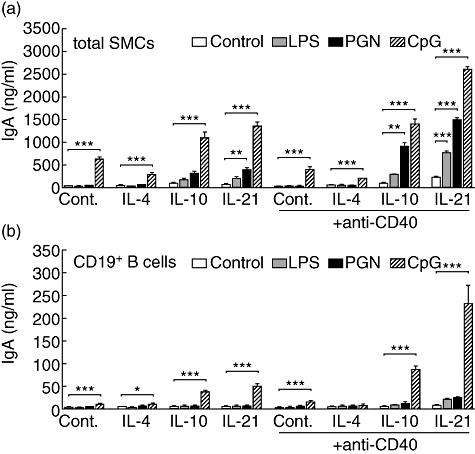
Effects of lipopolysaccharide (LPS), peptidoglycan (PGN) and cytosine-guanine dinucleotide (CpG) on immunoglobulin (Ig)A synthesis by total (a) and CD19+ isolated (b) spleen mononuclear cells. White bars: culture medium; grey bars: 1 µg/ml LPS; black bars: 2 µg/ml PGN; hatched bars: 2 µM CpG. IgA was measured in 12-day culture supernatants. Each bar represents mean ± standard error of the mean of three independent experiments. *P < 0·05, **P < 0·01, ***P < 0·001 according to one-way analysis of variance followed by Newman–Keuls post-test.
Absence of response to CpG on IgG and IgM synthesis by PBMCs from ALC patients
CpG was able to enhance the spontaneous production of IgM by HD PBMCs, and revealed or potentiated the effects of IL-4, IL-10 or IL-21 both in the presence or absence of anti-CD40 mAb (Fig. 5a). With regard to IgG synthesis by HD PBMCs, whereas TLR-9 ligation had no effect alone or with CD40 stimulation, CpG revealed the stimulating effects of IL-10 and IL-21 and vice versa in the presence or absence of anti-CD40 mAb (Fig. 5c). The effect of IL-21 in the presence of anti-CD40 mAb is already notable and cannot be enhanced further by CpG (Fig. 5c). Surprisingly, CpG was unable to enhance further either IgM or IgG synthesis induced or not by cytokines or anti-CD40 mAb in ALC PBMCs (Fig. 5b and d). As expected, IL-4 enhanced IgE synthesis strongly by CD40-stimulated HD PBMCs (Fig. 5e). In contrast to the other Igs, IgE-induced synthesis was inhibited strongly by CpG (Fig. 5e). Interestingly, the same profile was obtained with ALC PBMCs (Fig. 5f), showing that TLR-9 downstream signalling is still functional in ALC PBMCs.
Fig. 5.
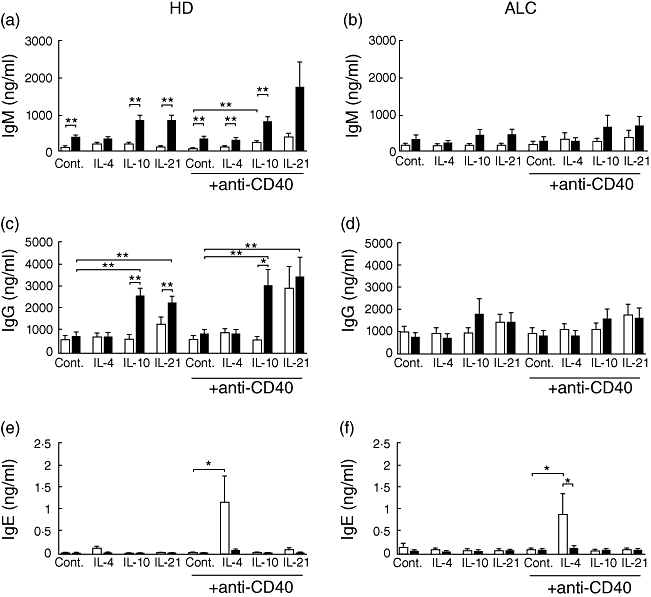
Effects of cytosine-guanine dinucleotide (CpG) on immunoglobulin (Ig)M (a,b), IgG (c,d), and IgE (e,f) synthesis by peripheral blood mononuclear cells (PBMCs) from alcoholic liver cirrhosis (ALC) patients (b,d,f) and healthy donors (HD) (a,c,e). White bars: control; black bars: 2 µM CpG. Igs were measured in 12-day culture supernatants by specific enzyme-linked immunosorbent assay. Each bar represents mean ± standard error of the mean (n = 8 for ALC, n = 7 for HD). *P < 0·05, **P < 0·01 according to Student's paired t-test (Wilcoxon's matched pairs t-test for IgE).
Purified B cells from ALC patients secrete more IgA than B cells from HD in response to CpG or R848
Although TLR-9 appeared to be functional in PBMCs from ALC patients, in a few conditions CpG had only a weak stimulatory effect on Ig secretion by PBMCs from ALC patients (Fig. 3f). Taking into account that such effects are mediated by direct stimulation of B cells, we studied TLR-9 stimulation on IgA secretion by purified CD19+ B cells from ALC patients compared to B cells from HD. CpG alone increased IgA synthesis significantly by ALC B cells; this effect was enhanced strongly by the addition of IL-21 (Fig. 6b), whereas IL-21 was necessary to increase IgA secretion significantly by B cells from HD in the presence of CpG (Fig. 6a). In the absence of stimulation, cultured CD19+ B cells from ALC patients always secreted a mean of 45 times more IgA (127 ± 41 ng/ml, Fig. 6b) than B cells from HD (2·8 ± 0·7 ng/ml, Fig. 6a). Further analysis of relative and absolute IgA production revealed that CpG alone enhanced IgA secretion 31-fold by CD19+ B cells from HD, whereas for ALC the enhancement was only sevenfold (Fig. 6a and b). This is accurate whatever the culture conditions: anti-CD40, IL-21 or a combination of anti-CD40 and IL-21. This analysis shows that the relative efficiency of CpG stimulation on IgA synthesis is less dramatic for ALC B cells. Absolute levels of IgA production by CpG-activated B cells from ALC patients were obviously higher than for HD, whatever the stimulation conditions (none, IL-21, anti-CD40 or both). For example, IgA levels in supernatant of IL-21- and anti-CD40-activated B cells from HD were 21 ± 6 ng/ml, and 473 ± 146 ng/ml when CpG was added; under the same conditions, for ALC patients the levels of IgA were 568 ± 177 ng/ml and 5023 ± 1582 ng/ml, respectively. Thus, the calculated increase in IgA was 452 ng/ml for HD and 4455 ng/ml for ALC. Overall, B cells from ALC patients secreted 10–20 times more IgA than B cells from HD under the same activation conditions (Fig. 6a and b, P < 0·01 according to Mann–Whitney U-test). The absolute IgA production by CpG-activated ALC B cells was enhanced greatly when compared to HD, although the relative CpG-induced IgA production was less important for ALC B cells. A very close IgA over-secretion pattern was observed for both HD and ALC B cells under TRL7 activation by R848 (Fig. 6c and d).
Fig. 6.
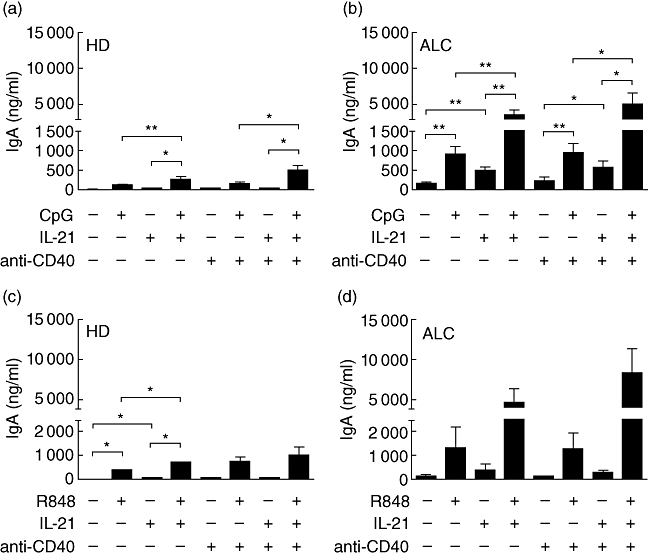
Effects of cytosine-guanine dinucleotide (CpG) (a,b) and R848 (c,d) on immunoglobulin (Ig)A synthesis by CD19+ B cells from healthy donors (HD) (a,c) and alcoholic liver cirrhosis (ALC) patients (b,d). IgA was measured in 12-day culture supernatants. Each bar represents mean ± standard error of the mean (a,b: n = 7 for ALC, n = 6 for HD; c,d: n = 3 for ALC and n = 3 for HD). *P < 0·05, **P < 0·01, according to Student's paired t-test.
Discussion
In alcoholic liver cirrhosis, liver injury is accompanied by a characteristic increase in serum levels of IgA, IgG and IgM, the origin of which remains to be elucidated fully. In parallel, ALC patients often undergo bacterial translocation, evidenced by circulating viable or non-viable bacterial products such as endotoxin or bacterial DNA, and caused by complex mechanisms including the increase of intestinal permeability [33]. Endotoxin, also known as LPS, belongs to the PAMPs family of microbial compounds, also including PGN and CpG, which are potent activators of TLR-4, -2 and -9, respectively. Activation of TLR-2 and -4 has been associated with proinflammatory cytokine secretion, while TLR-9 activation, due to its particular expression pattern, is implicated in B cell activation and Ig synthesis [8]. Riordan et al. showed previously that in peripheral monocytes from ALC patients, TLR-2, but not TLR-4, expression level was increased [28]. In our study, TLR-2 and -4 gene expression levels in PBMCs were increased slightly, while TLR-9 gene expression level was reduced significantly. In agreement, the percentage of B cells expressing TLR-9 as well as the mean fluorescence intensity (MFI) of TLR-9 labelling were significantly lower in B cells from ALC patients compared to HD. Such down-regulation of TLR-9 expression by B cells has been reported after in vitro CpG treatment, suggesting that the decrease in TLR-9 expression by B cells from ALC patients could be due to in vivo priming by bacterial DNA [14].
In mice, TLRs are linked closely to B cell activation and Ig secretion, as demonstrated by the critically impaired Ig synthesis against both T-dependent and T-independent antigens in MyD88 knock-out mice [22]. In humans, TLR implication in Ig synthesis has long been supported by the effectiveness of adjuvants in the success of vaccination [34], and more recently by the finding that B cells can be induced to produce Igs by bacterial CpG DNA or R848 through direct TLR-9 or TLR-7 stimulation, respectively [13,20,21,35]. In the present study, we have shown that CpG enhanced IgA, IgG and IgM synthesis by HD PBMCs. In contrast, CpG inhibited the secretion of IgE induced by anti-CD40 mAb and IL-4, according to previously reported results [15]. IgM secretion by HD PBMCs was also increased significantly by CpG in the absence or presence of exogenous IL-10 or IL-21. As described previously for IgG production [16], CpG enhanced IgA and IgG secretions by HD PBMCs only when combined with IL-10 or IL-21. In the presence of IL-10, LPS and PGN also enhanced IgA secretion by HD PBMCs. According to the well-documented expression patterns of TLR-2, -4 and -9 [12], and supported by our data obtained with purified spleen CD19+ B cells, we speculate that the enhancing effects of PGN and LPS on IgA secretion by HD PBMCs are mediated by the activation of non-B cells, such as cells from the monocytic lineage. In contrast, the action of CpG on Ig secretion by HD PBMCs is mediated, at least partially, by direct B cell activation. Nevertheless, we cannot exclude the participation of accessory cells such as plasmacytoid dendritic cells, the role of which in the induction of Ig synthesis by CpG has already been demonstrated [14].
In ALC, translocation of bacterial products leads to increased endotoxaemia, as evidenced previously by the presence of circulating PAMPs such as LPS or bacterial DNA [1,19]. Taking into account that PGN, LPS and CpG, in the presence of appropriate cytokines, enhance IgA production by HD PBMCs, and that ALC is characterized by hyper-IgA, we tested the influence of these PAMPs on Ig secretion by ALC PBMCs. Spontaneous IgA production by ALC PBMCs was increased significantly, as described previously [27]. As also observed for HD PBMCs, LPS, PGN and CpG enhanced IgA secretion by ALC PBMCs in the presence of anti-CD40 mAb and IL-10. CpG, which was the strongest enhancer of IgA, IgG and IgM synthesis by HD PBMCs, had no or very discrete effects on IgA, IgG and IgM secretion by ALC PBMCs. Nevertheless, TLR-9 remains functional in these cells, as evidenced by the fact that CpG inhibited IgE synthesis induced by anti-CD40 mAb and IL-4 in HD PBMCs, as reported [15], but also in ALC PBMCs.
Study of IgA production by purified CD19+ B cells from ALC patients revealed that IgA levels were always 10–20 times higher than those found in HD B cell supernatants, either with or without CpG stimulation. CpG alone increased IgA secretion by B cells from ALC patients (this effect was enhanced in combination with IL-21), while IL-21 was necessary to observe an over-increased IgA secretion by HD B cells in the presence of CpG. Because activators of TLR-7 have been reported recently to induce Ig synthesis [13,20,21], we also studied the ability of purified B cells from HD and ALC patients to produce IgA under R848 activation as a control. As expected, the IgA secretion patterns were very similar to those observed in the presence of CpG.
Further analysis showed that relative CpG-induced IgA production was weaker for ALC B cells when compared to HD B cells, in accordance with the lower TLR-9 expression on ALC B cells compared to HD B cells. The absolute IgA production by CpG-activated B cells was enhanced greatly for ALC when compared to HD, in agreement with their intrinsic ability to produce spontaneously more IgA than HD. The reason for this remains an open question. We suggest that in vivo priming of B cells from ALC patients (a non-exclusive hypothesis is that TLR-9 and/or TLR-7 could participate to this priming) enhances their ex vivo capacity to produce IgA spontaneously, and to over-produce high absolute IgA levels after ex vivo CpG stimulation.
Taken together, these data favour the hypothesis of an involvement of TLR pathways in IgA synthesis, contributing to the characteristic hyperimmunoglobulinaemia of ALC patients. Our previous study showed that the diminished expression and/or altered glycosylation of the Fcα receptor at the surface of monocytes, leading to defective clearance of IgA, could be involved in the hyper-IgA accompanying ALC [31]. In the present work, we also suggest that circulating PAMPs activate B cells further to produce IgA, IgG and IgM by either a direct or indirect pathway, and participate in ALC-associated hyperimmunoglobulinaemia.
Acknowledgments
We thank Dr Renato Monteiro and Professor Franck Morel for their careful review of the manuscript. This study was supported by grants from a clinical research program from Poitiers University Hospital.
Disclosure
The authors have no conflicts of interest including all relevant financial interests in any company or institution.
References
- 1.Bauer TM, Schwacha H, Steinbruckner B, et al. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol. 2002;97:2364–70. doi: 10.1111/j.1572-0241.2002.05791.x. [DOI] [PubMed] [Google Scholar]
- 2.von Baehr V, Docke WD, Plauth M, et al. Mechanisms of endotoxin tolerance in patients with alcoholic liver cirrhosis: role of interleukin 10, interleukin 1 receptor antagonist, and soluble tumour necrosis factor receptors as well as effector cell desensitisation. Gut. 2000;47:281–7. doi: 10.1136/gut.47.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guarner C, Soriano G. Bacterial translocation and its consequences in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:27–31. doi: 10.1097/00042737-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–5. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann A, Baltimore D. Circuitry of nuclear factor kappaB signaling. Immunol Rev. 2006;210:171–86. doi: 10.1111/j.0105-2896.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–91. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 9.Hemmi H, Takeuchi O, Kawai T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 10.Nakao Y, Funami K, Kikkawa S, et al. TLR6 participates in the recognition of diacylated lipopeptide and peptidoglycan in human cells. J Immunol. 2005;174:1566–73. doi: 10.4049/jimmunol.174.3.1566. [DOI] [PubMed] [Google Scholar]
- 11.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- 12.Hornung V, Rothenfusser S, Britsch S, et al. Quantitative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168:4531–7. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 13.Hanten JA, Vasilakos JP, Riter CL, et al. Comparison of human B cell activation by TLR7 and TLR9 agonists. BMC Immunol. 2008;9:39. doi: 10.1186/1471-2172-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poeck H, Wagner M, Battiany J, et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–64. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 15.Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003;4:687–93. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- 16.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173:4479–91. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 17.Blaas SH, Stieber-Gunckel M, Falk W, Obermeier F, Rogler G. CpG-oligodeoxynucleotides stimulate immunoglobulin A secretion in intestinal mucosal B cells. Clin Exp Immunol. 2009;155:534–40. doi: 10.1111/j.1365-2249.2008.03855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kikuchi K, Lian ZX, Yang GX, et al. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128:304–12. doi: 10.1053/j.gastro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Such J, Frances R, Munoz C, et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135–41. doi: 10.1053/jhep.2002.33715. [DOI] [PubMed] [Google Scholar]
- 20.Glaum MC, Narula S, Song D, et al. Toll-like receptor 7-induced naive human B-cell differentiation and immunoglobulin production. J Allergy Clin Immunol. 2009;123:224–30. e4. doi: 10.1016/j.jaci.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and Toll-like receptors. Allergy. 2008;63:1455–63. doi: 10.1111/j.1398-9995.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 22.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–8. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 23.Albillos A, de la Hera A, Gonzalez M, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208–17. doi: 10.1053/jhep.2003.50038. [DOI] [PubMed] [Google Scholar]
- 24.Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumor necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. 1997;26:1530–7. doi: 10.1002/hep.510260621. [DOI] [PubMed] [Google Scholar]
- 25.McClain CJ, Hill DB, Song Z, Deaciuc I, Barve S. Monocyte activation in alcoholic liver disease. Alcohol. 2002;27:53–61. doi: 10.1016/s0741-8329(02)00212-4. [DOI] [PubMed] [Google Scholar]
- 26.Le Moine O, Marchant A, De Groote D, Azar C, Goldman M, Deviere J. Role of defective monocyte interleukin-10 release in tumor necrosis factor-alpha overproduction in alcoholic cirrhosis. Hepatology. 1995;22:1436–9. [PubMed] [Google Scholar]
- 27.Deviere J, Content J, Denys C, et al. Immunoglobulin A and interleukin 6 form a positive secretory feedback loop: a study of normal subjects and alcoholic cirrhotics. Gastroenterology. 1992;103:1296–301. doi: 10.1016/0016-5085(92)91519-a. [DOI] [PubMed] [Google Scholar]
- 28.Riordan SM, Skinner N, Nagree A, et al. Peripheral blood mononuclear cell expression of Toll-like receptors and relation to cytokine levels in cirrhosis. Hepatology. 2003;37:1154–64. doi: 10.1053/jhep.2003.50180. [DOI] [PubMed] [Google Scholar]
- 29.van de Wiel A, Schuurman HJ, Kater L. Alcoholic liver disease: an IgA-associated disorder. Scand J Gastroenterol. 1987;22:1025–30. doi: 10.3109/00365528708991951. [DOI] [PubMed] [Google Scholar]
- 30.Gomez F, Ruiz P, Schreiber AD. Impaired function of macrophage Fc gamma receptors and bacterial infection in alcoholic cirrhosis. N Engl J Med. 1994;331:1122–8. doi: 10.1056/NEJM199410273311704. [DOI] [PubMed] [Google Scholar]
- 31.Silvain C, Patry C, Launay P, Lehuen A, Monteiro RC. Altered expression of monocyte IgA Fc receptors is associated with defective endocytosis in patients with alcoholic cirrhosis. Potential role for IFN-gamma. J Immunol. 1995;155:1606–18. [PubMed] [Google Scholar]
- 32.Nouri-Aria KT, Alexander GJ, Portmann BC, Hegarty JE, Eddleston AL, Williams R. T and B cell function in alcoholic liver disease. J Hepatol. 1986;2:195–207. doi: 10.1016/s0168-8278(86)80078-2. [DOI] [PubMed] [Google Scholar]
- 33.Campillo B, Pernet P, Bories PN, Richardet JP, Devanlay M, Aussel C. Intestinal permeability in liver cirrhosis: relationship with severe septic complications. Eur J Gastroenterol Hepatol. 1999;11:755–9. doi: 10.1097/00042737-199907000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Freund J, Casals J, Hosmer E. Sensitization and antibody formation after injection of tubercle bacilli and paraffin oil. Proc Soc Exp Biol Med. 1937;37:509–13. [Google Scholar]
- 35.Bauer S, Kirschning CJ, Hacker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–42. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levan-Petit I, Lelievre E, Barra A, et al. T(h)2 cytokine dependence of IgD production by normal human B cells. Int Immunol. 1999;11:1819–28. doi: 10.1093/intimm/11.11.1819. [DOI] [PubMed] [Google Scholar]
- 37.Pene J, Gauchat JF, Lecart S, et al. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J Immunol. 2004;172:5154–7. doi: 10.4049/jimmunol.172.9.5154. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 39.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT–PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. Epub 18 June 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]


