Abstract
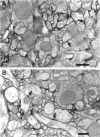
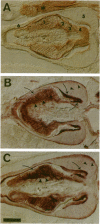
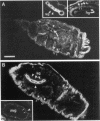
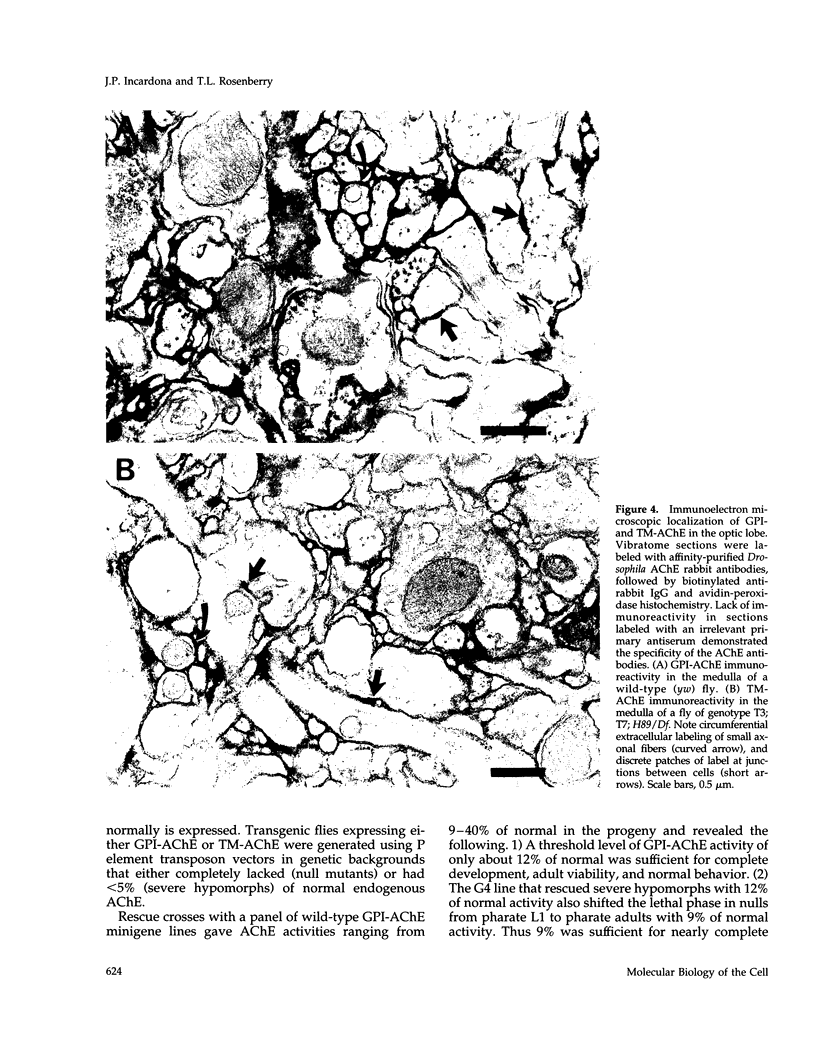
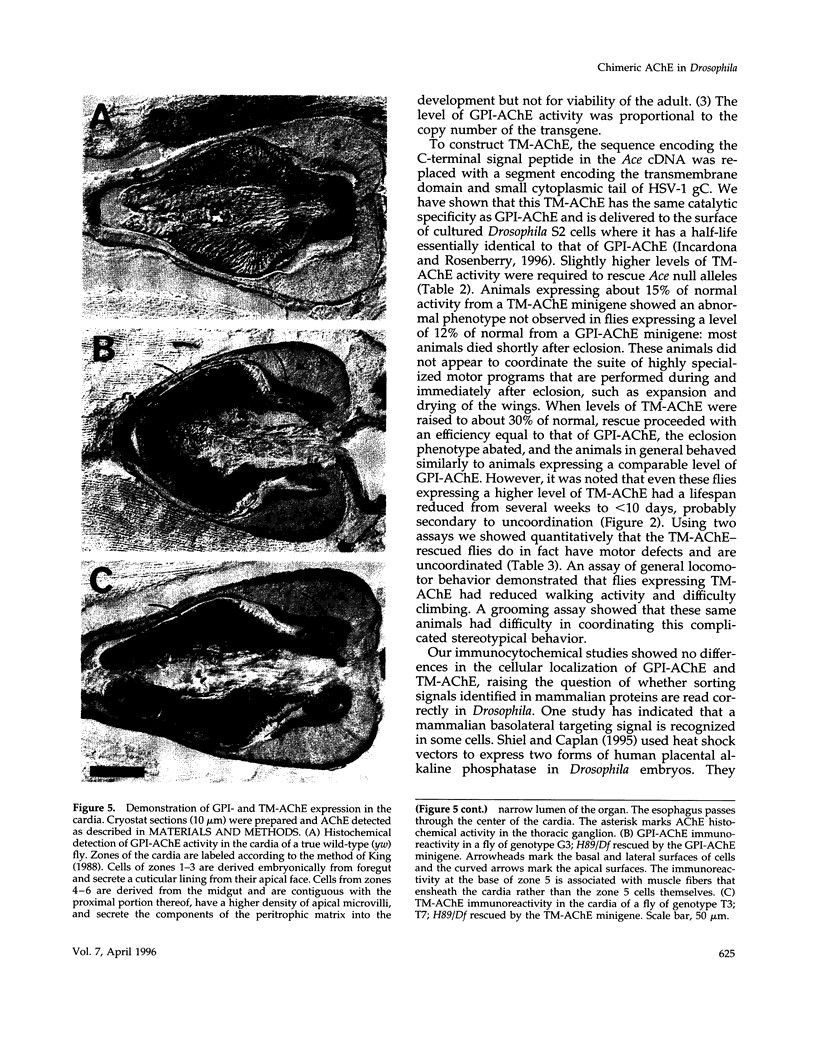
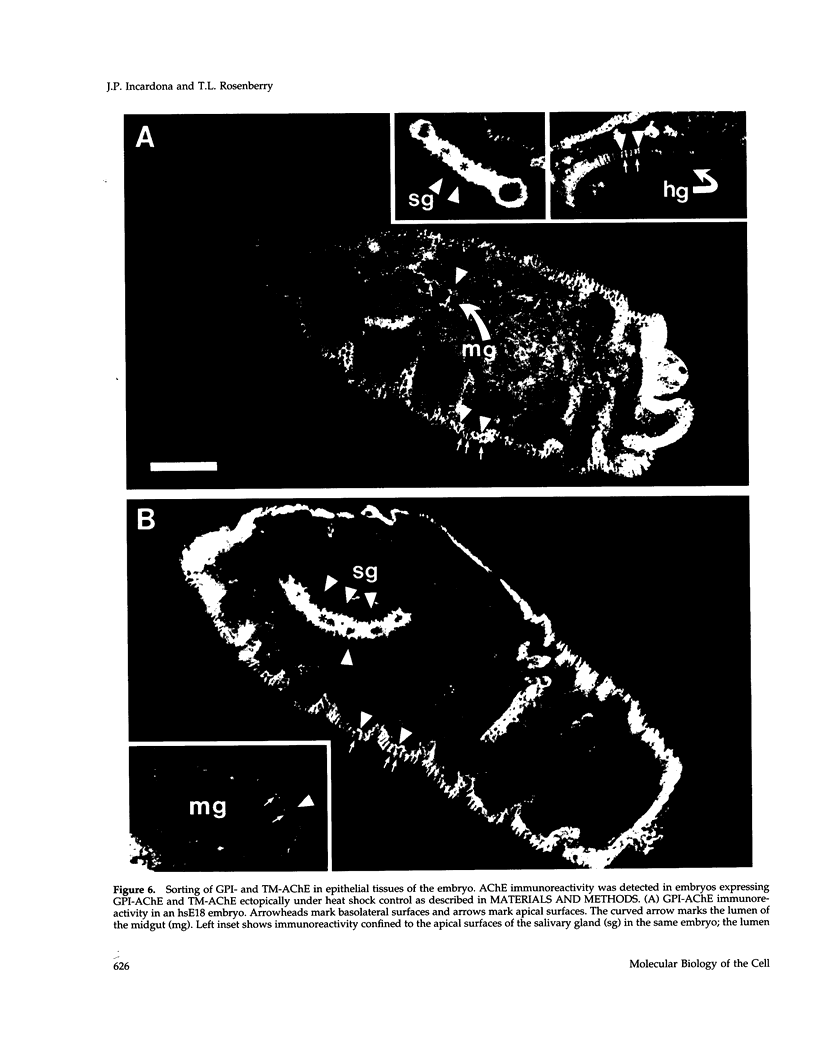
Drosophila has a single glycoinositol phospholipid (GPI)-anchored form of acetylcholinesterase (AChE) encoded by the Ace locus. To assess the role that GPI plays in the physiology, of AChE, we have replaced the wild-type GPI-AChE with a chimeric transmembrane form (TM-AChE) in the nervous system of the fly. Ace null alleles provided a genetic background completely lacking in endogenous GPI-AChE, and Ace minigene P transposon constructs were used to express both GPI- and TM-AChE forms in the tissues where AChE is normally expressed. Control experiments with the GPI-AChE minigene demonstrated a threshold between 9 and 12% of normal AChE activity for adult viability. Ace mutant flies were rescued by GPI-AChE minigene lines that expressed 12-40% of normal activity and were essentially unchanged from wild-type flies in behavior. TM-AChE minigene lines were able to rescue Ace null alleles, although with a slightly higher threshold than that for GPI-AChE. Although rescued flies expressing GPI-AChE at a level of 12% of normal activity were viable, flies expressing 13-16% of normal activity from the TM-AChE transgene died shortly after eclosion. Flies expressing TM-AChE at about 30% of normal levels were essentially unchanged from wild-type flies in gross behavior but had a reduced lifespan secondary to subtle coordination defects. These flies also showed reduced locomotor activity and performed poorly in a grooming assay. However, light level and electron microscopic immunocytochemistry showed no differences in the localization of GPI- and TM-AChE. Furthermore, endogenous and ectopic-induced expression of both AChEs in epithelial tissues of the adult and embryo, respectively, showed that they were sorted identically. Most epithelial cells sorted GPI- and TM-AChE to the apical surface, but cuticle-secreting epithelia sorted both proteins basolaterally. Our data suggest that rather than having a primary role in protein sorting, the GPI anchor or AChE plays some other more subtle cellular role in neuronal physiology.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth A., Müller-Taubenberger A., Taranto P., Gerisch G. Replacement of the phospholipid-anchor in the contact site A glycoprotein of D. discoideum by a transmembrane region does not impede cell adhesion but reduces residence time on the cell surface. J Cell Biol. 1994 Jan;124(1-2):205–215. doi: 10.1083/jcb.124.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Crise B., Rose J. K. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989 Sep 29;245(4925):1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brown D. The tyrosine kinase connection: how GPI-anchored proteins activate T cells. Curr Opin Immunol. 1993 Jun;5(3):349–354. doi: 10.1016/0952-7915(93)90052-t. [DOI] [PubMed] [Google Scholar]
- Buchanan R. L., Benzer S. Defective glia in the Drosophila brain degeneration mutant drop-dead. Neuron. 1993 May;10(5):839–850. doi: 10.1016/0896-6273(93)90200-b. [DOI] [PubMed] [Google Scholar]
- Bunch T. A., Brower D. L. Drosophila cell adhesion molecules. Curr Top Dev Biol. 1993;28:81–123. doi: 10.1016/s0070-2153(08)60210-0. [DOI] [PubMed] [Google Scholar]
- Cerneus D. P., Ueffing E., Posthuma G., Strous G. J., van der Ende A. Detergent insolubility of alkaline phosphatase during biosynthetic transport and endocytosis. Role of cholesterol. J Biol Chem. 1993 Feb 15;268(5):3150–3155. [PubMed] [Google Scholar]
- Chang W. S., Zachow K. R., Bentley D. Expression of epithelial alkaline phosphatase in segmentally iterated bands during grasshopper limb morphogenesis. Development. 1993 Jun;118(2):651–663. doi: 10.1242/dev.118.2.651. [DOI] [PubMed] [Google Scholar]
- Chase B. A., Kankel D. R. On the role of normal acetylcholine metabolism in the formation and maintenance of the Drosophila nervous system. Dev Biol. 1988 Feb;125(2):361–380. doi: 10.1016/0012-1606(88)90218-7. [DOI] [PubMed] [Google Scholar]
- Connolly K. Locomotor activity in drosophila. II. Selection for active and inactive strains. Anim Behav. 1966 Oct;14(4):444–449. doi: 10.1016/s0003-3472(66)80043-x. [DOI] [PubMed] [Google Scholar]
- DiAntonio A., Parfitt K. D., Schwarz T. L. Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell. 1993 Jul 2;73(7):1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- Dotti C. G., Parton R. G., Simons K. Polarized sorting of glypiated proteins in hippocampal neurons. Nature. 1991 Jan 10;349(6305):158–161. doi: 10.1038/349158a0. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Elkins T., Zinn K., McAllister L., Hoffmann F. M., Goodman C. S. Genetic analysis of a Drosophila neural cell adhesion molecule: interaction of fasciclin I and Abelson tyrosine kinase mutations. Cell. 1990 Feb 23;60(4):565–575. doi: 10.1016/0092-8674(90)90660-7. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C., Gennarini G., Goridis C., Rougon G. F3/F11 cell surface molecule expression in the developing mouse cerebellum is polarized at synaptic sites and within granule cells. J Neurosci. 1992 Jan;12(1):257–267. doi: 10.1523/JNEUROSCI.12-01-00257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier D., Bergé J. B., Cardoso de Almeida M. L., Bordier C. Acetylcholinesterases from Musca domestica and Drosophila melanogaster brain are linked to membranes by a glycophospholipid anchor sensitive to an endogenous phospholipase. J Neurochem. 1988 Apr;50(4):1158–1163. doi: 10.1111/j.1471-4159.1988.tb10587.x. [DOI] [PubMed] [Google Scholar]
- Fournier D., Karch F., Bride J. M., Hall L. M., Bergé J. B., Spierer P. Drosophila melanogaster acetylcholinesterase gene. Structure, evolution and mutations. J Mol Biol. 1989 Nov 5;210(1):15–22. doi: 10.1016/0022-2836(89)90287-8. [DOI] [PubMed] [Google Scholar]
- Ganetzky B., Wu C. F. Indirect Suppression Involving Behavioral Mutants with Altered Nerve Excitability in DROSOPHILA MELANOGASTER. Genetics. 1982 Apr;100(4):597–614. doi: 10.1093/genetics/100.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnagey A. L., Forte M., Rosenberry T. L. Isolation and characterization of acetylcholinesterase from Drosophila. J Biol Chem. 1987 Sep 25;262(27):13290–13298. [PubMed] [Google Scholar]
- Greenspan R. J., Finn J. A., Jr, Hall J. C. Acetylcholinesterase mutants in Drosophila and their effects on the structure and function of the central nervous system. J Comp Neurol. 1980 Feb 15;189(4):741–774. doi: 10.1002/cne.901890409. [DOI] [PubMed] [Google Scholar]
- Haas R., Marshall T. L., Rosenberry T. L. Drosophila acetylcholinesterase: demonstration of a glycoinositol phospholipid anchor and an endogenous proteolytic cleavage. Biochemistry. 1988 Aug 23;27(17):6453–6457. doi: 10.1021/bi00417a038. [DOI] [PubMed] [Google Scholar]
- Hall J. C., Alahiotis S. N., Strumpf D. A., White K. Behavioral and biochemical defects in temperature-sensitive acetylcholinesterase mutants of Drosophila melanogaster. Genetics. 1980 Dec;96(4):939–965. doi: 10.1093/genetics/96.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. C. Genetics of the nervous system in Drosophila. Q Rev Biophys. 1982 May;15(2):223–479. doi: 10.1017/s0033583500004844. [DOI] [PubMed] [Google Scholar]
- Hall J. C., Kankel D. R. Genetics of acetylcholinesterase in Drosophila melanogaster. Genetics. 1976 Jul;83(3 PT2):517–535. [PMC free article] [PubMed] [Google Scholar]
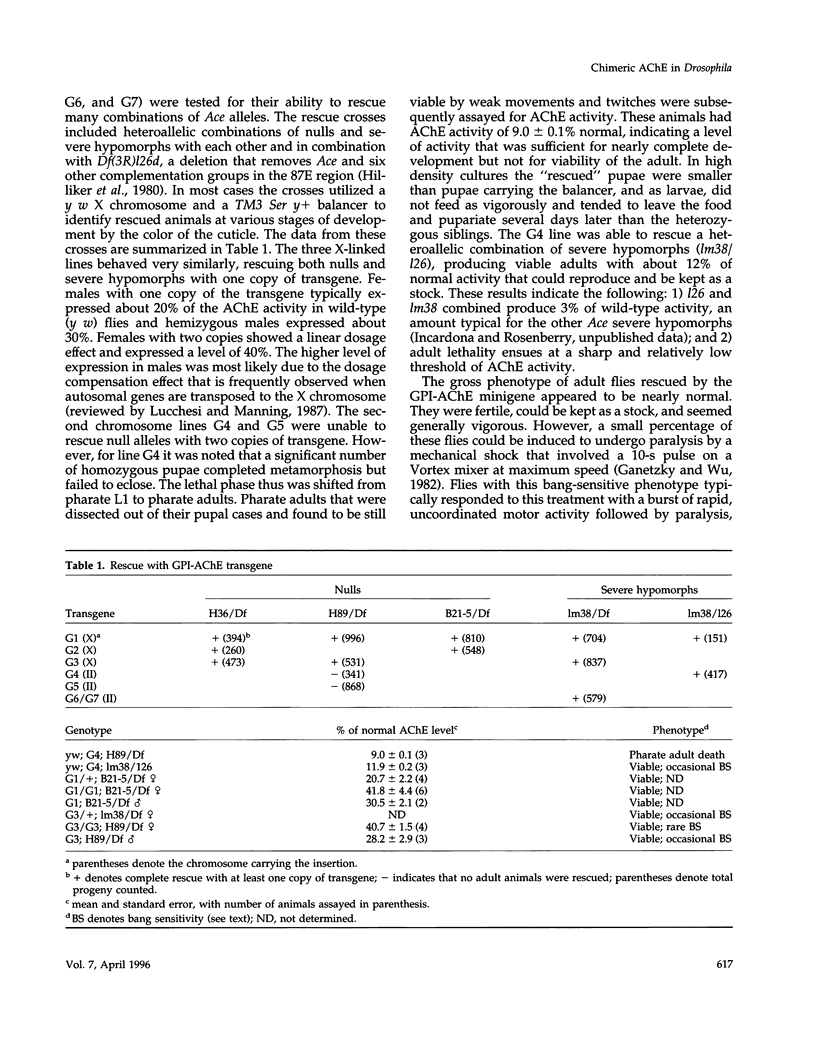
- Hilliker A. J., Clark S. H., Chovnick A., Gelbart W. M. Cytogenetic analysis of the chromosomal region immediately adjacent to the rosy locus in Drosophila melanogaster. Genetics. 1980 May;95(1):95–110. doi: 10.1093/genetics/95.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann F., Fournier D., Spierer P. Minigene rescues acetylcholinesterase lethal mutations in Drosophila melanogaster. J Mol Biol. 1992 Jan 5;223(1):17–22. doi: 10.1016/0022-2836(92)90710-2. [DOI] [PubMed] [Google Scholar]
- Incardona J. P., Rosenberry T. L. Construction and characterization of secreted and chimeric transmembrane forms of Drosophila acetylcholinesterase: a large truncation of the C-terminal signal peptide does not eliminate glycoinositol phospholipid anchoring. Mol Biol Cell. 1996 Apr;7(4):595–611. doi: 10.1091/mbc.7.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B., Grote E. Protein targeting in the neuron. Annu Rev Neurosci. 1993;16:95–127. doi: 10.1146/annurev.ne.16.030193.000523. [DOI] [PubMed] [Google Scholar]
- King D. G. Cellular organization and peritrophic membrane formation in the cardia (proventriculus) of Drosophila melanogaster. J Morphol. 1988 Jun;196(3):253–282. doi: 10.1002/jmor.1051960302. [DOI] [PubMed] [Google Scholar]
- Kitamoto T., Ikeda K., Salvaterra P. M. Analysis of cis-regulatory elements in the 5' flanking region of the Drosophila melanogaster choline acetyltransferase gene. J Neurosci. 1992 May;12(5):1628–1639. doi: 10.1523/JNEUROSCI.12-05-01628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. D., Kravchenko V., Kirkland T. N., Han J., Mackman N., Moriarty A., Leturcq D., Tobias P. S., Ulevitch R. J. Glycosyl-phosphatidylinositol-anchored or integral membrane forms of CD14 mediate identical cellular responses to endotoxin. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9930–9934. doi: 10.1073/pnas.90.21.9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Caras I. W., Davitz M. A., Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989 Nov;109(5):2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi J. C., Manning J. E. Gene dosage compensation in Drosophila melanogaster. Adv Genet. 1987;24:371–429. doi: 10.1016/s0065-2660(08)60013-9. [DOI] [PubMed] [Google Scholar]
- Matter K., Hunziker W., Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992 Nov 27;71(5):741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Matter K., Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994 Aug;6(4):545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen I. A., O'Neil S. D. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J Comp Neurol. 1991 Mar 8;305(2):232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- Petersen R. B., Lindquist S. Regulation of HSP70 synthesis by messenger RNA degradation. Cell Regul. 1989 Nov;1(1):135–149. doi: 10.1091/mbc.1.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis R. W., Bramlage A. T., Wotus C., Whittaker A., Gramates L. S., Seppala D., Farahanchi F., Caruccio P., Murphey R. K. Isolation of mutations affecting neural circuitry required for grooming behavior in Drosophila melanogaster. Genetics. 1993 Mar;133(3):581–592. doi: 10.1093/genetics/133.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. G., Richards P. A. The peritrophic membranes of insects. Annu Rev Entomol. 1977;22:219–240. doi: 10.1146/annurev.en.22.010177.001251. [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988 Mar;118(3):461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. J. Phosphatidylinositol membrane anchors and T-cell activation. Immunol Today. 1991 Jan;12(1):35–41. doi: 10.1016/0167-5699(91)90110-F. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L., Scoggin D. M. Structure of human erythrocyte acetylcholinesterase. Characterization of intersubunit disulfide bonding and detergent interaction. J Biol Chem. 1984 May 10;259(9):5643–5652. [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kamen B. A., Anderson R. G. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990 Dec;111(6 Pt 2):2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M., Sudol M., Tang Z., Lisanti M. P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993 Aug;122(4):789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubiger M., Feng Y., Fambrough D. M., Palka J. A mutation of the Drosophila sodium pump alpha subunit gene results in bang-sensitive paralysis. Neuron. 1994 Feb;12(2):373–381. doi: 10.1016/0896-6273(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Shiel M. J., Caplan M. J. Developmental regulation of membrane protein sorting in Drosophila embryos. Am J Physiol. 1995 Jul;269(1 Pt 1):C207–C216. doi: 10.1152/ajpcell.1995.269.1.C207. [DOI] [PubMed] [Google Scholar]
- Srinivas R. V., Balachandran N., Alonso-Caplen F. V., Compans R. W. Expression of herpes simplex virus glycoproteins in polarized epithelial cells. J Virol. 1986 May;58(2):689–693. doi: 10.1128/jvi.58.2.689-693.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. P transposons controlled by the heat shock promoter. Mol Cell Biol. 1986 May;6(5):1640–1649. doi: 10.1128/mcb.6.5.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R., Heisenberg M. A higher control center of locomotor behavior in the Drosophila brain. J Neurosci. 1993 May;13(5):1852–1861. doi: 10.1523/JNEUROSCI.13-05-01852.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tago H., Kimura H., Maeda T. Visualization of detailed acetylcholinesterase fiber and neuron staining in rat brain by a sensitive histochemical procedure. J Histochem Cytochem. 1986 Nov;34(11):1431–1438. doi: 10.1177/34.11.2430009. [DOI] [PubMed] [Google Scholar]
- Tepass U., Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994 Feb;161(2):563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Brewer C. B., Roth M. G. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993 Feb 15;268(5):3313–3320. [PubMed] [Google Scholar]
- Truman J. W., Booker R. Adult-specific neurons in the nervous system of the moth, Manduca sexta: selective chemical ablation using hydroxyurea. J Neurobiol. 1986 Nov;17(6):613–625. doi: 10.1002/neu.480170606. [DOI] [PubMed] [Google Scholar]
- Wolfgang W. J., Forte M. A. Expression of acetylcholinesterase during visual system development in Drosophila. Dev Biol. 1989 Feb;131(2):321–330. doi: 10.1016/s0012-1606(89)80005-3. [DOI] [PubMed] [Google Scholar]
- Zinsmaier K. E., Eberle K. K., Buchner E., Walter N., Benzer S. Paralysis and early death in cysteine string protein mutants of Drosophila. Science. 1994 Feb 18;263(5149):977–980. doi: 10.1126/science.8310297. [DOI] [PubMed] [Google Scholar]