Summary
Monoclonal gammopathy of undetermined significance (MGUS) is a common premalignant plasma cell proliferative disorder with a lifelong risk of progression to multiple myeloma. Since myeloma is an incurable malignancy, strategies to delay or prevent progression in high-risk patients are of considerable importance.
In this issue of Clinical Cancer Research, Golombick et al (1), report on preventive therapy for monoclonal gammopathy of undetermined significance (MGUS) using curcumin, the most active component of the commonly used Indian spice, turmeric. This small pilot trial uses non-standard response criteria and does not affect clinical practice, but is an important study that raises several major issues pertinent not just to the field of plasma cell disorders, but also to the overall concepts of premalignancy and chemoprevention. To fully appreciate the implications of this research it is important to understand the nature of MGUS, and the unique biologic and clinical dilemmas posed by this entity.
MGUS is a classic premalignant condition, present in over 3% of the general population over the age of 50.(2) The main clinical significance of MGUS is its lifelong risk of transformation to myeloma or related malignancy, at a fixed but unrelenting rate of 1% per year.(3) Since myeloma is a devastating, incurable malignancy, understanding why MGUS occurs, and what causes its progression is of considerable importance. Recent studies show that myeloma is almost always preceded by MGUS,(4, 5), lending additional impetus for testing preventive strategies such as the one undertaken by Golombick et al for patients with MGUS who are at high risk of progression.
The study by Golombick is among the first preventive studies in clinically defined MGUS, although others have used preventive strategies such as anakinra and thalidomide in a more advanced form of biologic MGUS, clinically referred to as smoldering multiple myeloma (SMM). The clinical distinction between MGUS and SMM is important for prognosis and for testing of preventive strategies, but SMM is likely not a discrete biologic entity. Most patients clinically recognized as SMM have biologic MGUS (premalignancy) while some likely have early stages of myeloma.
Etiologic Factors for MGUS and Prospects for Primary Prevention
Recent observations offer important clues, indicating that genetic predisposition and potentially preventable environmental factors may play a key role in the development of MGUS. African Americans, and blacks from Africa, have a 2-3 fold higher risk of MGUS compared with whites.(6, 7) In contrast, the risk is lower in Asians from Japan,(8)and in Mexicans. Age, hormonal factors, family history, immunosuppression, and exposure to certain pesticides are known risk factors. Understanding these factors, and the pathogenetic steps discussed below may allow for the development of primary prevention strategies in the future.
MGUS is likely the culmination of a cascade of events initiated by an abnormal response to antigenic stimulation mediated by factors such as aberrant expression of Toll-like receptors (TLRs) and overexpression of IL-6 receptors.(9) Any attempt at primary prevention may need to abort the abnormal response to antigenic stimulation, or perhaps the specific stimulus itself. The cascade results in the development of one of two types of primary cytogenetic abnormalities that are likely critical in establishing MGUS: hyperdiploidy or immunoglobulin heavy chain (IgH) translocations.
Implications of Discrete Cytogenetically Defined Sub-Types of MGUS
Approximately 50% of MGUS is associated with hyperdiploidy (Hyperdiploid or IgH non-translocated MGUS) (Figure 1), while the remaining 50% is associated with translocations involving the IgH locus on chromosome 14q32 (IgH translocated or non-hyperdiploid MGUS). In a small proportion of cases, neither hyperdiploidy nor IgH translocations are found. In non-hyperdiploid MGUS, IgH translocations commonly involve one of 5 recurrent partner chromosome loci: 11q13 (CCND1 [cyclin D1 gene]), 4p16.3 (FGFR-3 and MMSET), 6p21 (CCND3 [cyclin D3 gene]), 16q23 (c-maf), and 20q11 (mafB).(10) Although clinically MGUS is considered a single entity, it is likely cytogenetically at least 6 different entities comprising of hyperdiploid MGUS, and the 5 most common primary IgH translocations discussed above. It is more than likely that the age and racial disparities, pathogenesis and clinical course of these types of MGUS differ from each other. Future studies will need to examine each cytogenetic type separately.
Figure 1. Pathogenesis of MGUS and its Progression to Myeloma.
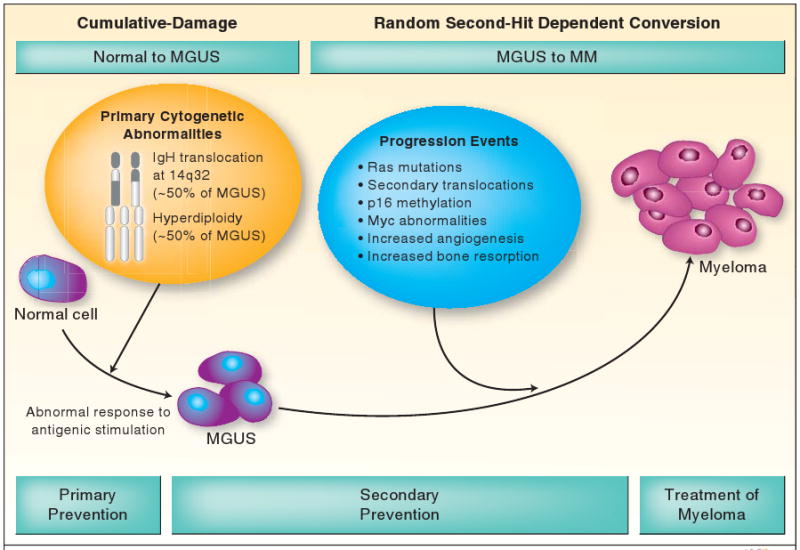
The initiation of limited clonal plasma cell proliferation in MGUS is likely triggered by an abnormal response to antigenic stimulation in a genetically or environmentally susceptible host, and development of typical cytogenetic abnormalities, hyperdiploidy or IgH translocations. Progression of MGUS to myeloma is accompanied by a variety of changes in the clonal cell and its microenvironment.
MGUS, monoclonal gammopathy of undetermined significance; IgH, immunoglobulin heavy chain.
Progression of MGUS to Myeloma and Secondary Prevention
While the initiation of MGUS appears to follow a cumulative damage model, the progression of MGUS to myeloma suggests a simple, random, two-hit genetic model of malignancy. (Figure 1) Unfortunately, the precise mechanisms of progression are unknown, although several potentially pathogenetic abnormalities have been described. These include Ras and p53 mutations, p16 methylation, myc abnormalities, and secondary translocations. Changes in the bone marrow microenvironment include induction of angiogenesis, and abnormal paracrine loops involving cytokines such as interleukin 6 (IL-6), which serves as a major growth factor for plasma cells. The main regulator of IL-6 signaling in myeloma is signal transducer and activator of transcription-3 (STAT3), and curcumin down regulates IL-6 induced STAT3 phosphorylation. In addition, curcumin also down regulates nuclear factor-kappaB (NF-κB) which is constitutively activated in myeloma cells, and felt to play a role in disease pathogenesis and drug resistance.(11)
The pathogenesis of lytic bone lesions associated with progression of MGUS is unclear. There is an increase in RANKL (receptor activator of nuclear factor κB ligand) expression by osteoblasts (and possibly plasma cells) accompanied by a reduction in the level of its decoy receptor, osteoprotegerin (OPG).(12) The resultant increase in RANKL/OPG ratio causes osteoclast activation and increased bone resorption and turnover. Curcumin inhibits RANKL signaling, and in this trial Golombick et al (1) were able to show a reduction in bone turnover in a subset of patients. Osteoclast activation in myeloma is also mediated by increased levels of macrophage inflammatory protein–1α (MIP-1α), IL-3 and IL-6, while increased levels of IL-3, IL-7 and dickkopf 1 (DKK1) inhibit osteoblast differentiation.
Clinical Implications
Any preventive strategy for MGUS, including trials with relatively non toxic agents such as curcumin need to consider the absolute risk of progression to malignancy. The true life-time probability of progression of MGUS is substantially lower than 1% per year when competing causes of death are taken into account, approximately 11% at 25 years.(13) Phase III studies with curcumin and other preventive strategies should be focused on patients who are at the highest risk of progression.
The main risk factors for progression of clinical MGUS are size and type of the serum M protein, and presence of an abnormal serum free light chain (FLC) ratio.(13) An abnormal FLC ratio (especially a more extreme value) most likely derives its prognostic value by being a surrogate marker of certain cytogenetic categories, eg., t(4;14) MGUS. Patients with an abnormal serum FLC ratio, non-IgG MGUS, and a high serum M protein level (≥15 gm/L) have a risk of progression at 20 years of 58% (high-risk MGUS) compared to 5% when none of the risk factors are present (low-risk MGUS). Patients with high-risk MGUS, and patients with biologic MGUS clinically identified as SMM are candidates for preventive strategies including phase III trials with curcumin.
Future Chemoprevention trials in MGUS and SMM
With the increasing availability of novel targeted therapies for myeloma, phase III clinical trials are ongoing to determine if the early use of drugs such as lenalidomide or bisphosphonates can delay progression in MGUS and SMM. Curcumin joins the list of other potential agents that needs further study. As discussed above, subsequent trials need to focus on those at the highest risk of progression. Trials should also stratify by cytogenetic subtype of MGUS and SMM. Therapeutic clinical trials in asymptomatic individuals are always a challenge, but in the right target population, they have the potential for substantial benefit.
Acknowledgments
Supported in part by Grants CA62242 and CA107476 from the National Cancer Institute, Bethesda, MD.
References
- 1.Golombick T, Diamond T, Badmaev V, Manoharan A, Ramakrishna R. The potential role of curcumin in patients with MGUS- its effect on paraproteinemia and the uNTx bone turnover marker. Clin Cancer Res. 2009;15 doi: 10.1158/1078-0432.CCR-08-2217. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of Monoclonal Gammopathy of Undetermined Significance. N Engl J Med. 2006;354:1362–9. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis of monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–69. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 4.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–7. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113:5418–22. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landgren O, Gridley G, Turesson I, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107:904–6. doi: 10.1182/blood-2005-08-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landgren O, Katzmann JA, Hsing AW, et al. Prevalence of Monoclonal Gammopathy of Undetermined Significance Among Men in Ghana. Mayo Clin Proc. 2007;82:1468–73. doi: 10.1016/S0025-6196(11)61089-6. [DOI] [PubMed] [Google Scholar]
- 8.Iwanaga M, Tagawa M, Tsukasaki K, Kamihira S, Tomonaga M. Prevalence of Monoclonal Gammopathy of Undetermined Significance: Study of 52,802 Persons in Nagasaki City, Japan. Mayo Clin Proc. 2007;82:1474–9. doi: 10.1016/S0025-6196(11)61090-2. [DOI] [PubMed] [Google Scholar]
- 9.Jego G, Bataille R, Geffroy-Luseau A, Descamps G, Pellat-Deceunynck C. Pathogen-associated molecular patterns are growth and survival factors for human myeloma cells through Toll-like receptors. Leukemia. 2006;20:1130–7. doi: 10.1038/sj.leu.2404226. [DOI] [PubMed] [Google Scholar]
- 10.Bergsagel PL, Kuehl WM. Chromosome translocations in multiple myeloma. Oncogene. 2001;20:5611–22. doi: 10.1038/sj.onc.1204641. [DOI] [PubMed] [Google Scholar]
- 11.Bharti AC, Shishodia S, Reuben JM, et al. Nuclear factor-{kappa}B and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood. 2004;103:3175–84. doi: 10.1182/blood-2003-06-2151. [DOI] [PubMed] [Google Scholar]
- 12.Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23:435–41. doi: 10.1038/leu.2008.336. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance (MGUS) Blood. 2005;106:812–7. doi: 10.1182/blood-2005-03-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]


