Abstract
Primary amines present in protonated polypeptides can be covalently modified via gas-phase ion/ion reactions using bifunctional reagent ions. The use of reagent anions with a charge bearing site that leads to strong interactions with the polypeptide, such as sulfonic acid, gives rise to the formation of a long-lived adduct. A distinct reactive functional group, an aldehyde in the present case, can then undergo a reaction with the peptide. Collisional activation of the adduct ion formed from a reagent with an aldehyde group and a peptide ion with a primary amine gives rise to water loss in conjunction with imine (Schiff base) formation. The covalently-bound modification is retained upon subsequent collisional activation. This work demonstrates the ability to selectively modify polypeptide ions in the gas-phase within the context of a multi-stage mass spectrometry experiment.
The structural characterization of polypeptides via tandem mass spectrometry has become a central activity in protein identification and quantification. Peptides and proteins are frequently modified in solution using selective derivatization reactions1 inter alia, to facilitate ionization,2 quantification,3 or structural characterization.4 We demonstrate here the formation of Schiff base derivatives of primary amine groups present in gaseous polypeptide ions via gas-phase ion/ion reactions. This work demonstrates that polypeptide ions can be modified in a functional group-selective fashion in the gas-phase and within the context of an MSn experiment.
Gas-phase ion/ion reactions have proved to be useful for a number of applications involving ions derived from biopolymers.5 The most widely studied reactions have involved proton transfer, primarily for charge state manipulation, or electron transfer, which has been demonstrated to be useful as a dissociation method,6 particularly for multiply-protonated peptides and proteins. Metal ion transfer reactions7 as well as multiple proton transfer reactions that lead to the inversion of the ion polarity8 have also been demonstrated, as has the formation of peptide metal coordination complexes involving rhenate anions, ReO3 −.9
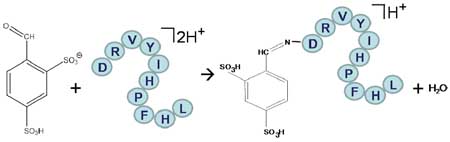
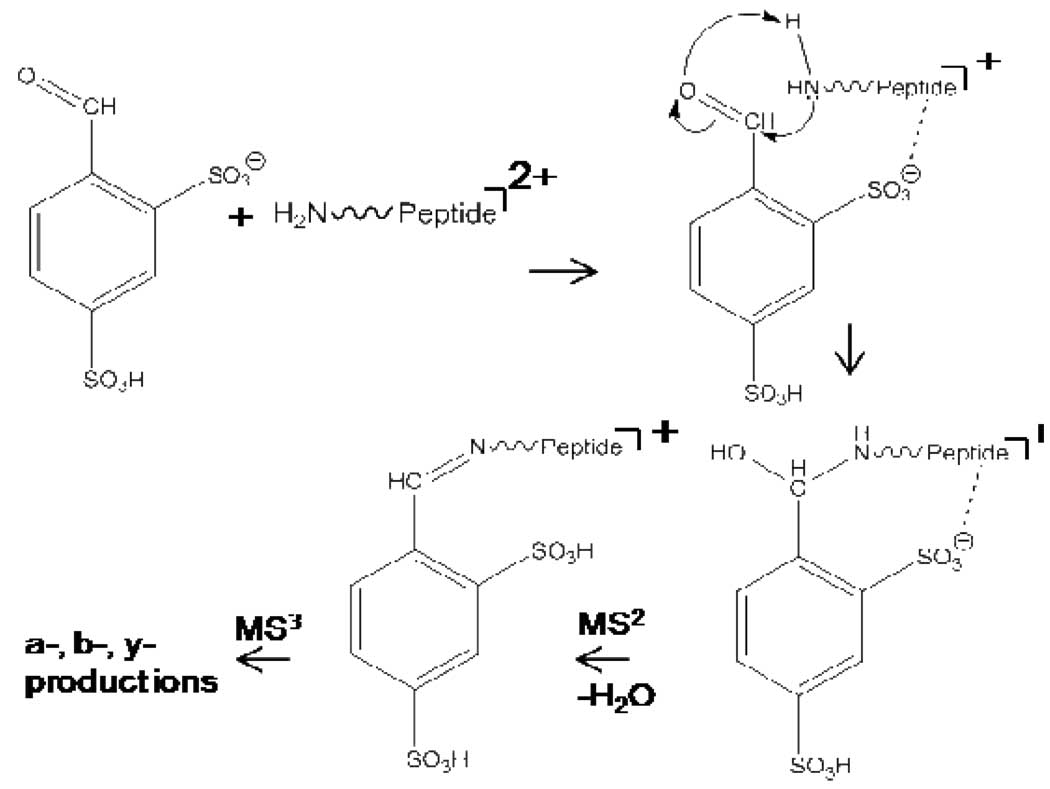
We examined the possibility for Schiff base formation, which results from the reaction of a primary amine (e.g., a free N-terminus or the ε-NH2 of lysine) and an aldehyde or ketone to yield an imine, as this has been noted via gas-phase ion/molecule reactions10 and, in particular, with protonated peptides in reaction with acetone10(a). The ability to make such a modification via ion/ion reactions is attractive in that it circumvents the volatility requirement for the reagent and therefore provides the potential for attaching a wide range of chemical functionalities to polypeptide ions. The reaction examined in this work is summarized in Scheme 1.
Scheme 1.
Ion/ion reaction scheme for Schiff base formation.
The first step in the process is the reaction of a peptide ion with at least one primary amine and at least two excess positive charges with singly deprotonated 4-formyl-1,3-benzenedisulfonic acid (FBDSA). It is important to use an anionic reagent that forms long-lived complexes. Otherwise, proton transfer is observed as the essentially exclusive ion/ion reaction channel. In this sense, the use of a sulfonic acid is important because the sulfonate group is known to lead to strong interactions with the cation11 leading to the formation of long-lived intermediates. Carboxylate groups, on the other hand, interact less strongly with protonated peptides. Reagents with a carboxylate group as the charge bearing site in the reagent anion showed no evidence for Schiff base formation. (See supplementary data for a list of reagents examined.) The next step of the process involves the collisional activation of the ion/ion reaction complex. Loss of a water molecule takes place upon Schiff base formation. Hence, formation of a long-lived complex followed by dehydration leads to the modified peptide.
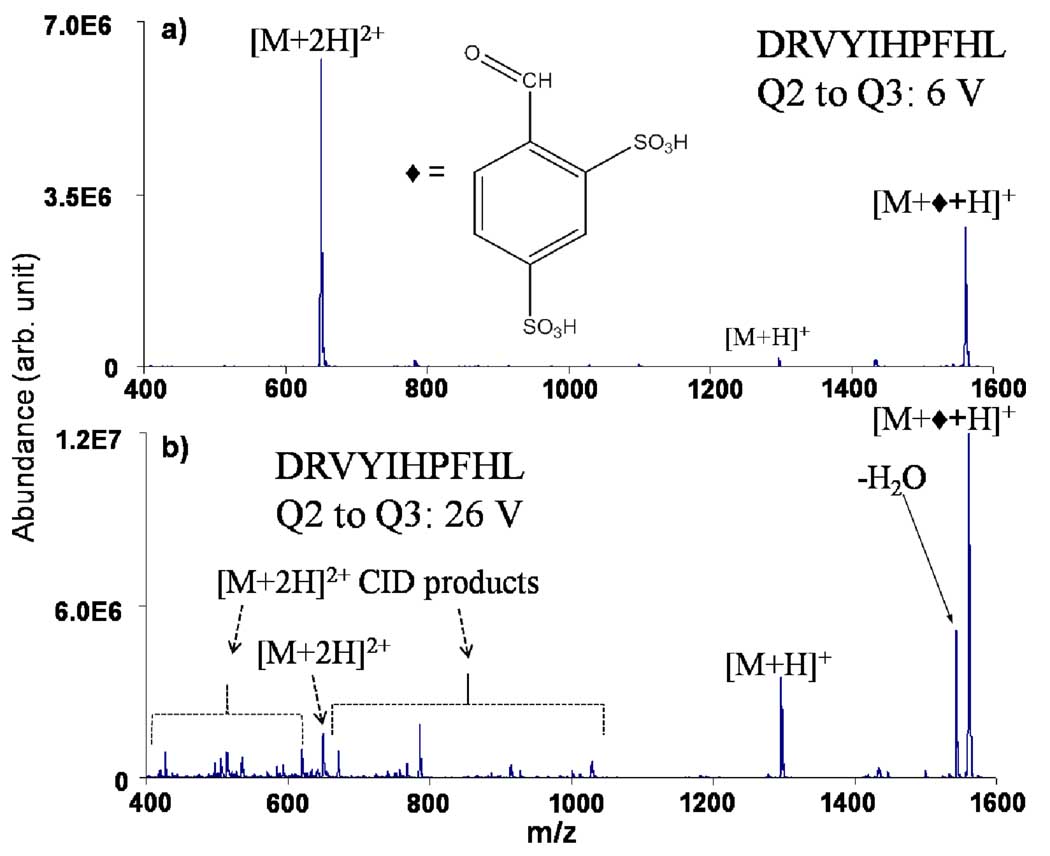
Ion/ion reactions involving a series of doubly and triply protonated peptides and with a variety of deprotonated acids with an aldehyde or ketone functional group were conducted with hybrid triple quadrupole/linear ion trap tandem mass spectrometer that has been modified for ion/ion reactions.12 The procedures for conducting ion/ion reaction experiments in this apparatus have been described.12 Figure 1 shows the post-ion/ion reaction results (50–500 ms reaction time, depending upon reagent anion signal level and pressure in the linear ion trap) for doubly-protonated angiotensin I (DRVYIHPFHL) in reaction with deprotonated FBDSA. Figure 1(a) shows the results after transfer from the ion/ion reaction region (quadrupole 2 of the instrument) to the mass analysis linear ion trap (quadrupole 3) under mild transfer conditions (6 V) while Figure 1(b) shows the results obtained after a significant degree of collisional activation induced by transferring the ions from quadrupole 2 to quadrupole 3 using a potential difference of 26 V. The larger energy gives rise to a greater degree of excitation via collisions in the relatively high pressure region (on the order of a millitorr) between the two quadrupoles.
Figure 1.
Post-ion/ion data for angiotensin I [M+2H]2+. (a) Low and (b) high energy transfer from the reaction region to the analyzer.
Both of the spectra show that adduct formation is the dominant ion/ion reaction product. Figure 1(b) shows water loss to be the major dissociation product upon collisional activation. There is also a significant increase in the signal due to [M+H]+, which reflects loss of the neutral reagent species. The doubly protonated N-terminally acetylated angiotensin also showed dominant adduct formation but yielded no significant water loss upon transfer to the analyzer quadrupole at 26 V (see supplementary information, Figure S1). Rather, loss of FBDSA was observed. This control experiment is consistent with Schiff base formation at the N-terminus.
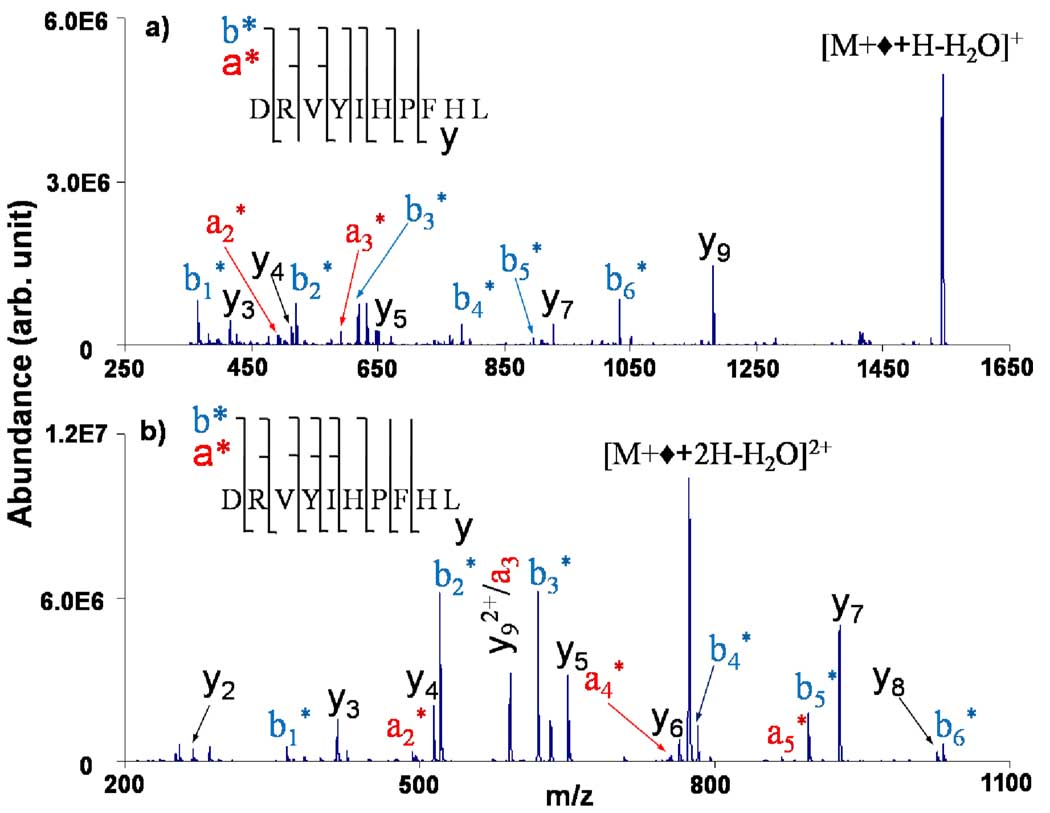
Collision-induced dissociation (CID) of the water loss product confirms Schiff base formation, as illustrated in Figure 2, which shows the product ion spectra from the water loss products formed from the complex between deprotonated FBDSA and doubly (Figure 2(a)) and triply (Figure 2(b)) protonated angiotensin. For both peptide charge states, the product ion spectra are fully consistent with Schiff base formation at the N-terminus, as reflected by the expected mass increase for the N-terminal fragments (i.e., the a- and b-type ions) and the lack of evidence for the modification on any C-terminal ions (i.e., the y-type ions). The presence of a modified b1 ion is fully consistent with modification at the N-terminus.13 For both charge states, significantly greater structural information is present in the Schiff base derivative spectrum than in the corresponding CID spectrum of the unmodified version of the peptide. (See supplementary information, Figure S2.)
Figure 2.
CID product ion spectra of the water loss products from the ion/ion reaction complexes between deprotonated FBDSA and doubly (a) and triply (b) protonated angiotensin I.
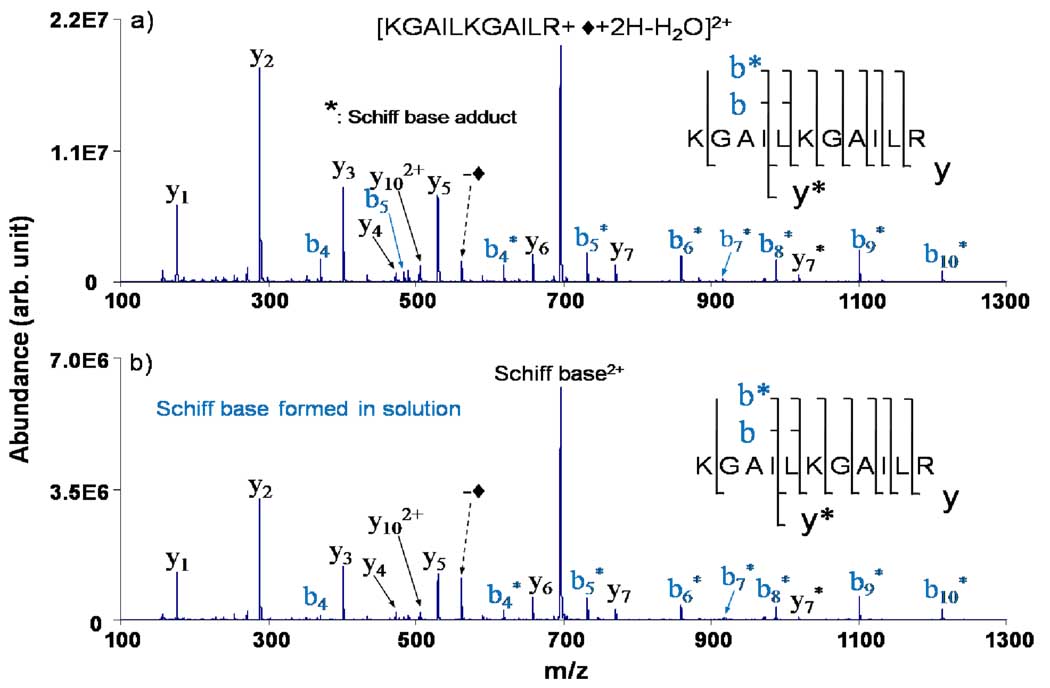
It is also of interest to consider the dissociation behavior of the peptide modified in the gas-phase with that of the peptide modified in solution. An example of such a comparison is provided in Figure 3, which shows the CID data for the doubly protonated nominal Schiff base conjugates of the peptide KGAILKGAILR. Figure 3(a) shows the product ion spectrum derived from the modification made via ion-ion reactions in the gas-phase from reaction of the triply protonated peptide with deprotonated FBDSA while Figure 3(b) shows the results for the Schiff base derivative formed in solution. The product ion spectra are indistinguishable within the experimental reproducibility of the measurement. No fragments reflect the presence of the modification on the five residues at the C-terminal end of the molecule. Rather, the products are consistent with a mixture of modification sites, presumably at the N-terminus or the ε-NH2 groups of Lys-1 or Lys-6.
Figure 3.
CID spectra of KGAILKGAILR a) modified in the gas-phase via ion/ion reaction and b) modified in solution.
The demonstration of Schiff base formation via ion/ion reactions opens up new possibilities for the structural characterization of gaseous ions. The use of multi-functional reagent ions, for example, allows for the selective attachment of chemical functionalities of interest, such as, for example, chromophores that can facilitate photodissociation or spectroscopy experiments. The use of selective reactive groups in reagent ions also opens up the possibility for functional group screening applications. This line of inquiry constitutes a significant potential expansion in utility of gas-phase ion/ion chemistry in identification and characterization applications.
Supplementary Material
ACKNOWLEDGMENT
Research supported by the Office of Basic Energy Sciences, Office of Sciences, U.S. Department of Energy, under Award No. DE-FG02-00ER15105 and the National Institutes of Health under Grant GM 45372.
Footnotes
Supporting Information Available: Complete Ref. 3(b), a list of other reagents examined, post-ion/ion reaction spectra for Nterminally acetylated angiotensin and CID spectra for singly and doubly protonated angiotensin I. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Mendoza VL, Vachet RW. Mass Spectrom. Rev. 2009 doi: 10.1002/mas.20203. In Press. DOI:10.1002/mas.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang WC, Mirzaei H, Liu XP, Regnier FE. Anal. Chem. 2006;78:4702–4708. doi: 10.1021/ac0600510. [DOI] [PubMed] [Google Scholar]
- 3.(a) Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Nature Biotech. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]; (b) Ross PL, et al. Mol. and Cell. Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.(a) Beardsley RL, Sharon LA, Reilly JP. Anal. Chem. 2005;77:6300–6309. doi: 10.1021/ac050540k. [DOI] [PubMed] [Google Scholar]; (b) Madsen JA, Brodbelt JS. Anal. Chem. 2009;81:3645–3653. doi: 10.1021/ac9000942. [DOI] [PubMed] [Google Scholar]
- 5.(a) Pitteri SJ, McLuckey SA. Mass Spectrom. Rev. 2005;24:931–958. doi: 10.1002/mas.20048. [DOI] [PubMed] [Google Scholar]; (b) McLuckey SA, Stephenson JL., Jr Mass Spectrom. Rev. 1998;17:369–407. doi: 10.1002/(SICI)1098-2787(1998)17:6<369::AID-MAS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 6.Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Proc. Nat. Acad. Sci. USA. 2004;101(26):9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton KA, McLuckey SA. J. Am. Chem. Soc. 2003;125:12404–12405. doi: 10.1021/ja036924e. [DOI] [PubMed] [Google Scholar]
- 8.He M, McLuckey SAJ. Am. Chem. Soc. 2003;125:7756–7757. doi: 10.1021/ja0354521. [DOI] [PubMed] [Google Scholar]
- 9.McAlister GC, Kiessel SEB, Coon JJ. Int. J. Mass Spectrom. 2008;276:149–152. doi: 10.1016/j.ijms.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Burinsky DJ, Campana JE, Cooks RG. Int. J. Mass Spectrom. Ion Processes. 1984;62:303–315. [Google Scholar]; (b) O'Hair RAJ, Reid GE. J. Am. Soc. Mass Spectrom. 2000;11:244–256. doi: 10.1016/S1044-0305(99)00142-7. [DOI] [PubMed] [Google Scholar]
- 11.He M, Emory JF, McLuckey SA. Anal. Chem. 2005;77:3173–3182. doi: 10.1021/ac0482312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Xia Y, Wu J, Londry FA, Hager JW, McLuckey SA. J. Am. Soc. Mass Spectrom. 2005;16:71–81. doi: 10.1016/j.jasms.2004.09.017. [DOI] [PubMed] [Google Scholar]; (b) Xia Y, Liang X, McLuckey SAJ. Am. Soc. Mass Spectrom. 2005;16:1750–1756. doi: 10.1016/j.jasms.2005.07.013. [DOI] [PubMed] [Google Scholar]; (c) Liang X, McLuckey SA. J. Am. Soc. Mass Spectrom. 2007;18:882–890. doi: 10.1016/j.jasms.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee YH, Kim M-S, Choie W-S, Min H-K, Lee S-W. Proteomics. 2004;4:1684–1694. doi: 10.1002/pmic.200300698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.