Summary
TLR2−/− mice immunized with Streptococcus pneumoniae (Pn) elicit normal IgM, but defective CD4+ T cell-dependent (TD) type 1 IgG isotype production, associated with a largely intact innate immune response. We studied the TD phosphorylcholine (PC)-specific IgG3 versus the T cell-independent IgM response to Pn to determine whether TLR2 signals directly via the adaptive immune system. Pn-activated TLR2−/− bone marrow dendritic cells (BMDC) have only a modest defect in cytokine secretion, undergo normal maturation, and when transferred into naive WT mice elicit a normal IgM and IgG3 anti-PC response, relative to WT BMDC. Pn synergizes with BCR and TCR signaling for DNA synthesis in purified WT B and CD4+ T cells, respectively, but is defective in cells lacking TLR2. Pn primes TLR2−/− mice for a normal CD4+ T cell IFN-γ recall response. Notably, TLR2−/− B cells transferred into RAG-2−/− mice with WT CD4+ T cells, or TLR2−/− CD4+ T cells transferred into athymic nude mice, each elicit a defective IgG3, in contrast to normal IgM, anti-PC response relative to WT cells. These data are the first to demonstrate a major role for B cell and CD4+ T cell expression of TLR2 for eliciting an anti-bacterial humoral immune response.
Keywords: Rodent, Bacterial, Antibodies, Toll-like receptor, Transgenic/Knockout Mice
Introduction
Initial host defense against infections by extracellular bacteria is mediated by the innate immune response, which is characterized by the use of germline-encoded pattern recognition receptors (PRRs) that bind distinct pathogen-associated molecular patterns (PAMPs) on microorganisms. The major PRRs in mammalian species are the Toll-like receptor (TLR) family of proteins [1]. Currently, 12 TLRs are described in the mouse. TLRs mediate the activation of nuclear transcription factors via one or more adaptor proteins, most critically MyD88. The ultimate result of TLR signaling is transcriptional activation of numerous genes, including those encoding various proinflammatory cytokines, chemokines, and immune receptors. A major component of innate immune activation, that serves as a link to the subsequent adaptive response, is the stimulation of dendritic cells (DC) that have internalized microorganisms at the site of infection [2]. Activated DC migrate to secondary lymphoid organs, undergo phenotypic maturation, present peptide/MHC complexes and secrete cytokines that promote T cell activation and differentiation [3]. In this manner, the innate immune system plays a major role in initiating and guiding the adaptive immune response.
Streptococcus pneumoniae [Pn], a Gram-positive extracellular bacteria, is the most important etiological agent of community-acquired pneumoniae and a major cause of morbidity and mortality in humans [4, 5]. Pn is known to contain ligands for TLR2, TLR4, TLR7/8, and TLR9 that can act collectively to initiate innate immunity. This is largely mediated through phagocytosis and intracellular killing by neutrophils and macrophages which are recruited to, and activated at, the site of infection [6]. Pn also elicits antibody specific for various structures on the bacterial surface, which includes the capsular polysaccharide PS [PPS] [7], the cell wall C-polysaccharide (C-PS, teichoic acid), including it’s phosphorylcholine (PC) moieties [8] and various pneumococcal proteins including pneumococcal surface protein (PspA) [9]. Adaptive immunity to pneumococcus is largely conferred by these antibodies, which synergize with the innate immune system to confer markedly enhanced host protection [10]
Mice genetically deficient in TLR2 (TLR2−/−) exhibit an increased [11–13], or no apparent change in [14, 15], sensitivity to infection with Pn, depending on the model system, in contrast to the more severe defect in host defense observed in MyD88−/− mice [15–17]. In particular, we previously demonstrated that spleen cells from TLR2−/− mice elicit a largely normal cytokine and chemokine response in vitro and in vivo in response to Pn, capsular type 14 (Pn14), associated with no increase in lethality upon i.p. infection, relative to wild-type (WT) mice [15]. More recently, we demonstrated that TLR2 synergizes with both TLR4 and TLR9 for induction of the MyD88-dependent splenic cytokine and chemokine response to Pn14 [18]. In contrast to their largely intact innate response to Pn14, TLR2−/− mice exhibited a significant decrease in the elicitation of type 1 IgG isotypes (IgG3, IgG2b, and IgG2a) specific for PPS, PC and a number of Pn proteins, whereas induced serum titers of specific IgG1 were equivalent to that observed in WT mice. Thus, the defective type 1 humoral immune response to Pn in TLR2−/− mice suggested that TLR2 might be playing an important role directly at the level of the adaptive immune system.
We previously demonstrated that the IgG, but not IgM, anti-PC and anti-PPS14 response to immunization with heat-inactivated Pn14, was dependent on CD4+ T cells, similar to that observed for the IgG response specific for several pneumococcal proteins [19–21]. Although TLRs have been shown to be critical in mediating innate immune cell activation, they are also expressed by cells participating directly in adaptive immunity (i.e. B cells [22], CD4+ T cells [23] and DC [24]) and could therefore possibly play a more direct role in an adaptive immune response. Indeed, two recent reports have indicated a critical role for B cell expression of MyD88 and TLR4 [25], or TLR9 [26] in promoting the humoral response to model protein antigens or virus-like particles, respectively. In this report we analyze the T cell-independent IgM anti-PC and T cell-dependent IgG3 anti-PC response to intact Pn14 and demonstrate, for the first time, that both B cell and CD4+ T cell expression of TLR2 is critical for optimal induction of a type 1 IgG response to an intact bacterium.
Results
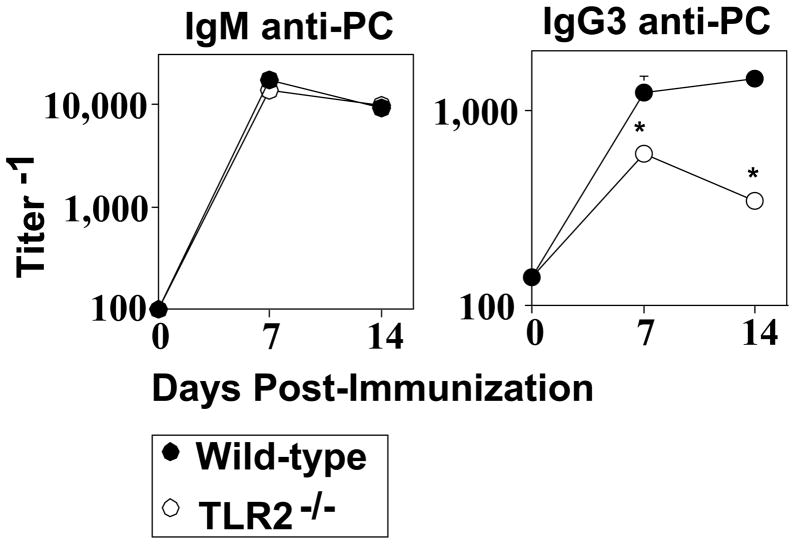
TLR2−/− mice elicit a defective IgG3, but normal IgM, anti-PC response to intact Pn14
TLR2−/− mice elicit a normal IgM, but defective type I IgG (IgG3, IgG2b, IgG2a) anti-phosphorylcholine (PC) response to intact Pn, capsular type 14 (Pn14), relative to WT mice [15]. The IgG, but not IgM, anti-PC response is TD [19–21]. The defective IgG anti-PC response in TLR2−/− mice was associated with a largely intact innate immune response [15], suggesting a role for TLR2 directly at the level of adaptive immunity. Since IgG3 is the predominant IgG anti-PC isotype we studied the PC-specific IgM and IgG3 response to Pn14 to determine the mechanism of TLR2 action. In light of our previous reports using heat-inactivated Pn14 to study the humoral response to Pn as an intact bacterial immunogen, and not as an infection per se [27], we continued with this approach in the current studies. WT and TLR2−/− mice made an equivalent IgM anti-PC response following i.p. immunization with heat-killed Pn14 (Fig. 1). In contrast, TLR2−/− mice exhibited a significant reduction (up to 4-fold) in serum titers of PC-specific IgG3.
Fig 1. TLR2−/− mice elicit a defective IgG3, but normal IgM, anti-PC response to intact Pn14.
WT or TLR2−/− (both C57BL/6 background) mice [seven per group] were injected i.p. with Pn14. Serum titers of PC-specific IgM and IgG3 were determined by ELISA. *represents significance (p<0.05) by Student’s t-test. One of two independent experiments is shown.
TLR2−/− bone marrow dendritic cells (BMDC) elicit normal IgM and IgG3 anti-PC response to Pn14
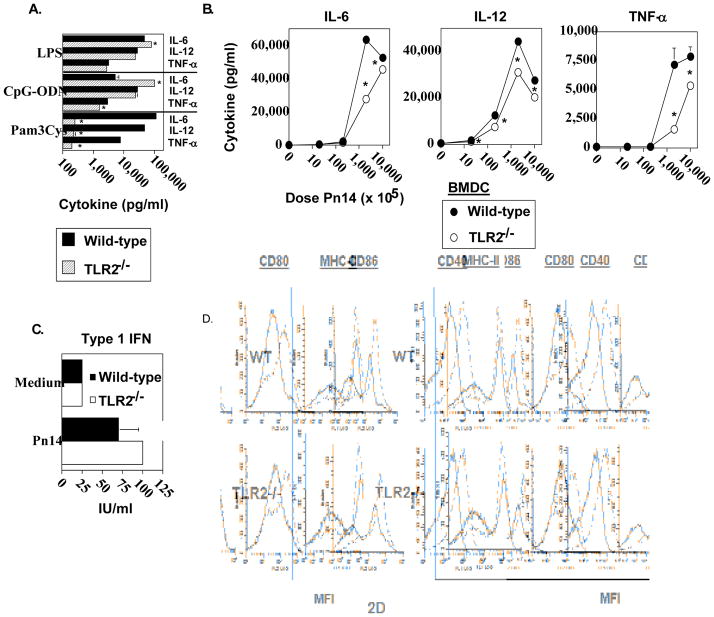
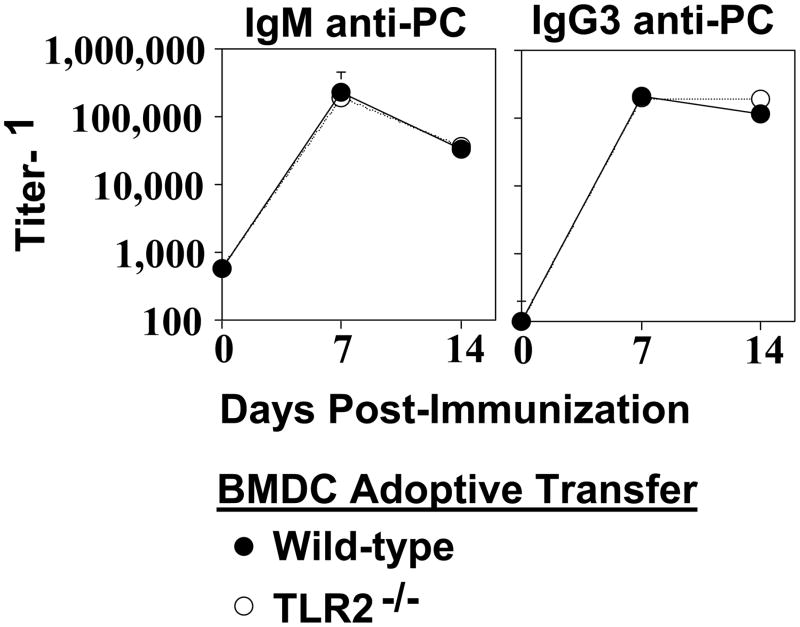
We previously demonstrated that BMDC pulsed with Pn14 in vitro secrete cytokines and undergo phenotypic maturation [28]. Upon adoptive transfer into naïve WT mice, in vitro Pn14-pulsed BMDC elicit both anti-polysaccharide (including anti-PC) and anti-protein responses. The absolute requirement for viable BMDC immediately prior to injection and for BMDC that are IL-6+/+ indicated that the transferred BMDC itself was playing an active role in this system, and not simply transferring antigen in a passive manner. We thus utilized this approach to determine the relative ability of in vitro Pn14-pulsed BMDC lacking TLR2 to elicit an IgM and IgG3 anti-PC response upon adoptive transfer into naïve WT recipients. As illustrated in Fig. 2A, Pam3Cys, a TLR2 ligand [29] failed to induce TLR2−/− BMDC to secrete IL-6, IL-12, and TNF-α, relative to TLR2−/− BMDC cultured in medium alone, whereas it induced all 3 cytokines in WT BMDC. In contrast, cytokine secretion by TLR2−/− BMDC in response to LPS (TLR4 ligand) [30] or CpG-ODN (TLR9 ligand) [31] was largely equivalent to WT BMDC. These data thus confirmed that BMDC obtained from mice genotyped as TLR2−/−, were indeed specifically deficient in TLR2 function. TLR2−/− BMDC cultured with varying doses of heat-killed Pn14 in vitro were found to have a significant, though partial, defect in IL-6, IL-12, and TNF-α secretion, more apparent at lower Pn14 doses (Fig. 2B). In contrast, there were no significant differences in type 1 IFN secretion between WT and TLR2−/− BMDC (Fig. 2C). Of interest, both IL-12 [32] and type 1 IFN [33] have been implicated in stimulating type 1 humoral immune responses. Moreover, phenotypic maturation of WT and TLR2−/− BMDC induced by Pn14 was found to be essentially equivalent (Fig. 2D). Thus, Pn14 induced, in WT and TLR2−/− BMDC, the surface expression of MHC-II, CD80, CD86, and CD40, molecules known to play an inductive role in APC-T cell interactions, to a similar degree. Pn14-pulsed WT and TLR2−/− BMDC were then adoptively transferred into naïve WT mice. No significant differences between WT and TLR2−/− BMDC in their capacity to induce an IgM or IgG3 anti-PC response were observed (Fig. 3). Collectively, these data suggest that the defective type 1 IgG response in TLR2−/− mice is not critically related to altered DC function in response to Pn14.
Fig 2. Analysis of in vitro cytokine secretion and phenotypic maturation of Pn14-stimulated TLR2−/− and wild-type bone marrow dendritic cells (BMDC).
BMDC from WT and TLR2−/− (both B6.129 background) mice were incubated with (A) LPS (2μg/mL), CpG (2μg/mL), Pam3CSK4 (150 ng/mL) or (B) varying doses of Pn14 for 24 h and the levels of IL-6, IL-12 and TNF-α were measured. The results are representative of two independent experiments. (C) BMDC from WT and TLR2−/− (both C57BL/6 background) mice were incubated with Pn14 for 24h and the levels of type I IFN were measured. The results are representative of two independent experiments. *represents significance (p<0.05) for A, B, and C, by Student’s t-test. (D) BMDC from WT and TLR2−/− (both B6.129 background) mice were stimulated with Pn14 for 24 h. Cell surface expression levels of MHC-II, CD80, CD86, and CD40 were measured by flow cytometry. Solid lines represent BMDC cultured in medium alone; dotted lines represent BMDC cultured with Pn14. One of two independent experiments is shown.
Fig 3. TLR2−/− bone marrow dendritic cells (BMDC) elicit normal IgM and IgG3 anti-PC response to Pn14.
Pn14-pulsed WT and TLR2−/− (both B6.129 background) BMDC were injected i.v. into WT (B6.129) mice (5 per group). Serum titers of PC-specific IgM and IgG3 were determined by ELISA. Significance (*p<0.05) was determined by Student’s t-test. One of two independent experiments is shown.
TLR2−/− B cells fail completely to respond to the co-mitogenic effects of Pn14, whereas TLR2−/− CD4+ T cells are partly TLR2-dependent
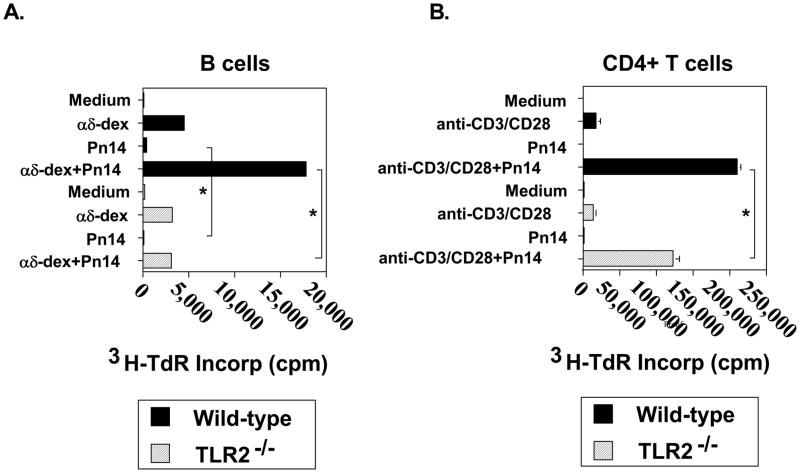
B cells activated with dextran-conjugated anti-IgD antibodies (αδ-dex), a polyclonal model for PS-specific multivalent membrane Ig crosslinking, synergizes with several distinct TLR ligands for proliferation [34]. We thus wished to determine whether Pn14 itself was directly co-mitogenic for αδ-dex-activated B cells. Purified splenic B cells from either WT or TLR2−/− mice were stimulated for 48 h with Pn14 and/or αδ-dex and then pulsed overnight with 3H-TdR for measurement of DNA synthesis. As illustrated in Fig. 4A, Pn14 alone had only a modest effect on inducing DNA synthesis by WT B cells that was nevertheless not observed for TLR2−/− B cells. In contrast, both WT and TLR2−/− B cells exhibited an equally strong proliferative response to αδ-dex. Importantly, whereas Pn14 synergized with αδ-dex for DNA synthesis by WT B cells, it had no effect on αδ-dex-activated TLR2−/− B cells. Thus, B cell expression of TLR2 is critical for the co-mitogenic effect of Pn14 on B cells activated via multivalent membrane Ig crosslinking in vitro.
Fig 4. TLR2−/−B cells fail completely to respond to the co-mitogenic effects of Pn14, whereas TLR2−/−CD4+ T cells are partly TLR2-dependent.
(A). B cells from WT and TLR2−/− (both B6.129 background) mice were cultured in the absence or presence of Pn14 and/or αδ-dex (1 ng/ml) for 48 h. (B) CD4+ T cells from WT and TLR2−/− (both C57BL/6 background) mice were cultured in the absence or presence of Pn14 and/or anti-CD3 mAb (0.3 μg/ml) + anti-CD28 (10 μg/ml) for 48 h. (A, B) 3H-TdR was added following stimulation for an additional 18h and cells were then harvested for 3H-TdR incorporation. *represents significance (p<0.05) by Student’s t-test. One of two independent experiments for both (A) and (B) is shown.
We also wished to determine whether Pn14 directly stimulated DNA synthesis in purified WT or TLR2−/− CD4+ T cells in the absence or presence of sub-mitogenic stimulation with anti-CD3 + anti-CD28 mAbs. As illustrated in Fig. 4B, Pn14 alone failed to stimulate DNA synthesis in either WT or TLR2−/− CD4+ T cells relative to cells cultured in medium alone. Both WT and TLR2−/− CD4+ T cells elicited comparable mitogenic responses following stimulation with anti-CD3 + anti-CD28 mAbs. The combination of anti-CD3 + anti-CD28 mAbs and Pn14 resulted in a synergistic increase in DNA synthesis in both WT and TLR2−/− CD4+ T cells, although the Pn14-mediated costimulation was significantly lower in TLR2−/− CD4+ T cells relative to WT. Additional studies using MyD88−/− CD4+ T cells gave similar results to that observed using cells from TLR2−/− mice (data not shown), indicating that Pn14 directly costimulates CD4+ T cell mitogenesis in both a MyD88-dependent and independent manner.
CD4+ T cell priming for IFN-γ secretion in response to Pn14 in vivo is similar in WT and TLR2−/− mice
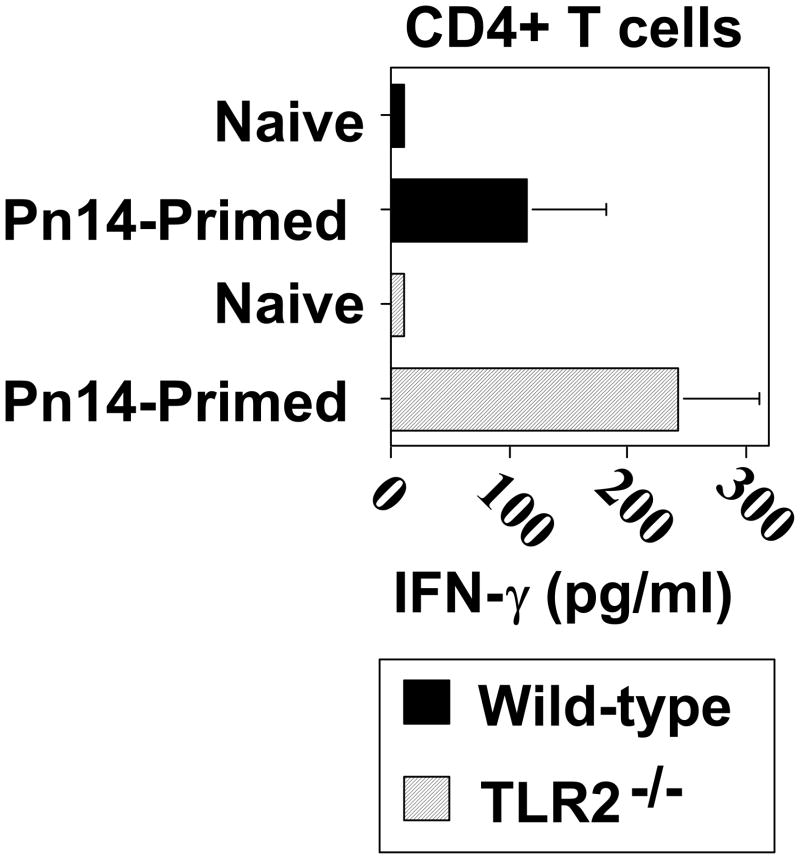
Since IFN-γ can stimulate switching to IgG2a [35] and IgG3 [36] and is a hallmark of a type 1 cytokine response, we next wished to determine whether Pn14-primed TLR2−/− mice exhibited diminished CD4+ T cell priming for IFN-γ. As illustrated in Fig. 5, spleen cells from Pn14-primed WT and TLR2−/− mice exhibited no significant differences in CD4+ T cell priming for IFN-γ secretion. Neither Pn14-primed WT nor TLR2−/− mice exhibit any detectable priming for IL-4, IL-5, or IL-13 (data not shown).
Fig 5. CD4+ T cell priming for IFN-γ secretion in response to Pn14 in vivo is similar in WT and TLR2−/− mice.
WT and TLR2−/− (both C57BL/6 background) mice were immunized i.p. with Pn14 and similarly boosted on day 14. On day 28 spleen cell suspensions from individual Pn14-immunized/boosted (Pn14-primed) and non-immunized (naïve) mice were cultured (triplicate wells) for 72 h with 30μg/ml of Pn14-derived protein extract (PnP). Cells cultured in medium alone showed IFN-γ concentrations below detection (<1 pg/ml, data not shown) The data show the geometric mean ± SEM of the individual SN for IFN-γ concentrations (IL-4, IL-5, and IL-13 were below detection in both groups [data not shown]). One of two independent experiments is shown.
B cell and CD4+ T cell expression of TLR2 is critical for stimulating an optimal TD IgG3, but not TI IgM, anti-PC response to Pn14
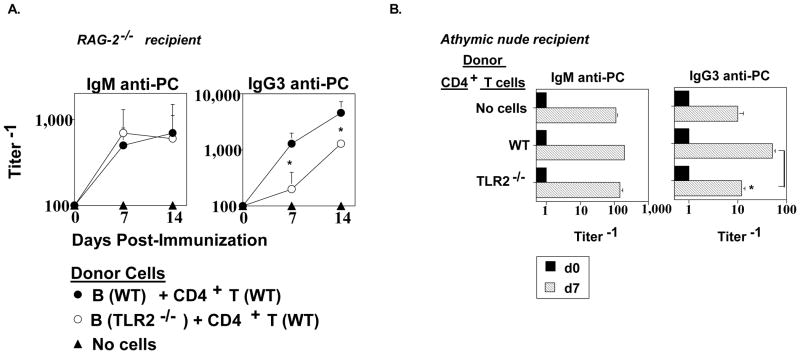
Our in vitro mitogenesis studies (Fig. 4) suggested a possible role for B cell and/or CD4+ T cells expression of TLR2 in the in vivo anti-PC response to Pn14. To determine this, we first purified splenic B cells from WT and TLR2−/− mice and co-injected them i.p. into RAG-2−/− mice with WT CD4+ T cells, followed 1 day later by i.p. immunization with Pn14. RAG-2−/− mice exhibit a critical defect in VDJ recombination, and thus lack B cells and T cells [37, 38]. As illustrated in Fig. 6A, no significant difference was observed in the ability of WT and TLR2−/− B cells to elicit an IgM anti-PC response. In contrast, WT B cells elicited a significantly higher IgG3 anti-PC response, on day 7 and 14, relative to TLR2−/− B cells in the RAG-2−/− recipients. This difference was similar to that observed in intact WT mice immunized with Pn14 (Fig. 1). Adoptive transfer of WT B cells into RAG-2−/− mice in the absence of WT CD4+ T cells resulted in 10-fold lower serum titers of IgG3 anti-PC relative to RAG-2−/− mice receiving both WT B and CD4+ T cells (data not shown), confirming the T cell-dependence of this response in this experimental system.
Fig 6. B cell expression of TLR2 has a significant role in stimulating the IgG3, but not IgM, anti-PC response to Pn14.
(A) RAG-2−/− (C57BL/6 background) mice (5 mice per group) were injected i.p. with WT (C57BL/6) CD4+ T cells + either WT or TLR2−/− (C57BL/6 background) B cells. (B) Athymic nude (C57BL/6 background) mice (5 mice per group) were injected i.p. with WT (C57BL/6) or TLR2−/− (C57BL/6 background) CD4+ T cells. After 24 h, mice in both (A) and (B) were injected i.p. with Pn14. Serum titers of PC-specific IgM and IgG3 were determined by ELISA at the indicated time points. *represents significance (p<0.05) by Student’s t-test One of two independent experiments is shown for both (A) and (B).
We next purified WT and TLR2−/− CD4+ T cells and injected them i.p. into separate groups of athymic nude mice which exhibit a profound T cell deficiency. The injection of either WT or TLR2−/− CD4+ T cells had no significant effect on the Pn14-mediated induction of the IgM anti-PC response observed in athymic nude mice to which no CD4+ T cells were transferred (Fig. 6B), confirming the T cell-independence of this response. In contrast, injection of WT CD4+ T cells resulted in a significant 5-fold enhancement in the IgG3 anti-PC response in Pn14-immunized athymic nude mice, relative to Pn14-immunized mice that received no cell transfer. In contrast, injection of TLR2−/− CD4+ T cells did not significantly enhance the Pn14-induced IgG3 anti-PC response relative to that observed in mice to which no CD4+ T cells were injected (Fig. 6B). The data obtained on day 14 was similar to that observed on day 7 (not shown). Collectively, these data indicate an important role for both B cell and CD4+ T cell expression of TLR2 in stimulating a primary humoral immune response to Pn14, in the setting of a largely intact innate immune system.
Discussion
In this report we investigated the mechanism underlying the defective TD type 1 IgG response to Pn14 in TLR2−/− mice. Although the initial innate immune response to a pathogen is known to play a major role in guiding CD4+ T cell subset differentiation [3], we were intrigued by our earlier finding that the in vitro and in vivo splenic cytokine and chemokine response of TLR2−/− mice, including IL-12 secretion, upon Pn14 challenge was largely equivalent to WT mice [15]. In addition, TLR2−/− mice exhibited no apparent increased susceptibility to i.p. challenge with live Pn14. In light of the relatively high and easily detectable IgM and IgG3 anti-PC responses to Pn14 in WT mice, and the selective decrease in IgG3 anti-PC serum titers in Pn14-immunized TLR2−/− mice, we studied this response in more detail to determine whether TLR2 acted directly at the level of the adaptive immune system. Whereas the IgM anti-PC response is T cell-independent, an optimal IgG3 anti-PC response requires CD4+ T cell help [19].
TLR2−/− BMDC underwent normal phenotypic maturation in response to Pn14 and secreted normal amounts of type 1 IFN, and substantial though somewhat reduced amounts of IL-12. Both these cytokines have been previously implicated in stimulating type 1 IgG responses in vivo by priming CD4+ T cells for IFN-γ production [32, 33]. IFN-γ, in turn can directly promote switching of IgM+ B cells to IgG3 [36]. In this regard, Pn14-pulsed TLR2−/− BMDC elicited a normal IgM and IgG3 anti-PC response when adoptively transferred into WT mice. Further, Pn14-immunized TLR2−/− mice exhibited a normal CD4+ T cell-dependent IFN-γ recall response upon in vitro re-stimulation with Pn14-derived protein. TLR2−/− BMDC secreted ~2-fold less IL-6 than WT BMDC in vitro, at the dose of Pn used for adoptive transfer studies, although both responses were relatively robust. Although we previously demonstrated a role of endogenous IL-6 in stimulating IgG, although not IgM, anti-PC responses to Pn [39], it is likely that both BMDC populations provided, and/or induced sufficient IL-6 for an optimal IgG3 anti-PC response upon adoptive transfer. The amount of BMDC-associated Pn14 delivered to the host in the BMDC transfer experiments (~3 × 107 CFU per mouse) [40] was lower than the amount of free Pn14 (2 × 108 CFU per mouse) used to immunize intact WT and TRL2−/− mice. We earlier demonstrated that Pn14-pulsed BMDC that are injected into naïve mice play an active role in Ig induction, and are not acting simply to passively release antigen to endogenous DC [28].
Pn14 was found to synergize with αδ-dex for DNA synthesis in purified WT B cells, and with sub-mitogenic doses of anti-CD3 + anti-CD28 mAbs in purified WT CD4+ T cells. In contrast, Pn14 had no co-mitogenic effects on TLR2−/− B cells, whereas it’s co-stimulatory effects were only partially, though significantly, reduced in TLR2−/− CD4+ T cells. These data are consistent with previous reports of TLR-mediated costimulation of DNA synthesis by BCR and TCR-activated B and CD4+ T cells, respectively [23, 41]. Of note, CD4+ Th1 cells, likely important for stimulating the IgG3 anti-PC response to Pn, directly respond to TLR2, but not other TLR, stimulation for enhanced IFN-γ release, proliferation and survival, in the absence of TCR signaling [42]. Most importantly, TLR2−/− B cells transferred into RAG-2−/− mice with WT CD4+ T cells, or TLR2−/− CD4+ T cells injected into athymic nude mice, elicited a defective IgG3, but normal IgM anti-PC response to Pn14, relative to WT cells. These data thus implicate B cell and CD4+ T cell expression of TLR2 as critical for mediating an optimal type 1 IgG primary response to intact Pn14.
Previous studies suggest that the induction of type 1 IgG responses might result from direct TLR-mediated B cell activation, in the absence of concomitant alterations in Th1 differentiation, as illustrated in this study. Thus, CpG-ODN, a TLR9 ligand [31], directly induce murine B cells to express germline CH transcripts specific for IgG3, IgG2b, and IgG2a, and switching to these isotypes [43]. Likewise, CpG-ODN stimulates human B cells to express CHγ1, CHγ2, and CHγ3 germline transcripts and switch recombination, associated with an increase in activation-induced cytidine deaminase (AID) [44], an enzyme critical for promoting switch recombination [45]. Membrane Ig crosslinking further elicited IgG production in CpG-ODN-activated human B cells. LPS, a TLR4 ligand, selectively induces class switching to IgG3 and IgG2b in murine B cells, although human papillomavirus-like particles, also signaling via TLR4, induce switching to IgG of all isotypes [46]. Stimulation of murine CD40-activated B cells via TLR7, utilizing R837, also selectively induces IgG2a/c secretion, which is further upregulated by type 1 IFN [47].
Murine B cells can be directly costimulated by a number of distinct TLR2 ligands, such as lipoprotein [48], Neisserial porins [49, 50], and macrophage-activating lipopeptide-2 (MALP-2) [51] to proliferate and differentiate into Ig-secreting cells. Lipoteichoic acid, which is expressed within the cell membrane of Pn, is known to bind and signal mammalian cells via TLR2 [52]. Pn also expresses lipoproteins such as pneumococcal surface adhesin A (PsaA) [53], which could potentially engage TLR2 as well. However, whether direct TLR2-mediated signaling in B cells selectively induces type 1 IgG isotype expression is unknown. We further speculate that the relative inability of TLR2−/− CD4+ T cells to deliver help for the IgG3 anti-PC response perhaps reflects deficient T cell-mediated stimulation of B cell proliferation. In this regard, a requirement for a minimal number of rounds of B cell proliferation for induction of the Ig class switch, depending on the isotype, has been demonstrated [54].
Our data are consistent with two recent reports utilizing model protein antigens [25] or virus-like particles [26]. Thus, B cell-deficient (μMT) mice were immunized with human serum albumin (HSA) conjugated to LPS to induce HSA-specific memory Th cells [25]. Purified B cells from WT, TLR4−/−, or MyD88−/− mice were then transferred into the primed μMT mice followed by immunization with HSA-LPS. The IgM and IgG1 anti-HSA response, in mice receiving TLR4−/− or MyD88−/− B cells was reduced, relative to mice receiving WT B cells demonstrating a B cell-intrinsic requirement for TLR signaling. Similarly, MyD88−/− B cells transferred into μMT mice elicited a defective TD IgM and IgG1 anti-flagellin response upon flagellin immunization, although a normal TI IgG3 anti-flagellin response, relative to WT B cells [25]. These data are analogous to ours in which B cell TLR2 expression was critical for the TD IgG3, but not TI IgM anti-PC response to Pn14. In another recent study, WT and TLR9−/− B cells were transferred into μMT mice and immunized with virus-like particles (VLP) loaded with CpG-ODN [26]. The IgG2a, although not IgG1, anti-VLP response in mice receiving TLR9−/− B cells was significantly reduced relative to mice in which WT B cells were transferred. Of note, CpG-ODN is a type 1 adjuvant [55], likely accounting for the selective change in IgG2a. Our data are the first to implicate TLR2, and to utilize an intact bacterial pathogen, to confirm and significantly extend the concept of a key role for B cell expression of TLR in humoral immune responses. These data, to our knowledge, are also the first to implicate a role for any TLR expressed by CD4+ T cells in mediating an in vivo primary Ig response.
Materials and methods
Mice
C57BL/6, B6129SF2/J, and RAG2−/− (C57BL/6 background) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Athymic nude mice (C57BL/6 background) were obtained from the National Cancer Institute (Frederick, MD). TLR2−/− mice [56] were provided by Dr. S. Akira (B6.129 background) and D. Golenbock [U. Mass. Med Ctr, Worcester, MA] (C57BL/6 background, backcrossed 9x). Both female and male mice were bred and maintained at U.S.U.H.S. in a pathogen-free environment and were used between 7–12 weeks of age. The experiments in this study were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals, Institute of Animal Resources, National Research Council, Department of Health, Education, and Welfare(National Institutes of Health) 78–23.
Reagents
Dextran-conjugated anti-IgD (αδ-dex) was prepared by conjugation of a “b” allotype-specific anti-IgD mAb (clone AF3) to dextran (2×106 MW) [57]. PC-keyhole limpet hemocyanin (KLH) was synthesized as described previously [19]. The resulting conjugate had a substitution degree of 19 PC/KLH. A protein extract from S. pneumoniae, capsular type 14 (Pn14) was prepared using B-PER (Bacterial Protein Extraction Reagent) [PIERCE, Rockford, IL]. The following mAbs were obtained from BD Pharmingen (San Diego, CA): APC-rat IgG2a, κ anti-mouse CD90.2 (clone 53–2.1), PE-rat IgG2a, κ anti-mouse CD4 (clone H129.19), FITC-rat IgG2a, κ anti-mouse CD45R/B220 (clone RA3-6B2), Syrian hamster IgG2, λ1 anti-mouse CD28 (clone 37.51), and Armenian hamster IgG1, κ anti-mouse CD3ε (clone 145-2C11).
Preparation and immunization of S. pneumoniae, capsular type 14 (Pn14)
Pn14 was prepared and stored as previously described [58]. Mice were immunized i.p. with 2 × 108 CFU of heat-killed bacteria in 250 μL of PBS. Serum samples for measurement of anti-PC Ab titers were prepared from blood obtained through the tail vein.
Purification of splenic B and CD4+ T cells
Single cell suspensions from spleen were prepared, and RBCs were lysed using ACK lysing buffer (Quality Biological, Inc., Gaithersburg, MD). B cells were positively selected by magnetic bead sorting using anti-mouse CD45R (B220) micro magnetic beads (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. Cell purities were checked by flow cytometry following each purification using a BD-LSR-II flow cytometer (BD Biosciences, San Jose, CA) and found to be 90–92% B220+ cells. CD4+ T cells (CD4+CD90+B220−) were purified by electronic cell sorting using a BD Biosciences FACSAria flow cytometer cell sorter. Purities of >99% CD4+ T cells were obtained.
Measurement of DNA synthesis by [3H]-TdR incorporation
Purified B220+ splenic B cells (2.5 × 105 cells/ml in 0.2 ml) or CD4+ T cells (1.0 × 106 cells/ml in 0.2. ml) were cultured in medium (RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 0.05 mM 2-ME, 50 μg/ml penicillin, and 50 μg/ml streptomycin), in the presence of various stimuli, in flat-bottom 96 well Costar plates (Corning Incorporated, Corning, NY). After 48 h in culture at 37°C in a 5% C02-containing incubator, [3H]-TdR (2 μCi; specific activity of 25Ci/mmol or 925GBq/mmol, Cat#TRK120) [Amersham Biosciences, Piscataway, NJ] was added to the cultures for an additional 18 h. Cultured cells were then harvested onto glass filter paper (Wallac, Turku, Finland, Cat # 1450-421) using a Harvester 96 (Tomtec, Hamden, CT). Specific incorporation of [3H]-TdR was determined using a 1450 Microbeta, “Wallac” Trilux scintillation counter.
Production and culture of bone marrow dendritic cells (BMDC)
BMDC were prepared as previously described [28]. Briefly, BM cells were cultured at 1.25 × 106 cells/ml (24-well plates) in cell culture medium supplemented with 10 ng/ml murine rGM-CSF (Sigma, St. Louis, MO). After 6–8 days of culture, non-adherent cells were harvested and were found to be 90% CD11c+ cells by flow cytometry.
Adoptive transfer of Pn14-pulsed BMDC
The method of Pn14-pulsed BMDC transfer into naïve mice has been described in detailed elsewhere [28]. Briefly, BMDC at 1 × 106/ml were incubated for 5 h in vitro with 8 × 108 CFU equivalents of heat-killed Pn14. Free bacteria was then removed from the BMDC cultures by washing ~6X in cold PBS. Pn14-pulsed BMDC were then resuspended in fresh medium at 1 × 106 BMDC/200μl, and 200 μl were injected i.v. into each mouse.
Adoptive transfer of B cells and/or CD4+ T cells into RAG-2−/− or athymic nude mice
RAG-2−/− mice were injected i.p. with a mixture of 2 × 107 purified B cells (derived from either wild-type or TLR2−/− mice) and 1 × 107 purified CD4+ T cells (derived from wild-type mice). Athymic nude mice were injected i.p. with 1 × 107 WT or TLR2−/− CD4+ T cells. Mice were then immunized 18 h later with 2 × 108 CFU equivalents of heat-killed Pn14 and sera were obtained 7 and/or 14 days later.
CD4+ T cell priming assay
This assay has been described previously [59]. Briefly, mice were immunized i.p. with 2 × 108 CFU heat-killed Pn14 in saline and similarly boosted on day 14. On day 28, spleen cell suspensions were prepared from individual mice and cultured for 72 h in the presence of 30 μg of Pn14-derived protein extract. SN was then obtained for measurement of secreted IFN-γ concentrations by ELISA. Cytokine secretion was eliminated when spleen cells were first depleted of CD4+ T cells using anti-CD4-coated magnetic beads.
Measurement of cytokine concentrations in culture SN by ELISA
The concentrations of specific cytokines released into the medium of cell cultures were measured using optimized standard sandwich ELISA. Recombinant cytokines used as standards, as well as the capture mAbs, biotinylated mAbs used for detection, and streptavidin-alkaline phosphatase (AP), were purchased from BD PharMingen (San Diego, CA). Streptavidin-AP was used in combination with p-nitrophenyl phosphate disodium (Sigma) as substrate to detect the specific binding. Standards were included in every plate, and the samples weretested in duplicate.
Measurement of type 1 interferon
Type 1 interferon concentrations in culture SN were measured as previously described [60]. Briefly, L cells were cultured with SN from Pn-activated BMDC cultures. L cell culture SN was then removed and cells were challenged with 100 μl encephalomyocarditis (EMC) virus at 10−3 dilution (multiplicity of infection of 0.1). Cultures were incubated overnight at 37°C until the viral cytopathic effect (CPE) reached 100% in medium-only control. The process was terminated with 20% formaldehyde and the monolayer was stained with 0.5% crystal violet to detect attached, viable cells. Plates were air dried and read in a plate reader equipped with a 540 nm filter. The reciprocal dilution of the well which exhibited a ~50% CPE was defined as the antiviral titer. All titers were compared to a reference type 1 IFN, and converted to international units/ml (IU/ml).
Flow cytometric analysis
All steps were performed on ice. Fcγ R receptors were initially blocked by addition of 10 μg/ml of rat IgG2b,κ anti-mouse Fcγ RI/II/III (clone 2.4G2). Cells were then stained 20 min later by incubation for an additional 30 min with PE-Armenian hamster IgG1,λ2 anti-mouse CD11c (clone HL3) plus either FITC-mouse IgG2a,κ anti-mouse MHC-IIb (clone AF6 120.1), FITC-rat IgG2a,κ anti-mouse CD40 (clone 3/23), PE-rat IgG2a,κ anti-mouse CD86 (clone GL1), or Armenian hamster IgG2a,κ anti-mouse (clone 16-10A1). All mAbs were purchased from BD Pharmingen. Irrelevant isotype- and species-matched mAbs were used as staining controls. Cells were analyzed on an EPICS XL-MCL (Beckman Coulter, Miami, FL). Dead cells and debris were eliminated from analysis by excluding cells positive for propidium iodide and gating on the appropriate forward and side scatter profile.
Measurement of serum IgM and IgG3 anti-PC titers
This assay has been previously described [58]. Briefly, Immulon 4 ELISA plates (Dynex Technologies, Inc., Chantilly, VA) were coated with PC-KLH and incubated with threefold dilutions of serum samples, starting at a 1/100 serum dilution. Alkaline phosphatase-conjugated polyclonal goat anti-mouse IgM or IgG3 Abs were then added, followed by addition of substrate (p-nitrophenyl phosphate, disodium; Sigma, St. Louis, MO). Color was read at an absorbance of 405 nm on a Multiskan Ascent ELISA reader (Labsystems, Finland).
Statistics
Data were expressed as geometric mean ± S.E.M. of the individual results. Student’s t-test was used to determine statistical significance between groups. P<0.05 was considered statistically significant.
Acknowledgments
Opinions and assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense or the Uniformed Services University of the Health Sciences.
We thank Dr. Andrew Lees (Lees BioConsulting, Gaithersburg, MD) for provision of αδ-dex and PC-KLH.
This study was supported by N.I.H. grants 1R01 AI49192 (CMS), U.S.U.H.S. grant RO74OQ-01 (CMS), and the U.S.U.H.S. Dean’s Research and Education Endowment Fund (CMS).
Abbreviations
- Pn14
Pn capsular type 14
- PC
phosphorylcholine
- PnP
Pn14-derived protein extract
- αδ-dex
dextran-conjugated anti-IgD antibodies
- TD
T cell-dependent
- TI
T cell-independent
- BMDC
bone marrow-derived dendritic cell
- SN
supernatant
References
- 1.Akira S. Mammalian Toll-like receptors. Curr Opin Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 2.Barton GM, Medzhitov R. Control of adaptive immune responses by Toll-like receptors. Curr Opin Immunol. 2002;14:380–383. doi: 10.1016/s0952-7915(02)00343-6. [DOI] [PubMed] [Google Scholar]
- 3.Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol. 2001;13:291–298. doi: 10.1016/s0952-7915(00)00218-1. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein JM, Campbell GD. Treatment of Pneumonia and Its Implications for Antimicrobial Resistance. Chest. 1999;115:1–2. [Google Scholar]
- 5.Musher DM, Alexandraki I, Graviss EA, Yanbeiy N, Eid A, Inderias L, Hoang P, Solomon E. Bacteremic and Nonbacteremic Pneumococcal Pneumonia: A Prospective Study. Medicine. 2000;79:210–221. doi: 10.1097/00005792-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Paterson GK, Mitchell TJ. Innate immunity and the pneumococcus. Microbiology. 2006;152:285–293. doi: 10.1099/mic.0.28551-0. [DOI] [PubMed] [Google Scholar]
- 7.AlonsoDeVelasco E, Verheul AF, Verhoef J, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol Rev. 1995;59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray BM, Dillon HC, Jr, Briles DE. Epidemiological studies of Streptococcus pneumoniae in infants: development of antibody to phosphocholine. J Clin Microbiol. 1983;18:1102–1107. doi: 10.1128/jcm.18.5.1102-1107.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briles DE, King JD, Gray MA, McDaniel LS, Swiatlo E, Benton KA. PspA, a protection-eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine. 1996;14:858–867. doi: 10.1016/0264-410x(96)82948-3. [DOI] [PubMed] [Google Scholar]
- 10.Briles DE, Forman C, Horowitz JC, Volanakis JE, Benjamin WHJ, McDaniel LS, Eldridge J, Brooks J. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect Immun. 1989;57:1457–1464. doi: 10.1128/iai.57.5.1457-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J Infect Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- 12.Koedel U, Angele B, Rupprecht T, Wagner H, Roggenkamp A, Pfister HW, Kirschning CJ. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J Immunol. 2003;170:438–444. doi: 10.4049/jimmunol.170.1.438. [DOI] [PubMed] [Google Scholar]
- 13.van Rossum AM, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005;73:7718–7726. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knapp S, Wieland CW, van‘t Veer C, Takeuchi O, Akira S, Florquin S, van der Poll T. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J Immunol. 2004;172:3132–3138. doi: 10.4049/jimmunol.172.5.3132. [DOI] [PubMed] [Google Scholar]
- 15.Khan AQ, Chen Q, Wu ZQ, Paton JC, Snapper CM. Both innate immunity and type 1 humoral immunity to Streptococcus pneumoniae are mediated by MyD88 but differ in their relative levels of dependence on toll-like receptor 2. Infect Immun. 2005;73:298–307. doi: 10.1128/IAI.73.1.298-307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albiger B, Sandgren A, Katsuragi H, Meyer-Hoffert U, Beiter K, Wartha F, Hornef M, Normark S, Normark BH. Myeloid differentiation factor 88-dependent signalling controls bacterial growth during colonization and systemic pneumococcal disease in mice. Cell Microbiol. 2005;7:1603–1615. doi: 10.1111/j.1462-5822.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 17.Koedel U, Rupprecht T, Angele B, Heesemann J, Wagner H, Pfister HW, Kirschning CJ. MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. Brain. 2004;127:1437–1445. doi: 10.1093/brain/awh171. [DOI] [PubMed] [Google Scholar]
- 18.Lee KS, Scanga CA, Bachelder EM, Chen Q, Snapper CM. TLR2 synergizes with both TLR4 and TLR9 for induction of the MyD88-dependent splenic cytokine and chemokine response to Streptococcus pneumoniae. Cell Immunol. 2007;245:103–110. doi: 10.1016/j.cellimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu ZQ, Vos Q, Shen Y, Lees A, Wilson SR, Briles DE, Gause WC, Mond JJ, Snapper CM. In vivo polysaccharide-specific IgG isotype responses to intact Streptococcus pneumoniae are T cell dependent and require CD40- and B7-ligand interactions. J Immunol. 1999;163:659–667. [PubMed] [Google Scholar]
- 20.Wu ZQ, Shen Y, Khan AQ, Chu CL, Riese R, Chapman HA, Kanagawa O, Snapper CM. The mechanism underlying T cell help for induction of an antigen-specific in vivo humoral immune response to intact Streptococcus pneumoniae is dependent on the type of antigen. J Immunol. 2002;168:5551–5557. doi: 10.4049/jimmunol.168.11.5551. [DOI] [PubMed] [Google Scholar]
- 21.Khan AQ, Lees A, Snapper CM. Differential regulation of IgG anti-capsular polysaccharide and antiprotein responses to intact Streptococcus pneumoniae in the presence of cognate CD4+ T cell help. J Immunol. 2004;172:532–539. doi: 10.4049/jimmunol.172.1.532. [DOI] [PubMed] [Google Scholar]
- 22.Fillatreau S, Manz RA. Tolls for B cells. Eur J Immunol. 2006;36:798–801. doi: 10.1002/eji.200636040. [DOI] [PubMed] [Google Scholar]
- 23.Kabelitz D. Expression and function of Toll-like receptors in T lymphocytes. Curr Opin Immunol. 2007;19:39–45. doi: 10.1016/j.coi.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 25.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 26.Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007;178:2415–2420. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- 27.Snapper CM. Differential regulation of protein- and polysaccharide-specific Ig isotype production in vivo in response to intact Streptococcus pneumoniae. Curr Protein Pept Sci. 2006;7:295–305. doi: 10.2174/138920306778017972. [DOI] [PubMed] [Google Scholar]
- 28.Colino J, Shen Y, Snapper CM. Dendritic cells pulsed with intact Streptococcus pneumoniae elicit both protein- and polysaccharide-specific immunoglobulin isotype responses in vivo through distinct mechanisms. J Exp Med. 2002;195:1–13. doi: 10.1084/jem.20011432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 31.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 32.Metzger DW, Buchanan JM, Collins JT, Lester TL, Murray KS, Cleave VHV, Vogel LA, Dunnick WA. Enhancement of humoral immunity by interleukin-12. Ann N Y Acad Sci. 1996;795:100–115. doi: 10.1111/j.1749-6632.1996.tb52659.x. [DOI] [PubMed] [Google Scholar]
- 33.Finkelman FD, Svetic A, Gresser I, Snapper C, Holmes J, Trotta PP, Katona I, Gause WC. Regulation by interferon α of immunoglobulin isotype selection and lymphokine production in mice. J Exp Med. 1991;174:1179–1188. doi: 10.1084/jem.174.5.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snapper CM, Yamaguchi H, Moorman MA, Mond JJ. An in vitro model for T cell-independent induction of humoral immunity. A requirement for NK cells. J Immunol. 1994;152:4884–4892. [PubMed] [Google Scholar]
- 35.Snapper CM, Paul WE. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 36.Snapper CM, McIntyre TM, Mandler R, Pecanha LMT, Finkelman FD, Lees A, Mond JJ. Induction of IgG3 secretion by interferon g: A model for T cell-independent class switching in response to T cell-independent type 2 antigens. J Exp Med. 1992;175:1367–1371. doi: 10.1084/jem.175.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1 deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 38.Shinkai Y, Rathbun G, Lam K-P, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 39.Khan AQ, Shen Y, Wu ZQ, Wynn TA, Snapper CM. Endogenous pro- and anti-inflammatory cytokines differentially regulate an in vivo humoral response to Streptococcus pneumoniae. Infect Immun. 2002;70:749–761. doi: 10.1128/iai.70.2.749-761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasilevsky S, Colino J, Puliaev R, Canaday DH, Snapper CM. Macrophages pulsed with Streptococcus pneumoniae elicit a T cell-dependent antibody response upon transfer into naive mice. J Immunol. 2008;181:1787–1797. doi: 10.4049/jimmunol.181.3.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snapper CM, Mond JJ. A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J Immunol. 1996;157:2229–2233. [PubMed] [Google Scholar]
- 42.Imanishi T, Hara H, Suzuki S, Suzuki N, Akira S, Saito T. Cutting Edge: TLR2 Directly Triggers Th1 Effector Functions. J Immunol. 2007;178:6715–6719. doi: 10.4049/jimmunol.178.11.6715. [DOI] [PubMed] [Google Scholar]
- 43.Lin L, Gerth AJ, Peng SL. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur J Immunol. 2004;34:1483–1487. doi: 10.1002/eji.200324736. [DOI] [PubMed] [Google Scholar]
- 44.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173:4479–4491. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 45.Kenter AL. Class-switch recombination: after the dawn of AID. Curr Opin Immunol. 2003;15:190–198. doi: 10.1016/s0952-7915(03)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang R, Murillo FM, Delannoy MJ, Blosser RL, Yutzy WHt, Uematsu S, Takeda K, Akira S, Viscidi RP, Roden RB. B Lymphocyte Activation by Human Papillomavirus-Like Particles Directly Induces Ig Class Switch Recombination via TLR4-MyD88. J Immunol. 2005;174:7912–7919. doi: 10.4049/jimmunol.174.12.7912. [DOI] [PubMed] [Google Scholar]
- 47.Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, Marsland BJ. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 48.Snapper CM, Rosas FR, Jin L, Wortham C, Kehry MR, Mond JJ. Bacterial lipoproteins may substitute for cytokines in the Humoral immune response to T cell-independent type II antigens. J Immunol. 1995;155:5582–5589. [PubMed] [Google Scholar]
- 49.Massari P, Henneke P, Ho Y, Latz E, Golenbock DT, Wetzler LM. Cutting edge: Immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J Immunol. 2002;168:1533–1537. doi: 10.4049/jimmunol.168.4.1533. [DOI] [PubMed] [Google Scholar]
- 50.Snapper CM, Rosas FR, Kehry MR, Mond JJ, Wetzler LM. Neisserial porins may provide critical second signals to polysaccharide-activated murine B cells for induction of immunoglobulin secretion. Infect Immun. 1997;65:3203–3208. doi: 10.1128/iai.65.8.3203-3208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borsutzky S, Kretschmer K, Becker PD, Muhlradt PF, Kirschning CJ, Weiss S, Guzman CA. The mucosal adjuvant macrophage-activating lipopeptide-2 directly stimulates B lymphocytes via the TLR2 without the need of accessory cells. J Immunol. 2005;174:6308–6313. doi: 10.4049/jimmunol.174.10.6308. [DOI] [PubMed] [Google Scholar]
- 52.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 53.De BK, Sampson JS, Ades EW, Huebner RC, Jue DL, Johnson SE, Espina M, Stinson AR, Briles DE, Carlone GM. Purification and characterization of Streptococcus pneumoniae palmitoylated pneumococcal surface adhesin A expressed in Escherichia coli. Vaccine. 2000;18:1811–1821. doi: 10.1016/s0264-410x(99)00481-8. [DOI] [PubMed] [Google Scholar]
- 54.Deenick E, Hasbold J, Hodgkin P. Switching to IgG3, IgG2b, and IgA is division linked and independent, revealing a stochastic framework for describing differentiation. J Immunol. 1999;163:4707–4714. [PubMed] [Google Scholar]
- 55.Sano K, Shirota H, Terui T, Hattori T, Tamura G. Oligodeoxynucleotides without CpG motifs work as adjuvant for the induction of th2 differentiation in a sequence-independent manner. J Immunol. 2003;170:2367–2373. doi: 10.4049/jimmunol.170.5.2367. [DOI] [PubMed] [Google Scholar]
- 56.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 57.Brunswick M, Finkelman FD, Highet PF, Inman JK, Dintzis HM, Mond JJ. Picogram quantities of anti-Ig antibodies coupled to dextran induce B cell proliferation. J Immunol. 1988;140:3364–3372. [PubMed] [Google Scholar]
- 58.Chattopadhyay G, Khan AQ, Sen G, Colino J, Dubois W, Rubtsov A, Torres RM, Potter M, Snapper CM. Transgenic Expression of Bcl-xL or Bcl-2 by Murine B Cells Enhances the In Vivo Antipolysaccharide, but Not Antiprotein, Response to Intact Streptococcus pneumoniae. J Immunol. 2007;179:7523–7534. doi: 10.4049/jimmunol.179.11.7523. [DOI] [PubMed] [Google Scholar]
- 59.Chen Q, Sen G, Snapper CM. Endogenous IL-1R1 signaling is critical for cognate CD4+ T cell help for induction of in vivo type 1 and type 2 antipolysaccharide and antiprotein Ig isotype responses to intact Streptococcus pneumoniae, but not to a soluble pneumococcal conjugate vaccine. J Immunol. 2006;177:6044–6051. doi: 10.4049/jimmunol.177.9.6044. [DOI] [PubMed] [Google Scholar]
- 60.Yeh TJ, McBride PT, Overall JC, Jr, Green JA. Automated, quantitative cytopathic effect reduction assay for interferon. J Clin Microbiol. 1982;16:413–415. doi: 10.1128/jcm.16.2.413-415.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]