Abstract
Prostanoids have a broad spectrum of biological activities in a variety of organs including the brain. However, their effects on synaptic plasticity in the brain, which have been recently revealed, are ambiguous in comparison to those in the other organs. Prostaglandin E2 (PGE2) is a prostanoid produced from arachidonic acid in the cellular membrane, and knowledge about its functions is increasing. Recently, a novel function of PGE2 in the brain has shed light on aspects of synaptic plasticity such as long-term potentiation (LTP). More recently, we have proposed a hypothesis for the mechanisms of this PGE2-related form of synaptic plasticity in the visual cortex. This involves the dynamics of two subtypes of PGE2 receptors that have opposing functions in intracellular signal transduction. Consequently, mechanisms that increase the level of cyclic AMP in the cytosol may explain for the mechanisms of LTP in the visual cortex. The current notion of bidirectional trafficking of PGE2 receptors under this hypothesis is reminiscent of the “silent synapse” mechanism of LTP on the trafficking of the AMPA receptors between the membrane and cytosol. Moreover, we propose the hypothesis that PGE2 acts as a “post-to-postsynaptic messenger” for the induction of LTP in the visual cortex. This review describes a complex mode of action of PGE2 receptors in synaptic plasticity in the brain.
Keywords: prostaglandin, LTP, visual cortex, cAMP, synaptic plasticity
Production of PGE2
Prostanoids are classified into prostaglandins and the thromboxanes. Prostaglandins consist of prostaglandin (PG)D2, PGE2, PGF2a, and PGI2, whereas thromboxanes consist of thromboxane A2 (TxA22). All of these products are metabolites of arachidonic acid, which originates from the phospholipid components of the cell membrane. Arachidonic acid is converted to PGG2 by the cyclooxygenases, which comprise cyclooxygenase(COX)-1, -2, and 3 (for review, see Schwab et al. 2003). COX-1 is ubiquitous and COX-3 is a splice variant of COX-1, whereas COX-2 is inducible in the brain by the activity in the physiological or pathological condition. For example, the level of COX-2 mRNA is enhanced in long-term potentiation (LTP), which is a form of synaptic plasticity and is defined as the persistent enhancement of synaptic transmission efficacy (Yamagata et al. 1993; Chen and Bazan, 2005). Because PGG22 is remarkably unstable, PGG2 is immediately converted to PGH2. Then PGH2 is metabolized to a specific prostanoid by a corresponding synthase; for example, PGE2 is produced by the PGE synthase.
In the prostanoids, PGG2, PGH2, PGI2 and TxA2 are degraded into inactive products within several minutes even under physiological conditions, whereas in in vivo the other prostaglandins are immediately inactivated through circulation in the lungs, where oxidative stress is strong. It is believed that because of the chemical and biological instability of prostanoids, the area, where the activity of prostanoids is essential for performing and maintaining the physiological conditions is believed to be restricted around the region where they prostanoids are produced. Prostanoids are hydrophobic, because they are synthesized from fatty acids in the cell membrane. This property allows them to pass through the membrane and reach neighboring cells. Thus, prostanoids are able to exert their functions approximately in spite of their short lifetime. This is consistent with the fact that the prostanoids which are produced in the brain do not circulate in the blood, and yet can act in the neighboring subcellular regions, such as synapses.
Receptors for PGE2
Four subtypes of receptor for PGE2 have been identified: EP1, EP2, EP3, and EP4 (EP1–4) (for review, see Narumiya et al. 1999; Narumiya and FitzGeraldl, 2001). These four receptor subtypes are expressed in different levels in the brain as well as in the other organs.
Although EP1 mRNA is distributed mainly in the kidneys, lungs, and stomach, EP1 is also found in the neurons in thalamus and dorsal root ganglia (DRG) (Watabe et al. 1993). However, a recent study showed that the levels of EP1 mRNA are the highest in the parietal cortex and cerebellum and that the protein level of EP1 is high in cerebellum (Candelario-Jalil et al. 2005). Although EP2 is the least abundant among the PGE2 receptor subtypes in the brain, its expression can be induced by stimulation with lipopolysaccharide (LPS) and gonadotropin (Katsuyama et al. 1998). A study involving Northern blot analysis and in situ hybridizatuion showed that EP3 is expressed abundantly and widely in the brain (Sugimoto et al. 1992). EP3 mRNA is expressed in the neurons of the cerebral cortex, hippocampus, thalamus, midbrain, and lower brain stem. However, EP4 mRNA is expressed only in the neurons of the DRG (Sugimoto et al. 1994; Oida et al. 1995; Burks et al. 2007).
Binding of agonists to these receptor subtypes induces signal transduction via secondary messengers, such as cyclic AMP (cAMP), in a G protein-coupled manner. The activation of EP1 elevates the levels of intracellular inositol phosphate and Ca2+ (Watabe et al. 1993), whereas the activation of EP2 or EP4 stimulates adenylate cyclase, resulting in increased levels of intracellular cAMP (Jumblatt and Paterson, 1991). Conversely, activation of EP3 inhibits adenylate cyclase (Sugimoto et al. 1992). Recently, we have shown that synaptic plasticity in the visual cortex is deeply involved in these opposing effects of EP2 and EP3 on the production of cAMP. This is a good way to acquire the sufficient cAMP for the induction of LTP (see below).
Functions of PGE2 in the Brain
PGE2 is a mediator of fever induction (Milton and Wendlandt 1970). It acts as a final mediator in the induction of fever by the pyrogens acting on the organum vasculosum of the lamina terminalis (OVLT) of the third lateral ventricle (Saper and Breder, 1994). An in situ hybridization study showed that EP3 mRNA is expressed at a particularly high level in the regions surrounding the OVLT (Sugimoto et al. 1994). Circulating pyrogens that may generate PGE2 have an easy access to the OVLT because it lacks blood-brain barrier. There have been increasing numbers of knockout (KO) studies on the four receptor subtypes, EP1–4, and in one of these studies EP3-deficient mice showed no febrile responses to any of the pyrogens tested (Ushikubi et al. 1998).
PGE2 and PGI2 promote bradykinin-induced pain (Katori et al. 1986). However, a KO study on the receptors involved revealed that this type of pain may require receptors for PGI2 rather than PGE2 (Murata et al. 1997). It is also known that PGE2 acts on the spinal dorsal horn to produce pain (Malmberg et al. 1995; Pitcher and Henry, 1999). Outside the central and peripheral nervous systems, it is known that PGE2 promotes the coagulation of platelets via EP3 (Ma et al. 2001), bone resorption and formation (Sakuma et al. 2000; Miyata et al. 2000; Li et al. 2000), cancer and carcinogenesis (Watanabe et al. 1999), hypertension (Kennedy et al. 1999; Tilley et al. 1999) and cardiovascular functions (Audoly et al. 1999; Zhang et al. 2000). Thus, PGE2 exerts a variety of functions in the body, including the brain. However, it is only recently that PGE2 has been shown to have functions in synaptic plasticity.
Knockdown for EP1–4
It is important to evaluate the functions of known and novel genes in order to understand the mechanisms involved. Genetic manipulation by KO is a useful tool to characterize a target gene. Indeed, KO studies in mice for EP1–4 in mice KO studies have been reported recently. The most eminent feature of genetic manipulation by KO is its specificity for the target gene, i.e. the genes whose expression is to be deduced. This is the most important reason why the method has become so popular and has produced so much information over the last decade. However, gene KO has shortcomings that hinder its use in ordinary laboratories. These include (1) the unpredictable effects of the knocked-out protein on other proteins that might in turn influence some other proteins especially during development. This makes it difficult to know whether the results obtained are caused because of the absence of the target protein or other proteins; (2) the high frequency of death before birth or during early postnatal development, resulting in reduced opportunity to conduct experiments; (3) the limitation of the knocked-out region (in most cases, the whole body). The effect of ordinary KO manipulation is systemic and begins at fertilization in almost all cases. It is difficult to predict the location and timing of the effect of the KO desired; (4) the fact that in most cases KO experiments are limited to mice, resulting in the restriction on the use of antibodies, DNA probes and so on; and (5) the large expenditure, labor, and time required to establish the KO model. With respect to reason (1), it is difficult to know whether the results obtained are caused by the absence of the target protein or by the absence or lack of other proteins. With respect to the reason (3), the effect of ordinary KO manipulation is systemic and begins at fertilization in almost all cases. It is difficult to predict exactly the location and timing for the effects of the KO desired. Nonetheless, KO manipulations have been used to analyze the function of the target proteins by many researchers. This is mainly because of the specificity of targeting, which presumably avoids none-specific actions, such as pharmacological actions.
An alternative way to inhibit the target expression is by RNA interference (RNAi), in which stretches of 21–23 nucleotides of RNA, termed short (or small) interfering RNA (siRNA), are used to inhibit the expression of the target mRNA, resulting in the inhibition of the target protein (Fire et al. 1998). The mechanisms of RNAi is believed to be as follows. The enzyme Dicer, a member of the RNase III family of nucleases, specifically cleaves double-stranded RNAs to form siRNAs. The siRNAs subsequently bind to RNAi-induced silencing protein complex (RISC), which contains an endo-nuclease; this is followed by targeting the perfectly complementary mRNA and cleaving the target within the sequences covered by the siRNA. Guidance of RISC to the target mRNA is highly sequence-specific; a difference of only one or two nucleotides in the targeting recognition sequence interferes with the RNAi process. The primary siRNA-template RNA can be used to support de novo RNA synthesis by an RNA-dependent RNA polymerase, resulting in the amplification of RNA. With respect to target specificity and the efficacy of maintenance of RNAi, the method using siRNA has great advantages over the use of the conventional antisense oligonucleotides to knock down the target.
For introducing exogenous molecules such as proteins, RNA, and DNA into the cells, electroporation is a convenient and efficient method (for review, see Neumann et al. 1999). The mechanisms comprises two steps: first, electrical shocks applied at the membrane produce pores that last for tens of seconds; external molecules that are negatively charged then enter the cytosol by electrophoresis. The efficiency of expression of exogenous genes has been reported to be 10–100% according to the parameters of electric impulses used. In most cases, however, the targets of the in vivo electroporation are whole organs, such as the liver and brain in the embryonic stages of the chick and mouse. Electroporation at the embryonic stage offers the possibility of influencing the destiny of the proteins other than the target as mentioned above.
To resolve the defects of systemic KO or knockdown manipulation mentioned above, we developed a novel method, termed RNAi-induced silencing by local electroporation, RISLE (Akaneya et al. 2005). It involves electroporation with the two needle electrodes after injecting siRNA into the target area of the brain to introduce the siRNA. In a recent paper, we showed that the target gene expression at the site where siRNA is injected between the two electrodes is exclusively knocked down, and that the effect is target-specific. We also found that, when using a combination of siRNA with local electroporation, the expression of certain proteins is significantly reduced in the restricted regions, such as the visual cortex and the CA1 region of the hippocampus, and use of the technique does not induce pathological changes. Moreover, we have shown that this model is available for in vitro and in vivo experiments such as electrophysiology.
Using the RISLE method, we knocked down EP1–4 in rats and analyzed the mechanisms of synaptic plasticity in the visual cortex with these knockdown rats (Akaneya and Tsumoto, 2006). We found that both EP2 and EP3, but not EP1 or EP4, are involved in the LTP in the visual cortex.
Differences in Site of Action of PGE2 between Hippocampus and Visual Cortex
Previous papers have reported the effect of PGE2 on synaptic plasticity. However, there is controversy regarding the sites of action of PGE2, i.e. presynaptic or postsynaptic sites of neurons. First, it has been reported that PGE2 increases excitatory postsynaptic potentials (EPSPs) in hippocampal slices and frequency, and that this action is via the EP2 which is located at the presynaptic sites (Sang et al. 2005; Zhu et al. 2005). This suggests the presynaptic action of PGE2. Immediately after this publication, we showed that PGE2 increases the magnitude of theta-burst stimulation (TBS; 5 stimulus trains at the interval of 10s, each train consisting of 10 bursts at 5 Hz and each burst consisting of 4 pulses at 100 Hz) induced LTP in the slices of rat visual cortex. Glutamate released from presynaptic sites by TBS activates N-methyl-D-aspartate receptor (NMDAR) at postsynaptic sites, followed by a Ca2+ influx. This activates Ca2+-dependent cPLA2, which then produces arachidonic acid from the membrane lipid substrate, in addition to the activation of Ca2+/calmudulin-dependent protein kinase II (CaMKII). Arachidonic acid is metabolized to PGH2 by COX-2 that has been activated by TBS concomitantly (Akaneya and Tsumoto, 2006). This action is mediated via EP2 at the postsynaptic sites in neurons of the visual cortex, which is inconsistent with the previous study in the hippocampus. The two studies depend mainly on the electrophysiological approach and the immunocytochemical analysis of the pre- and postsynaptic marker proteins, synaptophysin and postsynaptic density protein-95 (PSD-95), respectively. PGE2 increased the synaptic stimulus-evoked amplitudes of EPSPs in hippocampal slices and the frequency of miniature excitatory postsynaptic currents (mEPSCs) in hippocampal neurons in culture (Sang et al. 2005). In contrast, PGE2 has no significant effects on the paired-pulse ratio in the visual cortex slices or on the release of glutamate from synaptoneurosomes of the visual cortex (Akaneya and Tsumoto, 2006). Immunocytochemical analysis of the hippocampal neurons in culture revealed that EP2 highly colocalized with synaptophysin (71.0%), but partially with PSD-95 (14.2%), whereas EP3 partially colocalized with PSD-95 (45.3%) or synaptophysin (38.7%) (Zhu et al. 2005). The former and the other results suggest the pre- and postsynaptic actions of PGE2, respectively. In cultures of neurons from the visual cortex, however, both EP2 and EP3 are highly colocalized with PSD-95 (EP2, 71%; EP3, 82%), but less colocalized with synaptophysin (EP2, 25%; EP3, 13%) (Akaneya and Tsumoto, 2006). The difference may be due to the subcellular location of EP2, i.e. at the presynaptic site in the hippocampus and at the postsynaptic site in visual cortex. Moreover, both studies showed that EP2 and EP3, but not EP1 or EP4, are abundantly expressed in the hippocampus or visual cortex. This suggests the implication of EP2 and EP3 in the physiological functions of these regions of the brain. Another common point between these two studies is that PGE2 is synthesized at the postsynaptic sites by COX-2. Other papers have reported various findings about neuronal and synaptic functions which are common to the hippocampus and visual cortex. Further investigation, such as electron microscopic studies of the subcellular distribution of EP receptor subtypes, especially for EP2, is necessary in order to resolve this inconsistency.
A Hypothesis: Bidirectional Trafficking of PGE2 Receptor Subtypes
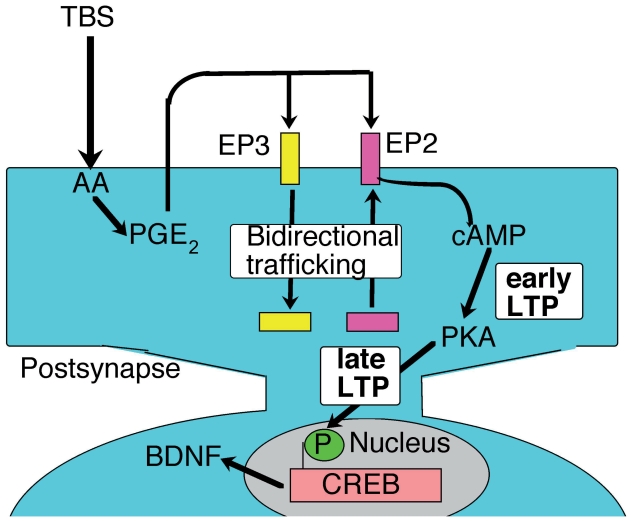
LTP comprises early- and late-LTP (E-LTP and L-LTP, respectively) (Frey et al. 1993; Abel et al. 1997; Huang et al. 2004). E-LTP lasts for a few hours after its initiation, and L-LTP lasts for hours, days or even months after the end of E-LTP. As mentioned above, we have shown that PGE2 increases the magnitude of both E- and L-LTP in the visual cortex (Akaneya and Tsumoto, 2006). This involves interesting and efficient trafficking of EP2 and EP3, both of which are located at the postsynaptic sites in the neurons of the visual cortex. We propose the following to account for the mechanism of PGE2-related LTP. In the stationary state, EP2 and EP3 are mainly located in the cytosol and at the membrane, respectively (Fig. 1). The TBS that induces LTP causes bidirectional trafficking of EP2 and EP3 into the membrane and the cytosol, respectively, which results in an increase in EP2 and a decrease in EP3 at the membrane. Simultaneously, TBS produces PGE2 by COX-2. PGE2-stimulation of EP2 or EP3 induces an increase or a decrease in the level of cAMP, respectively, in the cytosol via adenyl cyclase (Jumblatt and Paterson, 1991; Sugimoto et al. 1992). This shift in the composition of the PGE2 receptor subtypes at the membrane, i.e. the predominant location of EP2, can easily induce the production of a large amount of cAMP in the cytosol. Consequently, cAMP activates cAMP-dependent protein kinase (PKA), which may in turn activate cAMP response element binding protein (CREB), a transcription factor, in the nucleus. The maintenance of L-LTP depends on protein synthesis (Frey et al. 1993; Abel et al. 1997; Huang et al. 2004). This new protein synthesis resulting from the activation of CREB may therefore be involved in L-LTP. It is known that synaptogenesis occurs during L-LTP, and these newly synthesized proteins may be used for the formation of synapses.
Figure 1.
Hypothetical mechanism of PGE2-mediated LTP in the visual cortex. TBS produces arachidonic acid (AA) from the membrane lipid substrate. AA is metabolized to PGH2 by COX-2 that has been activated concomitantly by TBS. PGH2 is converted immediately to PGE2 by PGE2 synthase. EP2 translocates from the cytosol to the membrane, simultaneously, with the translocation of EP3 from the membrane to the cytosol. The PGE2 that is generated spreads from postsynaptic sites into the synaptic cleft, where it activates EP2 at the postsynaptic membrane, resulting in the production of cAMP. Subsequently, cAMP activates PKA, which in turn activates CREB in the nucleus of postsynaptic cells in the visual cortex. This activation of CREB may induce the synthesis of proteins such as BDNF, which is involved in the L-LTP.
A pharmacological analysis revealed that CaMKII may be involved in the trafficking of EP2 from the cytosol to the membrane, but the mechanism of EP3-trafficking is unknown (Akaneya and Tsumoto, 2006). The dynamic force causing this trafficking might be provided by as yet unknown EP2- and EP3-interacting proteins, similar to the AMPA receptor-interacting proteins involved in the so-called silent synapse mechanisms (Harris and Lim, 2001; Barry and Ziff, 2002; Collingridge et al. 2004). This bidirectional trafficking of EP2 and EP3 is similar to the trafficking of AMPA receptors in LTP.
A candidate for the product formed as a consequence of the activation of CREB is brain-derived neurotrophic factor (BDNF). Our previous work has shown that BDNF increases the efficacy of synaptic transmission and TBS-induced LTP in the visual cortex (Akaneya et al. 1997). Moreover, quantitative PCR analysis has shown that TBS induces the generation of BDNF mRNA in the visual cortex (Akaneya and Tsumoto, 2006). Taking these results together, we surmise that PGE2 has a direct action on E-LTP and an indirect action on L-LTP via the production of BDNF.
A Hypothesis: A Candidate for “Post-to-Postsynaptic Messenger”
The induction of LTP requires the activation of NMDA receptor at the postsynaptic cells. The maintenance of LTP requires, at least in part, a change in presynaptic function. In other words, some known or unknown factors, the so-called retrograde messengers, must be transmitted from the postsynaptic to the presynaptic cells, where they must affect presynaptic functions. Many candidates for these retrograde messengers have been proposed (for review, see Williams, 1996). It is possible to say that in the hippocampus PGE2 works as a retrograde messenger, because PGE2 is produced at the postsynaptic site and then diffuses across the postsynaptic membrane to the presynaptic site where EP2 is distributed (Chen et al. 2005; Sang et al. 2005; Zhu et al. 2005). In the visual cortex, on the other hand, it is possible to say that, as a new conception, PGE2 works as a “post-to-postsynaptic messenger,” because PGE2 is produced at the postsynaptic site and then passes across the membrane into the synaptic cleft where PGE2 activates EP2 at the postsynaptic membrane (Akaneya and Tsumoto, 2006).
Conclusion
PGE2 is generated from membrane phospholipids, which is ubiquitous in the central nervous systems. The prostanoids that is produced from this source is not only PGE2. Therefore, it is important to regulate the production of PGE2 for the maintenance and successful accomplishment of function. Here I want to propose “an intricate machinery” of neurons to yield the energy that is necessary for ordinary synaptic functions. Bidirectional trafficking of the two subtypes, EP2 and EP3, which have reciprocal actions is one of the ways by which synaptic functions may be regulated. In future, further elucidation of the mechanisms of action of PGE2 in the synaptic plasticity, in combination with new methods for introducing agents into the restricted brain regions, such as RISLE, may come to be useful for developing new therapies for psychoneurological diseases such as dementia and cerebrovascular diseases.
Acknowledgement
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
References
- Abel T, Nguyen PV, Barad M, et al. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–26. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Akaneya Y, Tsumoto T, Kinoshita S, et al. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J Neurosci. 1997;17:6707–16. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaneya Y, Jiang B, Tsumoto T. RNAi-induced gene silencing by local electroporation in targeting brain region. J Neurophysiol. 2005;93:594–602. doi: 10.1152/jn.00161.2004. [DOI] [PubMed] [Google Scholar]
- Akaneya Y, Tsumoto T. Bidirectional trafficking of prostaglandin E2 receptors involved in long-term potentiation in visual cortex. J Neurosci. 2006;26:10209–21. doi: 10.1523/JNEUROSCI.3028-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audoly LP, Tilley SL, Goulet J, et al. Identification of specific EP receptors responsible for the hemodynamic effects of PGE2. Am J Physiol. 1999;277:H924–30. doi: 10.1152/ajpheart.1999.277.3.H924. [DOI] [PubMed] [Google Scholar]
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–86. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Burks SR, Wright CL, McCarthy MM. Exploration of prostanoid receptor subtype regulating estradiol and prostaglandin E(2) induction of spinophilin in developing preoptic area neurons. Neroscience. 2007;146:1117–1127. doi: 10.1016/j.neuroscience.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candelario-Jalil E, Slawik H, Ridelis I, et al. Regional distribution of the prostaglandin E2 receptor EP1 in the rat brain: accumulation in Purkinje cells of the cerebellum. J Mol Neurosci. 2005;27:303–10. doi: 10.1385/JMN:27:3:303. [DOI] [PubMed] [Google Scholar]
- Chen C, Bazan NG. Lipid signaling: sleep, synaptic plasticity, and neuroprotection. Prostaglandins Other Lipid Mediat. 2005;77:65–76. doi: 10.1016/j.prostaglandins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JTR, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–62. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–4. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Harris BZ, Lim WA. Mechanism and role of PDZ domains in signaling complex assembley. J Cell Sci. 2001;114:3219–31. doi: 10.1242/jcs.114.18.3219. [DOI] [PubMed] [Google Scholar]
- Huang YY, Pittenger C, Kandel ER. A form of long-lasting, learning-related synaptic plasticity in the hippocampus induced by heterosynaptic low-frequency pairing. Proc Natl Acad Sci USA. 2004;101:859–64. doi: 10.1073/pnas.2237201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumblatt MM, Paterson CA. Prostaglandin E2 effects on corneal endothelial cyclic adenosine monophosphate synthesis and cell shape are mediated by a receptor of the EP2 subtype. Invest Ophthalmol Vis Sci. 1991;32:360–5. [PubMed] [Google Scholar]
- Katori M, Hori Y, Uchida Y, et al. Different modes of interaction of bradykinin with prostaglandins in pain and acute inflammation. Adv Exp Med Biol. 1986;198:393–8. doi: 10.1007/978-1-4757-0154-8_50. [DOI] [PubMed] [Google Scholar]
- Katsuyama M, Ikegami R, Karahashi H, et al. Characterization of the LPS-stimulated expression of EP2 and EP4 prostaglandin E receptors in mouse macrophage-like cell line, J774.1. Biochem Biophys Res Commun. 1998;251:727–31. doi: 10.1006/bbrc.1998.9540. [DOI] [PubMed] [Google Scholar]
- Kennedy CR, Zhang Y, Brandon S, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5:217–20. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- Li X, Okada Y, Pilbeam CC, et al. Knockout of the murine prostaglandin EP2 receptor impairs osteoclastogenesis in vitro. Endocrinology. 2000;141:2054–61. doi: 10.1210/endo.141.6.7518. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Hamberger A, Hedner T. Effects of prostaglandin E2 and capsaicin on behavior and cerebrospinal fluid amino acid concentrations of unanesthetized rats: a microdialysis study. J Neurochem. 1995;65:2185–93. doi: 10.1046/j.1471-4159.1995.65052185.x. [DOI] [PubMed] [Google Scholar]
- Milton AS, Wendlandt SA. A possible role for prostaglandin E1 as a modulator for temperature regulation in the central nervous system of the cat. J Physiol. 1970;207:76–7. [PubMed] [Google Scholar]
- Miyaura C, Inada M, Suzawa T, et al. Impaired bone resorption to prostaglandin E2 in prostaglandin E receptor EP4-knockout mice. J Biol Chem. 2000;275:19819–23. doi: 10.1074/jbc.M002079200. [DOI] [PubMed] [Google Scholar]
- Murata T, Ushikubi F, Matsuoka T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–82. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Inv. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann E, Kakorin S, Toensing K. Fundamentals of electroporative delivery of drugs and genes. Bioelectrochem Bioenerg. 1999;48:3–16. doi: 10.1016/s0302-4598(99)00008-2. [DOI] [PubMed] [Google Scholar]
- Oida H, Namba T, Sugimoto Y, et al. In situ hybridization studies of prostacyclin receptor mRNA expression in various mouse organs. Br J Pharmacol. 1995;116:2828–37. doi: 10.1111/j.1476-5381.1995.tb15933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher GM, Henry JL. NSAID-induced cyclooxygenase inhibition differentially depresses long-lasting versus brief synaptically-elicited responses of rat spinal dorsal horn neurons in vivo. Pain. 1995;82:173–86. doi: 10.1016/S0304-3959(99)00050-0. [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Tanaka K, Suda M, et al. Crucial involvement of the EP4 subtype of prostaglandin E receptor in osteoclast formation by proinflammatory cytokines and lipopolysaccharide. J Bone Miner Res. 2000;15:218–27. doi: 10.1359/jbmr.2000.15.2.218. [DOI] [PubMed] [Google Scholar]
- Saper CB, Breder CD. The neurologic basis of fever. N Engl J Med. 1994;330:1880–6. doi: 10.1056/NEJM199406303302609. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Marcheselli V, et al. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci. 2005;25:9858–70. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab JM, Schluesener HJ, Meyermann R, et al. COX-3 the enzyme and the concept: steps towards highly specialized pathways and precision therapeutics? Prostaglandins Leukot Essent Fatty Acids. 2003;69:339–43. doi: 10.1016/j.plefa.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Namba T, Honda A, et al. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. J Biol Chem. 1992;267:6463–6. [PubMed] [Google Scholar]
- Sugimoto Y, Shigemoto R, Namba T, et al. Distribution of the messenger RNA for the prostaglandin E receptor subtype EP3 in the mouse nervous system. Neuroscience. 1994;62:919–28. doi: 10.1016/0306-4522(94)90483-9. [DOI] [PubMed] [Google Scholar]
- Tilley SL, Audoly LP, Hicks EH, et al. Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J Clin Invest. 1999;103:1539–45. doi: 10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushikubi F, Segi E, Sugimoto Y, et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–4. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, et al. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–86. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Watabe A, Sugimoto Y, Honda A, et al. Cloning and expression of cDNA for a mouse EP1 subtype of prostaglandin E receptor. J Biol Chem. 1993;268:20175–8. [PubMed] [Google Scholar]
- Watanabe K, Kawamori T, Nakatsugi S, et al. Role of the prostaglandin E receptor subtype EP1 in colon carcinogenesis. Cancer Res. 1999;59:5093–6. [PubMed] [Google Scholar]
- Williams JH. Retrograde messengers and long-term potentiation: a progress report. J Lipid Mediat Cell Signal. 1996;14:331–9. doi: 10.1016/0929-7855(96)00542-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guan Y, Schneider A, et al. Characterization of murine vasopressor and vasodepressor prostaglandin E(2) receptors. Hypertension. 2000;35:1129–34. doi: 10.1161/01.hyp.35.5.1129. [DOI] [PubMed] [Google Scholar]
- Zhu P, Genc A, Zhang X, et al. Heterogeneous expression and regulation of hippocampal prostaglandin E2 receptors. J Neurosci Res. 2005;81:817–26. doi: 10.1002/jnr.20597. [DOI] [PubMed] [Google Scholar]