Abstract
Vascular basement membrane (VBM) derived molecules are regulators of certain biological activities such as cell growth, differentiation and angiogenesis. Angiogenesis is regulated by a systematic controlled balance between VBM derived antiangiogenic factors and proangiogenic growth factors. In the normal physiological state, equilibrium is maintained between the antiangiogenic and proangiogenic factors. The antiangiogenic factors (molecules), which are generated by the proteolytic cleavage of the VBM, include; α1 chain non-collagenous (NC1) domain of type XVIII collagen (endostatin) and the NC1 domains from the alpha chains of Type IV collagen considered as endogenous angiogenesis inhibitors. These collagen derived NC1 domains have a pivotal role in the regulation of tumor angiogenesis, thus making them attractive alternate candidates for cancer therapies. In this review we illustrate a comprehensive overview of the knowledge gained from the signaling mechanisms of Type IV collagen derived endogenous inhibitors in angiogenesis.
Introduction
Angiogenesis, the sprouting of capillaries from pre-existing blood vessels, or by splitting of blood vessels is among the key events in destructive pathological processes such as tumor growth, metastasis, arthritis, age related macular degeneration etc., as well as in physiological processes such as development, organ growth, reproduction and wound healing (Folkman, 1995a). Folkman’s group first reported a hypothesis that tumor growth is dependent on neovascularization or angiogenesis (Folkman, 1995a; Folkman, 1995b). The growth of tumors is strictly dependent on the neovascularization, and the inhibition of vascular supply to tumors can suppress tumor growth (Folkman, 1971; Hanahan and Folkman, 1996). Solid tumors cannot grow beyond 2 to 3 mm in diameter without recruitment of their own blood supply, thus tumor angiogenesis results from a balance between endogenous activators [vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF) etc.] and inhibitors [various antiangiogenic peptides generated from VBM or extracellular matrix (ECM) degradation by proteases] (Folkman, 1995a; Kieran et al. 2003; Folkman, 2003).
Endogenous angiogenesis inhibitors from ECM includes a large multifunctional ECM glycoproteins such as thrombospondin (Good et al. 1990), Endorepellin, a COOH terminal end of perlecan, (or perlecan domain V) (Yurchenco and O’Rear, 1994), Anastellin, a fibronectin fragment, Fibulins (COOH terminal fragments corresponding to fibulin 1D and the domain 111 of fibulin 5) (Yi and Ruoslahti, 2001; Albig and Schiemann, 2004). Endostatin, a 20 kDa fragment derived from the COOH-terminal non-collagenous domain of α1 chain of type XVIII collagen (O’Reilly et al. 1997) and Type IV collagen derived α1 chain non-collagenous α1(IV)NC1, α2(IV)NC1, α3(IV)NC1 and α6(IV)NC1 domains (Petitclerc et al. 2000).
Non-ECM derived endogenous angiogenesis inhibitors includes angiostatin, a 38 to 45 kDa peptide from plasminogen, that contain homologous triple-disulfide bridged kringle domains, 1 to 4 or 1 to 3 (Patterson and Sang, 1997; Cornelius et al. 1998). Circulating clotting factors in the blood are also known to play an important role in angiogenesis. These factors include Antithrombin III, a latent form of intact antithrombin (O’Reilly et al. 1999), Prothrombin kringle-2, is derived from cleavage of the COOH-terminal loop of antithrombin and the cleaved conformational changed molecule showing antiangiogenic and antitumorogenic activity (Lee et al. 1998). Tissue inhibitors of matrix metalloproteinases-2 (TIMP-2) suppress MMP activity and ECM turnover (Brew et al. 2000; Jiang et al. 2002), 2-Methoxyestradiol (2-ME) an endogenous estradiol metabolite (Mabjeesh et al. 2003), Vasostatin, a NH2-terminal domain of human Calreticulin inclusive of 1,180 amino acids (Pike et al. 1998; Pike et al. 1999), soluble Fms-like tyrosine kinase 1 (sFlt-1) or VEGFR1 (Kendall and Thomas, 1993), Troponin I (Tn I) derived from cartilage (Moses et al. 1999), Pigment epithelium-derived factor (PEDF), a non-inhibitory member of the serpin superfamily (Volpert et al. 2002), Interferon α/β (INFα/β) (Lingen et al. 1998; Dinney et al. 1998), Chondromodulin-I, a 25 kDa cartilage specific Non-Collagenous-1 matrix protein (Kusafuka et al. 2002), PEX, a non-catalytic COOH terminal hemopexin-like domain of MMP-2 (Brooks et al. 1998), Prolactin fragment, 16 kDa and 8 kDa fragments generated from 23 kDa intact prolactin (Ferrara et al. 1991), Interleukins (a family of leukocyte-derived proteins) (Strieter et al. 1995b; Strieter et al. 1995a) and platelet factor-4 (release from platelet α-granules during platelet aggregation) (Maione et al. 1990) etc.
This review will highlight some of the important features of Type IV collagen-derived angiogenic inhibitor molecules and address their integrin mediated signaling mechanisms in the regulation of abnormal neovascularization in tumors, that would explain how these endogenous angiogenesis inhibitors regulate angiogenic balance in the tumor bed.
Type IV Collagen Derived Angiogenesis Inhibitors
Type IV collagen is the most abundant constituent of the basement membrane (BM) that forms a network like structure in the extracellular matrix. Type IV collagen providing a scaffold in the BM with other macromolecules, such as laminins, heparan sulfate proteoglycans, fibronectin, entactin and regulates the interaction with adhering cells (Timpl et al. 1981; Kuhn et al. 1981; Timpl, 1996). Type IV collagen is found normally only in the BM, but during pathogenesis, it is associated with tumor fibrosis and accumulates in the tumor inter-stitium (Timpl et al. 1981; Kuhn et al. 1981). Type IV collagen is composed of six (α1 to α6) distinct gene products and their genomic localization shows a pair-wise head-to-head arrangements with a bi-directional promoter, that were mapped onto three different chromosomes (Hudson et al. 1993; Hudson et al. 1994; Kuhn, 1995). α1 and α2 chains are most abundant forms of Type IV collagen found in most basement membranes (BM) (Hudson et al. 2003). Where as α3–α6 chains are found in kidney a specialized glomerular basement membrane with specific functional properties (Hudson et al. 2003).
The [α1(IV)]2α2(IV) trimers contain a triple helical domain with binding sites for α1β1 and α2β1 integrins (Vandenberg et al. 1991). Initially in 1986, cells binding to Type IV collagen and its inhibition with Type IV collagen peptides has been demonstrated (Aumailley and Timpl, 1986; Tsilibary et al. 1990; Chelberg et al. 1990). Tsilibary in 1990, first reported a peptide that was derived from non-collagenous domain (NC1) of the α1(IV) chain could promote adhesion of bovine aortic endothelial cells (Tsilibary et al. 1990). The functional α1 and α2 Type IV collagen chains isolated from the Engelbreth Holm Swarm Sarcoma tumors inhibited capillary endothelial cell proliferation (Ries et al. 1995; Madri, 1997).
The new functions for α2, α3 and α6 NC1 domains of type IV collagen and their integrin ligands inhibiting angiogenesis and tumor growth in vivo reported in 2000 (Petitclerc et al. 2000). Later several laboratories worked on these molecules and further supported antiangiogenic and antitumorogenic activities of these NC1 domains (Kamphaus et al. 2000; Maeshima et al. 2000; Pasco et al. 2000; Colorado et al. 2000; Marneros and Olsen, 2001; Maeshima et al. 2002; Sudhakar et al. 2003; Hamano et al. 2003; Sudhakar et al. 2005; Roth et al. 2005; Magnon et al. 2005; Borza et al. 2006; Boosani and Sudhakar, 2006; Boosani et al. 2007; Magnon et al. 2007). The molecular signaling mechanisms for regulation of angiogenesis by α1, α2, α3 and α6 NC1 domains of Type IV collagen are updated in this review. Understanding the mechanism(s) of action of such molecules would aid in unraveling their therapeutic applications.
α1(IV)NC1 or arresten
α1(IV)NC1 is one of the recently identified endogenous inhibitors of angiogenesis. It is a 26-kDa molecule derived from the NC1 domain of the α1 chain of Type IV collagen by proteases (Colorado et al. 2000; Sudhakar et al. 2005; Boosani et al. 2006). The extensive studies from my laboratory and others suggest that α1(IV)NC1 functions via α1β1 integrin and blocks the binding of α1β1 integrin to the Type IV collagen (Colorado et al. 2000; Sudhakar et al. 2005). Integrin α1β1 is a collagen binding receptor that also binds to other basement membrane components such as laminin (Zutter and Santoro, 1990; Keely et al. 1995). Both α1 and β1 integrins are involved in angiogenesis (Senger et al. 2002). Using the neutralizing antibodies for α1 integrin, angiogenesis associated with tumor growth could be suppressed. Blocking of α1β1 integrin interactions with ECM inhibits angiogenesis, which indicates that the integrins α1β1 acts as proangiogenic receptors (Senger et al. 2002). Among the integrin receptors for collagen, α1β1 integrin activates the Ras/Shc mitogen activated protein kinase (MAPK) pathway promoting cell proliferation (Senger et al. 2002). We demonstrated that α1(IV)NC1 binds to α1β1 integrin in a collagen type IV dependent manner and mediates all of its antiangiogenic functions through this integrin and inhibits angiogenesis by inhibiting endothelial cell proliferation, migration and tube formation (Sudhakar et al. 2005; Boosani et al. 2006). α1(IV)NC1 might also function via binding to heparan sulfate proteoglycans. Previously heparan sulfate proteoglycan was reported to bind to α1(IV)NC1 domain (Colorado et al. 2000). Significant halt in pathological angiogenesis and tumor growth was reported in α1 integrin knockout mice (Pozzi et al. 2000; Sudhakar et al. 2005). Whereas, α1(IV)NC1 had no effect in α1 integrin knockout mouse lung endothelial cells (Sudhakar et al. 2005). On the contrary, it significantly inhibited proliferation of wild type mouse lung endothelial cells. Thus confirms the significance of integrin mediated signaling of α1(IV)NC1 (Sudhakar et al. 2005).
In endothelial cells, ligand upon binding to integrins induces FAK phosphorylation, which serves as a platform for different downstream signals (Hynes, 2002; Kim et al. 2002; Sudhakar et al. 2003). Classical integrin ligand interactions are known to initiate intracellular signaling pathways, however some of such signaling events are reported to be inhibited by α1(IV)NC1 by binding to α1β1 integrin (Sudhakar et al. 2005). α1(IV)NC1 inhibits phosphorylation of FAK when mouse lung endothelial cells (MLEC) are plated on collagen type IV matrix (Sudhakar et al. 2005). Similar inhibition of FAK phosphorylation was not observed with α1(IV)NC1 treatment in α1 integrin knockout MLEC cells (Sudhakar et al. 2005). Downstream to FAK, protein kinase B (Akt/PKB) plays an important role in endothelial cell survival signaling (Shiojima and Walsh, 2002; Sudhakar et al. 2003; Sudhakar et al. 2005). α1(IV)NC1 does not inhibit Akt or phosphatidyl-3-kinase (PI3 kinase) phosphorylation suggesting that α1(IV) NC1 regulates migration of endothelial cells in an Akt-independent manner (Sudhakar et al. 2005).
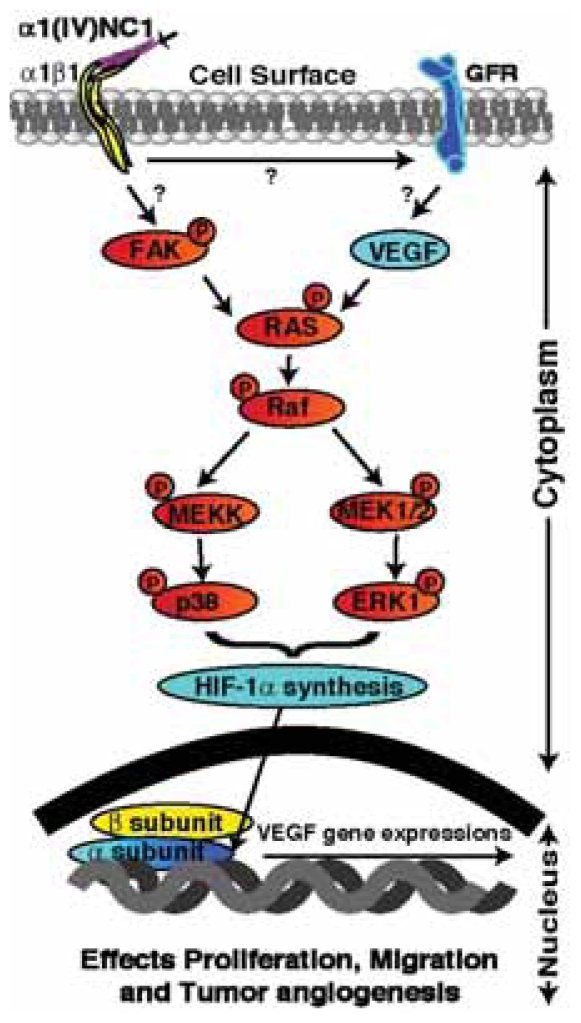
Interestingly hypoxia induced factor alpha (HIF-1α) expression was inhibited by treatment of α1(IV)NC1 in hypoxic (lack of oxygen) endothelial cells (Sudhakar et al. 2005). HIF-1α is an oxygen-dependent transcriptional activator, which plays crucial roles in the tumor angiogenesis (Semenza, 2003; Lee et al. 2004). HIF-1α regulates cellular responses to physiological and pathological hypoxia, and studies demonstrate that HIF-1α is a potential target for tumor angiogenesis (Wu et al. 2003; Unruh et al. 2003). HIF-1α transcriptionally regulates VEGF expression in hypoxic cells and promotes angiogenesis in solid tumors (Kung et al. 2000; Miller et al. 1994; Carmeliet et al. 1998; Sudhakar et al. 2005). These findings suggest that HIF-1α is a prime target for anticancer therapies. Our recently published findings demonstrate that α1(IV)NC1 binds to α1β1 integrin on endothelial cells and inhibits MAPK signaling, which results in inhibition of HIF-1α expression (Fig. 1) (Sudhakar et al. 2005). Wild type tumor bearing mice when treated with α1(IV)NC1, decreased circulating VEGFR2 positive endothelial cells, and such observations were not seen in MLECs of integrin α1 knockout mice. Measuring the number of circulating endothelial cells is being evaluated as pharmacodynamic marker (Hurwitz et al. 2004). These studies provide a rationale for the use of α1(IV)NC1 as an inhibitor of HIF-1α and VEGF in hypoxic endothelial cells (Sudhakar et al. 2005). This hypoxic inhibitory activity might be exploited for antiangiogenic therapy in the treatment of cancer, but more pre-clinical laboratory studies are needed.
Figure 1.
Schematic illustration of signaling pathway mediated by α1(IV)NC1. α1(IV)NC1 binds to α1β1 integrin and cross talk with growth factor receptors, and inhibit phosphorylation of FAK. Inhibition of FAK activation leads to inhibition of Raf/MEK/ERK1/2/p38 MAP kinase pathways that leads to inhibition of HIF-1α and VEGF expression which in turn results in inhibition of endothelial cell migration, proliferation and tube formation in proliferating endothelial cells.
α2(IV)NC1 or canstatin
Proteolytic degradation of type IV collagen liberates a 24-kDa peptide from α2 chain, called α2(IV)NC1, this peptide was reported to inhibit tumor associated angiogenesis (Petitclerc et al. 2000). The exact mechanisms by which this NC1 domain of TypeIV collagen inhibits tumor angiogenesis is not completely understood. α2(IV)NC1 binds to the endothelial and tumor cell surface in an αVβ3 and αVβ5 integrin dependent manner (Panka and Mier, 2003; Roth et al. 2005; Magnon et al. 2005; Magnon et al. 2007). α2(IV)NC1 competes with Type IV collagen of ECM for cell surface integrin binding and reverses the proliferative and migratory effects induced by cell-ECM interactions (Kamphaus et al. 2000). Thus, αVβ3 and αVβ5 integrins appear to mediate the antiangiogenic and antitumorgenic properties of α2(IV)NC1 (Magnon et al. 2005). In addition, researchers also determined that α2(IV)NC1 binds to αVβ3 and αVβ5 integrins and induce apoptosis in endothelial and certain tumor cells (Magnon et al. 2005). α2(IV)NC1 inhibits the growth of many tumors in human xenograft mouse models, histological studies revealed decreased CD31 positive vasculature (Petitclerc et al. 2000; Kamphaus et al. 2000; Roth et al. 2005; Magnon et al. 2005; Magnon et al. 2007).
α2(IV)NC1 strongly inhibits the migration and proliferation of endothelial cells. Moreover, these events are mediated by an upstream event involving α2(IV)NC1 binding to αVβ3 and αVβ5 integrins. Recent findings have shown that α2(IV)NC1 inhibits the phosphorylation of Akt, FAK, mammalian target of rapamycin (mTOR), eukaryotic initiation factor 4E binding protein-1 (4E-BP1), and ribosomal S6 kinase in cells (Panka and Mier, 2003). Collectively, the available research information suggests that, α2(IV)NC1 binds to αVβ3 and αVβ5 integrins and inactivates FAK down stream signaling, leading to suppression of cell proliferation and migration and thus leading to apoptosis (Kamphaus et al. 2000; Panka and Mier, 2003).
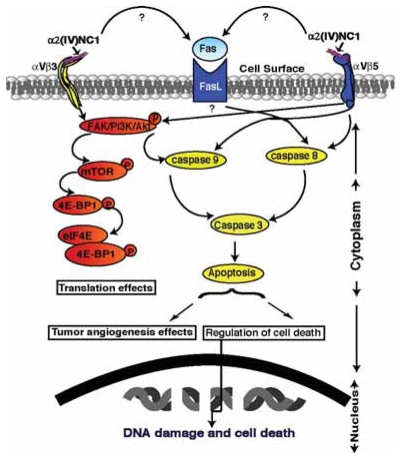
α2(IV)NC1 binds to αVβ3 and αVβ5 integrins and initiates two apoptotic pathways that include activation of caspase-8 and -9, (both initiators of the downstream apoptotic process) and leads to activation of caspase-3 (Roth et al. 2005, Magnon et al. 2005). α2(IV)NC1 activates caspase-8 by downregulation of Flip levels. Upregulation of Fas/Fas ligand triggers not only cell death directly through caspase-3 activation but also indirectly through mitochondrial damage via activation of caspase-9 within the apoptosome. On the other hand, phosphorylated FAK/PI3K is known to inactivate the mitochondrial apoptotic pathway by inhibition of caspase-9 (Magnon et al. 2005). So, α2(IV)NC1 directly activates procaspase-9 through inhibition of the FAK/PI3K pathway and amplifies the Fas-dependent pathway in mitochondria. Caspase activation might be exploited for antitumorogenic therapy in the treatment of cancer.
Overall α2(IV)NC1 inhibits FAK/Akt signaling by binds to αVβ3 and αVβ5 integrins and induces distinct signaling pathways to activate caspase-3 in endothelial or in tumoral cells. α2(IV)NC1 initiates two apoptotic pathways, involving activation of caspase-8 and -9, leading to activation of caspase-3. (a) α2(IV)NC1 activates procaspase-9 directly through inhibition of the FAK/PI3K/Akt pathway, and (b) activates caspase-3 by amplifying indirectly the mitochondrial pathway through Fas-dependent caspase-8 activation. Where as in tumor cells α2(IV)NC1 activates caspase-3 only the mitochondrial pathway (Magnon et al. 2005) (Fig. 2).
Figure 2.
Schematic illustration of distinct signaling pathways induced by α2(IV)NC1. α2(IV)NC1 binds to αVβ3 and αVβ5 integrins on endothelial and tumor cells, and initiates two distinct signaling pathways. (1) Inhibition of phosphorylation of FAK/PI-3K/eIF4E/4E-BP1. (2) Activation of caspase-8 and -9 leading to activation of caspase-3. α2(IV)NC1 activates pro-caspase-8 and -9 directly through inhibition of FAK/PI3K/Akt/mTOR pathway. α2(IV)NC1 also indirectly enhances the mitochondrial pathway through Fas dependent caspase-8 activation, which results in inhibition of protein synthesis, DNA damage and cell death.
α3(IV)NC1 or tumstatin
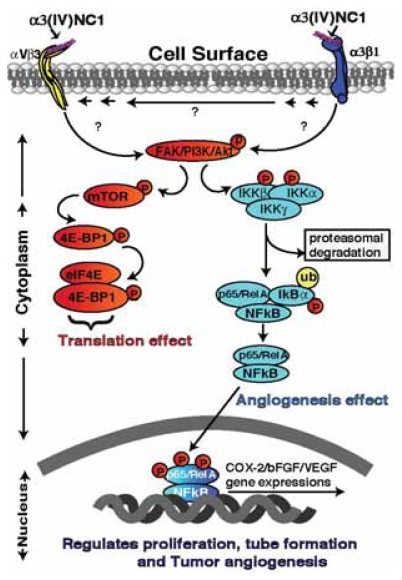
A 28-kDa proteolytic peptide liberated from the NC1 domain of α3 chain of Type IV collagen by MMP-9 and 2, has been shown to inhibit the proliferation of melanoma and other epithelial tumor cell lines in vitro by binding to the CD47/αVβ3 integrin complex (Monboisse et al. 1994; Han et al. 1997; Shahan et al. 1999; Petitclerc et al. 2000; Hamano et al. 2003). In vivo over expression of α3(IV)NC1 domain in tumor cells inhibited their invasive properties in mouse melanoma model (Pasco et al. 2004; Pasco et al. 2005). α3(IV)NC1 inhibits formation of new blood vessels in Matrigel plugs and suppresses tumor growth of human renal cell carcinoma and prostate carcinoma in mouse xenograft models and this is associated with in vivo endothelial cell specific apoptosis (Petitclerc et al. 2000; Maeshima et al. 2000). The antiangiogenic activity of α3(IV)NC1 is localized to two distinct integrin binding region of the molecule that is separate from the region responsible for the antitumor cell activity (Maeshima et al. 2000; Borza et al. 2006; Boosani et al. 2007). αVβ3 binds in the NH2-terminal end (54–132 amino acid region) of the α3(IV)NC1 that is associated with the antiangiogenic activity and α3β1 binds in the COOH-terminal end (185–203 amino acid region) that is associated with the antitumor activity (Shahan et al. 1999; Floquet et al. 2004). These two distinct integrin binding sites of α3(IV)NC1 mediating two distinct antiangiogenic and antitumorogenic activities was recently reported by Boosani et al. (Fig. 3) (Boosani et al. 2007).
Figure 3.
Schematic illustration of different signaling pathway mediated by α3(IV)NC1. α3(IV)NC1 binds to αVβ3 and α3β1 integrins, and inhibits phosphorylation of FAK. Inhibition of FAK activation leads to inhibition of FAK/PI-3K/eIF4E/4E-BP1 cap dependent translation. In addition α3(IV)NC1 inhibits NFκB mediated signaling in hypoxic conditions leading to inhibition of COX-2/VEGF/bFGF expression, resulting in inhibition of hypoxic tumor angiogenesis.
The signaling mechanism involving inhibition of endothelial cell-specific protein synthesis by α3(IV)NC1 binding to αVβ3 integrin was reported previously (Maeshima et al. 2002; Sudhakar et al. 2003). This mechanism has since been implicated in inhibition of tumor growth from several tumor cell lines such as CT26 (colon adenocarcinoma), LLC (Lewis lung carcinoma), renal cell carcinoma (786-O), prostate carcinoma (PC3), human prostate cancer (DU145), human lung cancer (H1299), and human fibrosarcoma (HT1080), by inhibiting tumor angiogenesis (Petitclerc et al. 2000; Miyoshi et al. 2006; Borza et al. 2006; Maeshima et al. 2000). The antiangiogenic activity of α3(IV)NC1 upon its interaction with αVβ3 integrin, inhibit activation of FAK, PI3K, Akt/protein kinase B, mTOR pathways and prevents the dissociation of eIF4E protein from 4E-BP1 leading to the inhibition of Cap-dependent translation (Maeshima et al. 2002; Sudhakar et al. 2003). Furthermore, these findings indicate the role for integrins in mediating cell specific inhibition of protein translation that suggests a potential mechanism for the specific effects of α3(IV)NC1 on endothelial cells (Sudhakar et al. 2003).
Recently our laboratory has identified the signaling mechanism mediated by α3(IV)NC1 that inhibits hypoxia induced cyclo-oxygenase-2 (COX-2) expression in endothelial cells via FAK/Akt/NFκB pathways, and leads to decreased tumor angiogenesis and tumor growth in an α3β1 integrin dependent manner (Boosani et al. 2007). COX-2 is a key enzyme involved in conversion of arachidonic acid to prostaglandins (PGs) and other eicosanoids (Hla and Neilson, 1992). Two isoforms of COX were identified; COX-1 is expressed constitutively, whereas COX-2 is induced by a variety of factors, including cytokines, growth factors, and tumor promoters (Hla and Neilson, 1992; DuBois et al. 1994). Mitogens such as tumor necrosis factor, phorbol ester, lipopolysaccharide, or interleukin-1 are known to increase the steady-state levels of COX-2 (Jones et al. 1993; Michiels et al. 1993). Hypoxia induces COX-2 expression by nuclear transcription factor-kappa B (NFκB) (Schmedtje et al. 1997; Tamura et al. 2002). There is ample evidence that COX-2 over expression contributes to carcinogenesis and that COX-2 disruption can both prevent and treat a variety of solid tumors (Wu et al. 2003; Wu et al. 2004; Tamura et al. 2002; Subbaramaiah et al. 1997). NFκB plays an essential role in many diseases such as AIDS, atherosclerosis, asthma, arthritis, diabetes, inflammatory bowel disease, muscular dystrophy, stroke, viral infections, cancer and is a possible target of therapeutic intervention (Kumar et al. 2004; Shishodia and Aggarwal, 2004). NFκB may facilitate the induction of COX-2 by lipopolysaccharide and phorbol ester in concert with the nuclear factor-interleukin-6 expression site and a cAMP responsive element site in bovine aortic endothelial cells (Inoue et al. 1995; Yamamoto et al. 1995).
In endothelial cells, α3(IV)NC1 binds to α3β1 integrins and inhibits NFκB signaling resulting in inhibition of COX-2 mediated signaling. It was further proved that expression of COX-2 was inhibited in β3 integrin knockout endothelial cells upon treatment with α3(IV)NC1, indicating that COX-2 mediated signaling is regulated through α3β1 and not by αVβ3 integrin (Boosani et al. 2007). Interestingly COX-2 expression was not affected when hypoxic α3 integrin knockout ECs were treated with α3(IV)NC1 protein, confirming that COX-2 expression was regulated by α3β1 integrin (Boosani et al. 2007). These findings strongly suggest that α3(IV)NC1 has the ability to inhibit pro-inflammatory factor COX-2, and inhibit tumor vasculature and tumor growth in an α3β1 integrin dependent manner (Boosani et al. 2007). In addition to COX-2 inhibition, the COX-2 regulated down stream VEGF and bFGF protein expression was also inhibited upon α3(IV)NC1 treatment to endothelial cells (Boosani et al. 2007). COX-2 was also reported to play a key role in tumor angiogenesis (Leung et al. 2003; Harris, 2002). Moreover, several investigators have demonstrated that blockade of the COX-2 mediated pathway serves as a therapeutic benefit in different cancer models (Gately and Kerbel, 2003; Panka and Mier, 2003; Kunz and Ibrahim, 2003). COX-2 regulates cellular responses to pathological conditions and studies have demonstrated that COX-2 is a potential target for tumor angiogenesis (Kunz and Ibrahim, 2003; Gately and Kerbel, 2003; Kunz et al. 2003).
The antitumorogenic activity of α3(IV)NC1 under hypoxic conditions in solid tumors was not clearly understood earlier. Our studies shed light on this mechanism by demonstrating that α3(IV)NC1 binds to α3β1 integrins which inhibit COX-2 expression both in vitro and in vivo (Boosani et al. 2007). It is clear that inhibition of hypoxia induced angiogenesis by α3(IV)NC1 is a complex process requiring further investigation. Our previous findings indicate that there may be several targets for the inhibitory effects of α3(IV)NC1 on tumor-angiogenesis, including or in addition to COX-2, VEGF and bFGF (Boosani et al. 2007).
In summary, the in vitro and in vivo observations support the role of αVβ3 and α3β1 integrins for the antiangiogenic activity of α3(IV)NC1. While both these integrins mediate tube formation in cultured ECs, α3β1 integrin mediates signaling events that influences downstream effects of COX-2 expression which appears to be central to the mechanism of α3(IV)NC1 antitumor activities. Our studies also demonstrate that α3(IV)NC1 inhibits hypoxia induced angiogenesis by (1) inhibiting NFκB activation, leading to (2) inhibition of COX-2 expression, which in turn results in (3) down regulation of hypoxia induced VEGF/bFGF expression (Fig. 3) (Boosani et al. 2007). These findings have potential implications of α3(IV)NC1 for treatment of solid tumor growth, which depend critically on hypoxic angiogenesis. The decrease in COX-2 expression under hypoxia that results in decreased VEGF/bFGF expression will likely represent a primary molecular mechanism by which α3(IV)NC1 inhibit the pathological angiogenesis that is essential to the growth of tumors (Boosani et al. 2007).
α6(IV)NC1
In addition to the NC1 domains of collagen IV α1,α2, α3 chains, α6(IV)NC1 domain also possesses antiangiogenic activity and inhibits tumor growth (Petitclerc et al. 2000), but a clear and extensive analysis of this molecule are yet to be unraveled.
Conclusions and Future Directions
Type IV collagen derived endogenous angiogenesis inhibitors bind to different cell surface integrins and exert their effects through multiple mechanisms that include induction of endothelial cells apoptosis, inhibition of migration, proliferation, tube formation of endothelial cells, and inhibit or alter the functions of proangiogenic growth factors. Three possible conclusions can be drawn from the signaling mechanisms of Type IV collagen derived angiogenic inhibitors that are shown in Table 1. (1) All these collagen type IV derived inhibitors appears to exert their antiangiogenic effects by binding to specific cell surface integrins. (2) These inhibitors also block the binding of natural ligand/binding partners for proangiogenic receptors/molecules. (3) In addition, possibly by binding to its receptors, these inhibitors crosstalk with other cell surface receptors and activate specific caspase mediated signaling to regulate cell function (Panka and Mier, 2003; Magnon et al. 2005).
Table 1.
Signaling mechanisms mediated by type IV collage derived angiogenesis inhibitors.
| Angiogenesis inhibitor name | Human α1(IV)NC1 | Human α2(IV)NC1 | Human α3(IV)NC1 |
|---|---|---|---|
| Inhibitor origin | α1 Type IV collagen | α2 Type IV collagen | α3 Type IV collagen |
| Generation of inhibitor | By MMP-9 and -2 | By MMP-9 and -2 | By MMP-9 and -2 |
| Receptors | α1β1 integrin | αVβ5/αVβ3 integrins | αVβ3/α3β1 integrins |
| Proliferation | Inhibition | Inhibition | Inhibition |
| Migration | Inhibition | Inhibition | No effect |
| Tube formation | Inhibition | Inhibition | Inhibition |
| Mechanism of action | FAK, Ras, c-Raf, MEK1/2, p38, ERK1/2, HIF1α mediated signaling | FAK, Akt, PI3K/mTOR/eIF-4E/4E-BP1 signaling and FasL mediated apoptosis | FAK, Akt, PI3K/mTOR/eIF-4E/4E-BP1 and NFkB/COX-2 mediated signaling |
Currently, more than 25 different endogenous circulating molecules (small proteins or peptides) are found to exist in the human body that functions as angiogenesis inhibitors. Circulating physiological concentration of α3(IV)NC1 was reported in normal mice to be about 336 ng/ml, that was absent in α3 chain of Type IV Collagen null mice (Hamano et al. 2003). Administration of 300 ng of recombinant α3(IV)NC1 to physiological levels in α3 chain of Type IV Collagen null mice with LLC tumors showed decrease tumor growth, the number of blood vessels and circulating endothelial cells to the wild-type baseline levels (Hamano et al. 2003; Sund et al. 2005). It is quite possible that genetic control of the physiologic levels of these endogenous angiogenesis inhibitors might contribute to a critical line of defense against the conversion of dormant neoplastic events into a malignant phenotype of cancer.
Several angiogenic inhibitors including integrin αV antagonist EMD 121974, 2-methoxyestradiol (panzam) and, MMP-2 and -9 inhibitor COL-3 etc are currently in phase 1/2 human clinical trails (Jansen et al. 2004). Questions regarding resistance to these angiogenesis inhibitors do remain unanswered; however, a combination of radiation therapy with other antiangiogenic therapies may also prove to be clinically useful and effective. Further evaluation through extensive laboratory studies on these molecules are needed to address the function of Type IV collagen derived endogenous inhibitors of angiogenesis to be considered for the clinical trials. Earlier lessons from preclinical trials of angiostatin, endostatin, Thrombospondin-1 (ABT-510) and 2-ME suggest that more basic laboratory research studies are required to better understand the mechanism of actions associated with each of these endogenous angiogenesis inhibitor molecules. Presently, some of the anti-angiogenic agents such as Bevacizumab and several other VEGFR tyrosine kinase inhibitors; Vatalanib (PTK787/ZK 222584), Semaxanib (SU5416), Sunitinib (SU11248), Sorafenib (BAY 43-9006) are in clinical trials (Hurwitz et al. 2004; Morabito et al. 2006). In the past few years several advances were made VBM derived endogenous angiogenesis inhibitors functional studies. VBM not only is an important structural component of the blood capillary, but it is also an important functional regulator of tumor angiogenesis and tumor growth. VBM in an assembled form performs completely new role compared with degraded form (exposed to different proteases). The degraded VBM modulate cellular behavior, hiding or exposing basement membrane integrin binding sequences. Therefore, VBM has become very good source of a collection of peptides or proteins that posses distinct activities with in the same primary sequence. These sequences are available at different stages during VBM structural changes; just like as the coagulation pathway proteins. Our understanding of how these collagen Type IV derived angiogenesis inhibitors regulate angiogenesis has just began compared to type XVIII collagen derived angiogenesis inhibitor or endostatin. Further extensive laboratory studies are required to know how Type IV collagen derived molecules regulating cellular functions to halt tumor growth and tumor angiogenesis.
Acknowledgements
Grant support: Flight Attendant Medical Research Institute Young Clinical Scientist Award Grant (FAMRI# 062558 to S. A), Dobleman Head and Neck Cancer Institute Grant and Cell Signaling and Tumor Angiogenesis Laboratory at Boys Town National Research Hospital research funds to S. A.
Keywords
- VBM
vascular basement membrane
- ECM
extra cellular matrix
- MMP
matrix metalloproteinase
- HUVEC
human umbilical vein endothelial cell
- MLEC
mouse lung endothelial cells
- SCC-PSA1
teratocarcinoma cell line
- VEGF
vascular endothelial cell growth factor
- bFGF
basic fibroblast growth factor
- α1–α6(IV)NC1
non-collagenous α1–α6 chains of Type IV collagen domains
References
- Albig AR, Schiemann WP. DNA Cell Biol. 2004;23:367–79. doi: 10.1089/104454904323145254. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Timpl R. J Cell Biol. 1986;103:1569–75. doi: 10.1083/jcb.103.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boosani CS, Mannam AP, Cosgrove D, Silva R, Hodivala-Dilke KM, Keshamouni VG, Sudhakar A. Blood. 2007;110:1168–77. doi: 10.1182/blood-2007-01-066282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boosani CS, Sudhakar A. Protein Expr Purif. 2006;49:211–8. doi: 10.1016/j.pep.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Borza CM, Pozzi A, Borza DB, Pedchenko V, Hellmark T, Hudson BG, Zent R. J Biol Chem. 2006 doi: 10.1074/jbc.M601147200. [DOI] [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H. Biochim Biophys Acta. 2000;1477:267–83. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Cell. 1998;92:391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E, Keshet E. Nature. 1998;394:485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- Chelberg MK, McCarthy JB, Skubitz AP, Furcht LT, Tsilibary EC. J Cell Biol. 1990;111:261–70. doi: 10.1083/jcb.111.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, Dhanabal M, Simons M, Post M, Kufe DW, Weichselbaum RR, Sukhatme VP, Kalluri R. Cancer Res. 2000;60:2520–6. [PubMed] [Google Scholar]
- Cornelius LA, Nehring LC, Harding E, Bolanowski M, Welgus HG, Kobayashi DK, Pierce RA, Shapiro SD. J Immunol. 1998;161:6845–52. [PubMed] [Google Scholar]
- Dinney CP, Bielenberg DR, Perrotte P, Reich R, Eve BY, Bucana CD, Fidler IJ. Cancer Res. 1998;58:808–14. [PubMed] [Google Scholar]
- DuBois RN, Tsujii M, Bishop P, Awad JA, Makita K, Lanahan A. Am J Physiol. 1994;266:G822–7. doi: 10.1152/ajpgi.1994.266.5.G822. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Clapp C, Weiner R. Endocrinology. 1991;129:896–900. doi: 10.1210/endo-129-2-896. [DOI] [PubMed] [Google Scholar]
- Floquet N, Pasco S, Ramont L, Derreumaux P, Laronze JY, Nuzillard JM, Maquart FX, Alix AJ, Monboisse JC. J Biol Chem. 2004;279:2091–100. doi: 10.1074/jbc.M307736200. [DOI] [PubMed] [Google Scholar]
- Folkman J. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Folkman J. Nat Med. 1995a;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J. Mol Med. 1995b;1:120–2. [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Semin Cancer Biol. 2003;13:159–67. doi: 10.1016/s1044-579x(02)00133-5. [DOI] [PubMed] [Google Scholar]
- Gately S, Kerbel R. Prog Exp Tumor Res. 2003;37:179–92. doi: 10.1159/000071373. [DOI] [PubMed] [Google Scholar]
- Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. Proc Natl Acad Sci USA. 1990;87:6624–8. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Ohno N, Pasco S, Monboisse JC, Borel JP, Kefalides NA. J Biol Chem. 1997;272:20395–401. doi: 10.1074/jbc.272.33.20395. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Harris AL. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Hla T, Neilson K. Proc Natl Acad Sci USA. 1992;89:7384–8. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson BG, Kalluri R, Gunwar S, Noelken ME. Contrib Nephrol. 1994;107:163–7. doi: 10.1159/000422975. [DOI] [PubMed] [Google Scholar]
- Hudson BG, Reeders ST, Tryggvason K. J Biol Chem. 1993;268:26033–6. [PubMed] [Google Scholar]
- Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG. N Engl J Med. 2003;348:2543–56. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Nat Med. 2002;8:918–21. doi: 10.1038/nm0902-918. [DOI] [PubMed] [Google Scholar]
- Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T. J Biol Chem. 1995;270:24965–71. doi: 10.1074/jbc.270.42.24965. [DOI] [PubMed] [Google Scholar]
- Jansen M, de Witt Hamer PC, Witmer AN, Troost D, van Noorden CJ. Brain Res Brain Res Rev. 2004;45:143–63. doi: 10.1016/j.brainresrev.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Goldberg ID, Shi YE. Oncogene. 2002;21:2245–52. doi: 10.1038/sj.onc.1205291. [DOI] [PubMed] [Google Scholar]
- Jones DA, Carlton DP, McIntyre TM, Zimmerman GA, Prescott SM. J Biol Chem. 1993;268:9049–54. [PubMed] [Google Scholar]
- Kamphaus GD, Colorado PC, Panka DJ, Hopfer H, Ramchandran R, Torre A, Maeshima Y, Mier JW, Sukhatme VP, Kalluri R. J Biol Chem. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- Keely PJ, Wu JE, Santoro SA. Differentiation. 1995;59:1–13. doi: 10.1046/j.1432-0436.1995.5910001.x. [DOI] [PubMed] [Google Scholar]
- Kendall RL, Thomas KA. Proc Natl Acad Sci USA. 1993;90:10705–9. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran MW, Folkman J, Heymach J. Nat Med. 2003;9:1104. doi: 10.1038/nm0903-1104a. author reply 1104–5. [DOI] [PubMed] [Google Scholar]
- Kim YM, Hwang S, Pyun BJ, Kim TY, Lee ST, Gho YS, Kwon YG. J Biol Chem. 2002;277:27872–9. doi: 10.1074/jbc.M202771200. [DOI] [PubMed] [Google Scholar]
- Kuhn K. Matrix Biol. 1995;14:439–45. doi: 10.1016/0945-053x(95)90001-2. [DOI] [PubMed] [Google Scholar]
- Kuhn K, Wiedemann H, Timpl R, Risteli J, Dieringer H, Voss T, Glanville RW. FEBS Lett. 1981;125:123–8. doi: 10.1016/0014-5793(81)81012-5. [DOI] [PubMed] [Google Scholar]
- Kumar A, Takada Y, Boriek AM, Aggarwal BB. J Mol Med. 2004;82:434–48. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM. Nat Med. 2000;6:1335–40. doi: 10.1038/82146. [DOI] [PubMed] [Google Scholar]
- Kunz M, Ibrahim SM. Mol Cancer. 2003;2:23. doi: 10.1186/1476-4598-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz M, Moeller S, Koczan D, Lorenz P, Wenger RH, Glocker MO, Thiesen HJ, Gross G, Ibrahim SM. J Biol Chem. 2003;278:45651–60. doi: 10.1074/jbc.M301373200. [DOI] [PubMed] [Google Scholar]
- Kusafuka K, Hiraki Y, Shukunami C, Kayano T, Takemura T. Acta Histochem. 2002;104:167–75. doi: 10.1078/0065-1281-00642. [DOI] [PubMed] [Google Scholar]
- Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- Lee TH, Rhim T, Kim SS. J Biol Chem. 1998;273:28805–12. doi: 10.1074/jbc.273.44.28805. [DOI] [PubMed] [Google Scholar]
- Leung WK, To KF, Go MY, Chan KK, Chan FK, Ng EK, Chung SC, Sung JJ. Int J Oncol. 2003;23:1317–22. [PubMed] [Google Scholar]
- Lingen MW, Polverini PJ, Bouck NP. Cancer Res. 1998;58:5551–8. [PubMed] [Google Scholar]
- Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P. Cancer Cell. 2003;3:363–75. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- Madri JA. Transpl Immunol. 1997;5:179–83. doi: 10.1016/s0966-3274(97)80035-4. [DOI] [PubMed] [Google Scholar]
- Maeshima Y, Colorado PC, Torre A, Holthaus KA, Grunkemeyer JA, Ericksen MB, Hopfer H, Xiao Y, Stillman IE, Kalluri R. J Biol Chem. 2000;275:21340–8. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- Maeshima Y, Sudhakar A, Lively JC, Ueki K, Kharbanda S, Kahn CR, Sonenberg N, Hynes RO, Kalluri R. Science. 2002;295:140–3. doi: 10.1126/science.1065298. [DOI] [PubMed] [Google Scholar]
- Magnon C, Galaup A, Mullan B, Rouffiac V, Bouquet C, Bidart JM, Griscelli F, Opolon P, Perricaudet M. Cancer Res. 2005;65:4353–61. doi: 10.1158/0008-5472.CAN-04-3536. [DOI] [PubMed] [Google Scholar]
- Magnon C, Opolon P, Ricard M, Connault E, Ardouin P, Galaup A, Metivier D, Bidart JM, Germain S, Perricaudet M, Schlumberger M. J Clin Invest. 2007;117:1844–1855. doi: 10.1172/JCI30269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione TE, Gray GS, Petro J, Hunt AJ, Donner AL, Bauer SI, Carson HF, Sharpe RJ. Science. 1990;247:77–9. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- Marneros AG, Olsen BR. Matrix Biol. 2001;20:337–45. doi: 10.1016/s0945-053x(01)00151-2. [DOI] [PubMed] [Google Scholar]
- Michiels C, Arnould T, Knott I, Dieu M, Remacle J. Am J Physiol. 1993;264:C866–74. doi: 10.1152/ajpcell.1993.264.4.C866. [DOI] [PubMed] [Google Scholar]
- Miller JW, Adamis AP, Shima DT, D’Amore PA, Moulton RS, O’Reilly MS, Folkman J, Dvorak HF, Brown LF, Berse B, et al. Am J Pathol. 1994;145:574–84. [PMC free article] [PubMed] [Google Scholar]
- Miyoshi T, Hirohata S, Ogawa H, Doi M, Obika M, Yonezawa T, Sado Y, Kusachi S, Kyo S, Kondo S, Shiratori Y, Hudson BG, Ninomiya Y. Faseb J. 2006;20:1904–6. doi: 10.1096/fj.05-5565fje. [DOI] [PubMed] [Google Scholar]
- Monboisse JC, Garnotel R, Bellon G, Ohno N, Perreau C, Borel JP, Kefalides NA. J Biol Chem. 1994;269:25475–82. [PubMed] [Google Scholar]
- Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Oncologist. 2006;11:753–64. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- Moses MA, Wiederschain D, Wu I, Fernandez CA, Ghazizadeh V, Lane WS, Flynn E, Sytkowski A, Tao T, Langer R. Proc Natl Acad Sci USA. 1999;96:2645–50. doi: 10.1073/pnas.96.6.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- O’Reilly MS, Pirie-Shepherd S, Lane WS, Folkman J. Science. 1999;285:1926–8. doi: 10.1126/science.285.5435.1926. [DOI] [PubMed] [Google Scholar]
- Panka DJ, Mier JW. J Biol Chem. 2003;278:37632–6. doi: 10.1074/jbc.M307339200. [DOI] [PubMed] [Google Scholar]
- Pasco S, Brassart B, Ramont L, Maquart FX, Monboisse JC. Cancer Detect Prev. 2005;29:260–6. doi: 10.1016/j.cdp.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Pasco S, Monboisse JC, Kieffer N. J Biol Chem. 2000;275:32999–3007. doi: 10.1074/jbc.M005235200. [DOI] [PubMed] [Google Scholar]
- Pasco S, Ramont L, Venteo L, Pluot M, Maquart FX, Monboisse JC. Exp Cell Res. 2004;301:251–65. doi: 10.1016/j.yexcr.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Patterson BC, Sang QA. J Biol Chem. 1997;272:28823–5. doi: 10.1074/jbc.272.46.28823. [DOI] [PubMed] [Google Scholar]
- Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Jr, Hudson BG, Brooks PC. J Biol Chem. 2000;275:8051–61. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- Pike SE, Yao L, Jones KD, Cherney B, Appella E, Sakaguchi K, Nakhasi H, Teruya-Feldstein J, Wirth P, Gupta G, Tosato G. J Exp Med. 1998;188:2349–56. doi: 10.1084/jem.188.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike SE, Yao L, Setsuda J, Jones KD, Cherney B, Appella E, Sakaguchi K, Nakhasi H, Atreya CD, Teruya-Feldstein J, Wirth P, Gupta G, Tosato G. Blood. 1999;94:2461–8. [PubMed] [Google Scholar]
- Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Proc Natl Acad Sci USA. 2000;97:2202–7. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries A, Engel J, Lustig A, Kuhn K. J Biol Chem. 1995;270:23790–4. doi: 10.1074/jbc.270.40.23790. [DOI] [PubMed] [Google Scholar]
- Roth JM, Akalu A, Zelmanovich A, Policarpio D, Ng B, MacDonald S, Formenti S, Liebes L, Brooks PC. Am J Pathol. 2005;166:901–11. doi: 10.1016/s0002-9440(10)62310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmedtje JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS. J Biol Chem. 1997;272:601–8. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Senger DR, Perruzzi CA, Streit M, Koteliansky VE, de Fougerolles AR, Detmar M. Am J Pathol. 2002;160:195–204. doi: 10.1016/s0002-9440(10)64363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahan TA, Ziaie Z, Pasco S, Fawzi A, Bellon G, Monboisse JC, Kefalides NA. Cancer Res. 1999;59:4584–90. [PubMed] [Google Scholar]
- Shiojima I, Walsh K. Circ Res. 2002;90:1243–50. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- Shishodia S, Aggarwal BB. Biochem Pharmacol. 2004;68:1071–80. doi: 10.1016/j.bcp.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL. Shock. 1995a;4:155–60. doi: 10.1097/00024382-199509000-00001. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. J Biol Chem. 1995b;270:27348–57. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Zakim D, Weksler BB, Dannenberg AJ. Proc Soc Exp Biol Med. 1997;216:201–10. doi: 10.3181/00379727-216-44170. [DOI] [PubMed] [Google Scholar]
- Sudhakar A, Nyberg P, Keshamouni VG, Mannam AP, Li J, Sugimoto H, Cosgrove D, Kalluri R. J Clin Invest. 2005;115:2801–10. doi: 10.1172/JCI24813. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sudhakar A, Sugimoto H, Yang C, Lively J, Zeisberg M, Kalluri R. Proc Natl Acad Sci USA. 2003;100:4766–71. doi: 10.1073/pnas.0730882100. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Sund M, Hamano Y, Sugimoto H, Sudhakar A, Soubasakos M, Yerramalla U, Benjamin LE, Lawler J, Kieran M, Shah A, Kalluri R. Proc Natl Acad Sci USA. 2005;102:2934–9. doi: 10.1073/pnas.0500180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Sebastian S, Gurates B, Yang S, Fang Z, Bulun SE. J Clin Endocrinol Metab. 2002;87:3504–7. doi: 10.1210/jcem.87.7.8796. [DOI] [PubMed] [Google Scholar]
- Timpl R. Curr Opin Cell Biol. 1996;8:618–24. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- Timpl R, Wiedemann H, van Delden V, Furthmayr H, Kuhn K. Eur J Biochem. 1981;120:203–11. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Tsilibary EC, Reger LA, Vogel AM, Koliakos GG, Anderson SS, Charonis AS, Alegre JN, Furcht LT. J Cell Biol. 1990;111:1583–91. doi: 10.1083/jcb.111.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unruh A, Ressel A, Mohamed HG, Johnson RS, Nadrowitz R, Richter E, Katschinski DM, Wenger RH. Oncogene. 2003;22:3213–20. doi: 10.1038/sj.onc.1206385. [DOI] [PubMed] [Google Scholar]
- Vandenberg P, Kern A, Ries A, Luckenbill-Edds L, Mann K, Kuhn K. J Cell Biol. 1991;113:1475–83. doi: 10.1083/jcb.113.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpert OV, Zaichuk T, Zhou W, Reiher F, Ferguson TA, Stuart PM, Amin M, Bouck NP. Nat Med. 2002;8:349–57. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- Wu AW, Gu J, Li ZF, Ji JF, Xu GW. World J Gastroenterol. 2004;10:2323–6. doi: 10.3748/wjg.v10.i16.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Mannam AP, Wu J, Kirbis S, Shie JL, Chen C, Laham RJ, Sellke FW, Li J. Am J Physiol Heart Circ Physiol. 2003;285:H2420–9. doi: 10.1152/ajpheart.00187.2003. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Arakawa T, Ueda N, Yamamoto S. J Biol Chem. 1995;270:31315–20. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- Yi M, Ruoslahti E. Proc Natl Acad Sci USA. 2001;98:620–4. doi: 10.1073/pnas.98.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD, O’Rear JJ. Curr Opin Cell Biol. 1994;6:674–81. doi: 10.1016/0955-0674(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Zutter MM, Santoro SA. Am J Pathol. 1990;137:113–20. [PMC free article] [PubMed] [Google Scholar]