Abstract
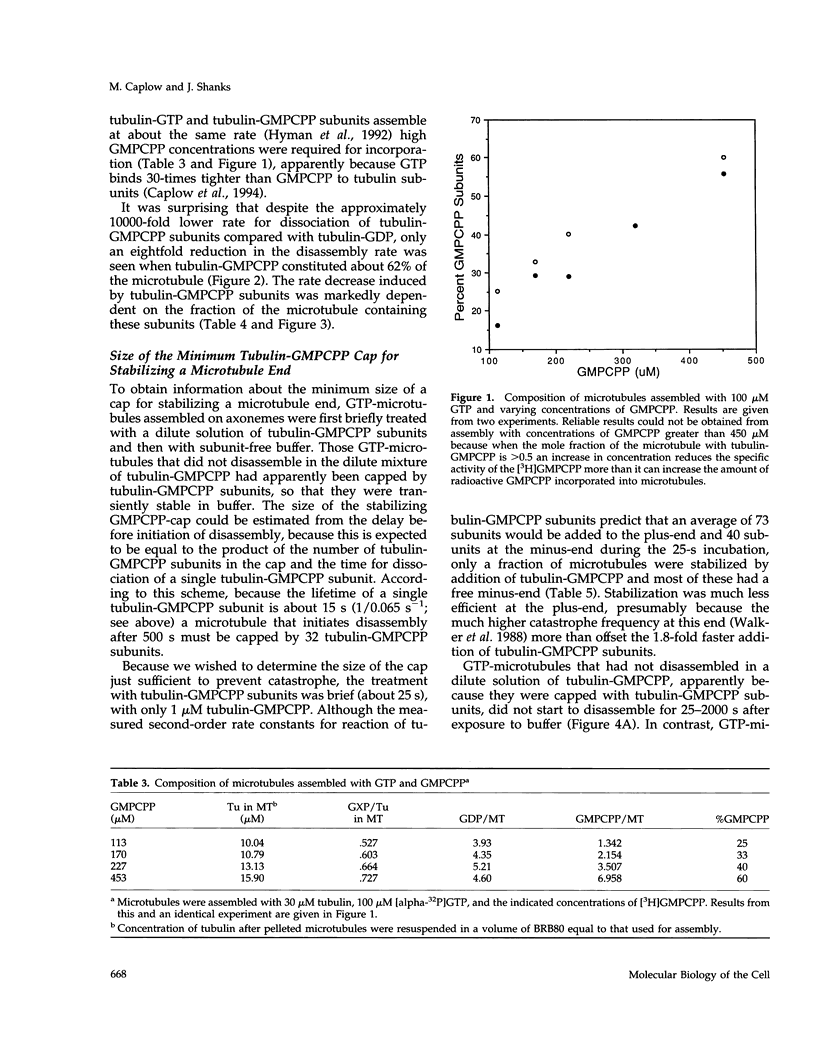
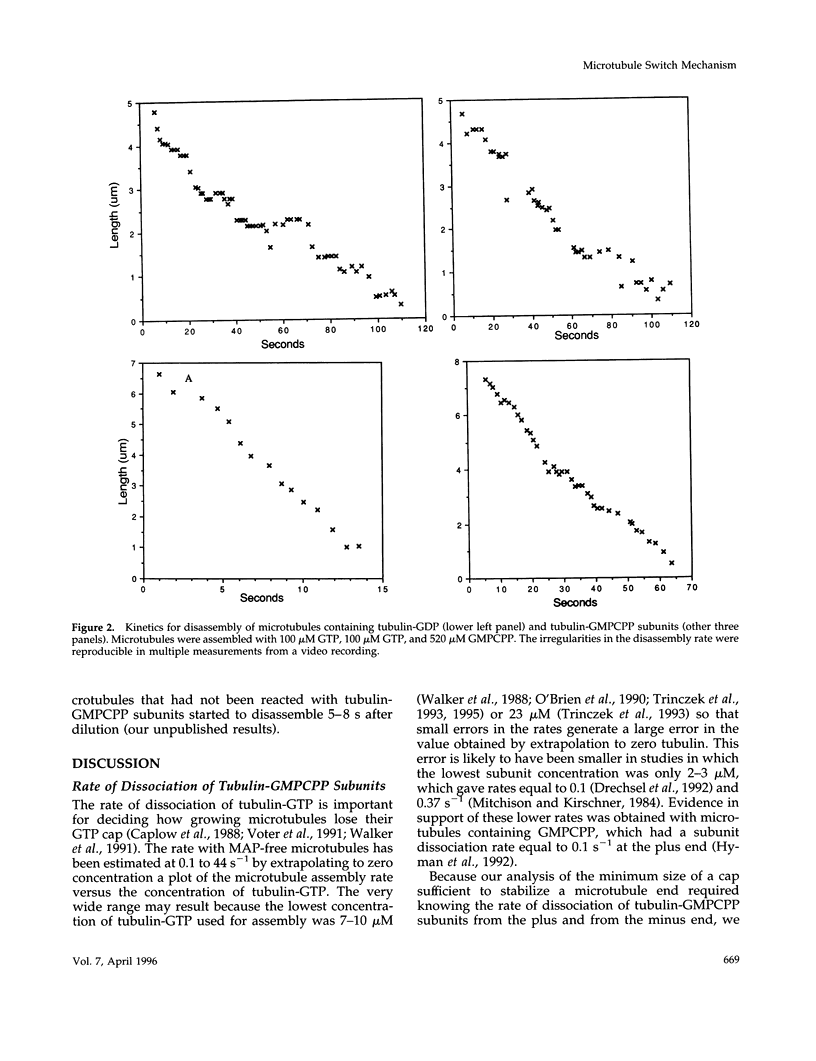
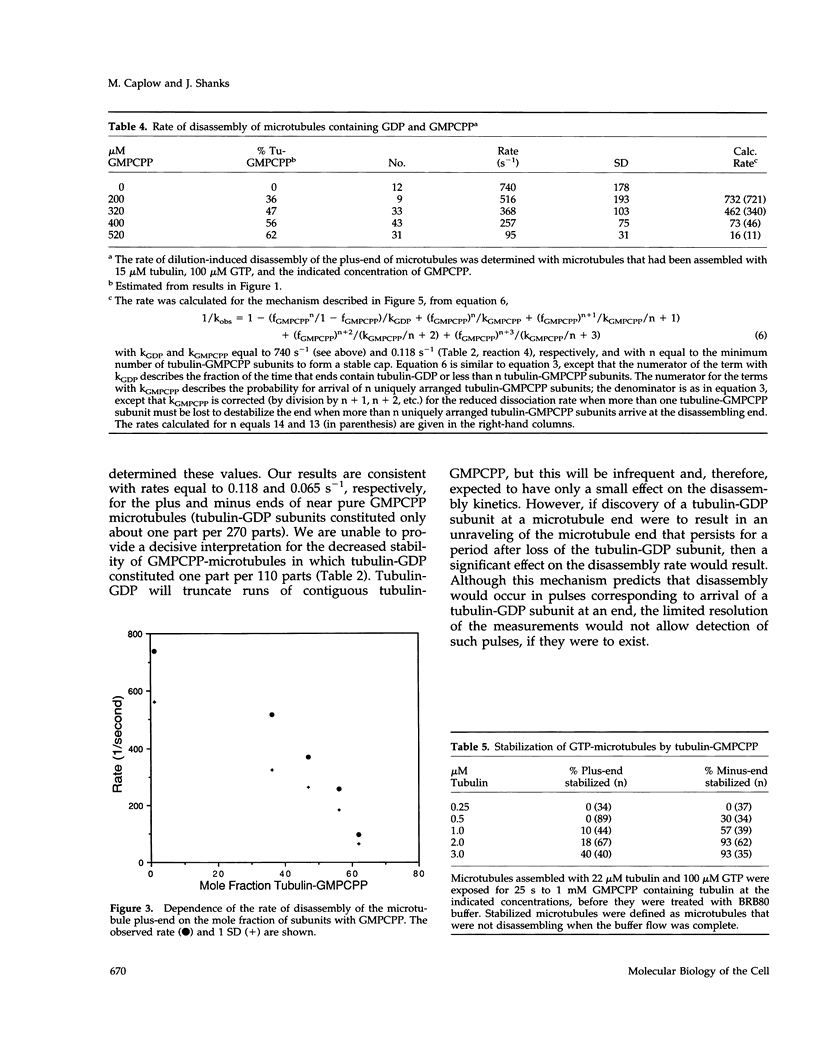
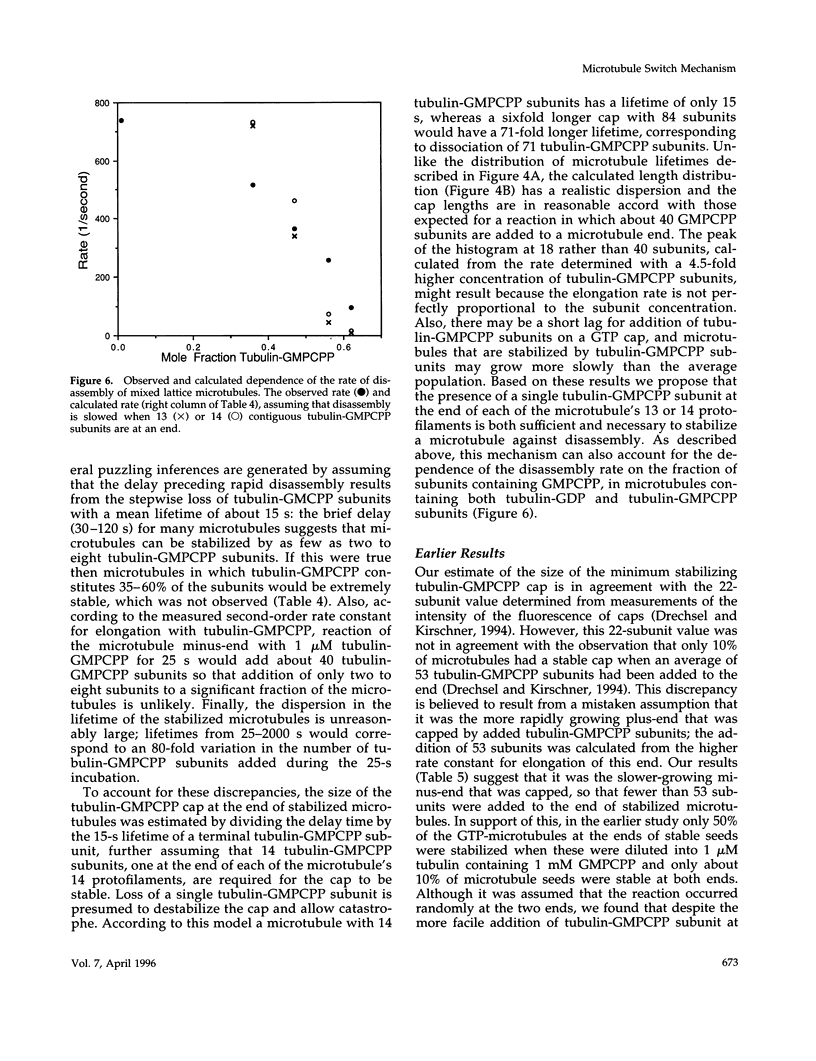
Evidence that 13 or 14 contiguous tubulin-GTP subunits are sufficient to cap and stabilize a microtubule end and that loss of only one of these subunits results in the transition to rapid disassembly(catastrophe) was obtained using the slowly hydrolyzable GTP analogue guanylyl-(a,b)-methylene-diphosphonate (GMPCPP). The minus end of microtubules assembled with GTP was transiently stabilized against dilution-induced disassembly by reaction with tubulin-GMPCPP subunits for a time sufficient to cap the end with an average 40 subunits. The minimum size of a tubulin-GMPCPP cap sufficient to prevent disassembly was estimated from an observed 25- to 2000-s lifetime of the GMPCPP-stabilized microtubules following dilution with buffer and from the time required for loss of a single tubulin-GMPCPP subunit from the microtubule end (found to be 15 s). Rather than assuming that the 25- to 2000-s dispersion in cap lifetime results from an unlikely 80-fold range in the number of tubulin-GMPCpP subunits added in the 25-s incubation, it is proposed that this results because the minimum stable cap contains 13 to 14 tubulin-GMPCPP subunits. As a consequence, a microtubule capped with 13-14 tubulin-GMPCPP subunits switches to disassembly after only one dissociation event (in about 15 s), whereas the time required for catastrophe of a microtubule with only six times as many subunits (84 subunits) corresponds to 71 dissociation events (84-13). The minimum size of a tubulin-GMPCPP cap sufficient to prevent disassembly was also estimated with microtubules in which a GMPCPP-cap was formed by allowing chance to result in the accumulation of multiple contiguous tubulin-GMPCPP subunits at the end, during the disassembly of microtubules containing both GDP and GMPCPP. Our observation that the disassembly rate was inhibited in proportion to the 13-14th power of the fraction of subunits containing GMPCPP again suggests that a minimum cap contains 13-14 tubulin-GMPCPP subunits. A remeasurement of the rate constant for dissociation of a tubulin-GMPCPP subunit from the plus-end of GMPCPP microtubules, now found to be 0.118 s-1, has allowed a better estimate of the standard free energy for hydrolysis of GMPCPP in a microtubule and release of Pi: this is +0.7 kcal/mol, rather than -0.9 kcal/mol, as previously reported.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley P. M., Schilstra M. J., Martin S. R. Microtubule dynamic instability: numerical simulation of microtubule transition properties using a Lateral Cap model. J Cell Sci. 1990 Jan;95(Pt 1):33–48. doi: 10.1242/jcs.95.1.33. [DOI] [PubMed] [Google Scholar]
- Belmont L. D., Hyman A. A., Sawin K. E., Mitchison T. J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990 Aug 10;62(3):579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Caplow M. Microtubule dynamics. Curr Opin Cell Biol. 1992 Feb;4(1):58–65. doi: 10.1016/0955-0674(92)90059-l. [DOI] [PubMed] [Google Scholar]
- Caplow M., Ruhlen R. L., Shanks J. The free energy for hydrolysis of a microtubule-bound nucleotide triphosphate is near zero: all of the free energy for hydrolysis is stored in the microtubule lattice. J Cell Biol. 1994 Nov;127(3):779–788. doi: 10.1083/jcb.127.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplow M., Ruhlen R., Shanks J., Walker R. A., Salmon E. D. Stabilization of microtubules by tubulin-GDP-Pi subunits. Biochemistry. 1989 Oct 3;28(20):8136–8141. doi: 10.1021/bi00446a026. [DOI] [PubMed] [Google Scholar]
- Caplow M., Shanks J. Induction of microtubule catastrophe by formation of tubulin-GDP and apotubulin subunits at microtubule ends. Biochemistry. 1995 Dec 5;34(48):15732–15741. doi: 10.1021/bi00048a018. [DOI] [PubMed] [Google Scholar]
- Caplow M., Shanks J. Mechanism of the microtubule GTPase reaction. J Biol Chem. 1990 May 25;265(15):8935–8941. [PubMed] [Google Scholar]
- Caplow M., Shanks J., Ruhlen R. L. Temperature-jump studies of microtubule dynamic instability. J Biol Chem. 1988 Jul 25;263(21):10344–10352. [PubMed] [Google Scholar]
- Drechsel D. N., Hyman A. A., Cobb M. H., Kirschner M. W. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992 Oct;3(10):1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel D. N., Kirschner M. W. The minimum GTP cap required to stabilize microtubules. Curr Biol. 1994 Dec 1;4(12):1053–1061. doi: 10.1016/s0960-9822(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., O'Brien E. T. Microtubule dynamic instability and GTP hydrolysis. Annu Rev Biophys Biomol Struct. 1992;21:145–166. doi: 10.1146/annurev.bb.21.060192.001045. [DOI] [PubMed] [Google Scholar]
- Gliksman N. R., Parsons S. F., Salmon E. D. Okadaic acid induces interphase to mitotic-like microtubule dynamic instability by inactivating rescue. J Cell Biol. 1992 Dec;119(5):1271–1276. doi: 10.1083/jcb.119.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A. A., Salser S., Drechsel D. N., Unwin N., Mitchison T. J. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP. Mol Biol Cell. 1992 Oct;3(10):1155–1167. doi: 10.1091/mbc.3.10.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski R. J., Williams R. C., Jr Unambiguous classification of microtubule-ends in vitro: dynamic properties of the plus- and minus-ends. Cell Motil Cytoskeleton. 1993;26(4):282–290. doi: 10.1002/cm.970260403. [DOI] [PubMed] [Google Scholar]
- Martin S. R., Schilstra M. J., Bayley P. M. Dynamic instability of microtubules: Monte Carlo simulation and application to different types of microtubule lattice. Biophys J. 1993 Aug;65(2):578–596. doi: 10.1016/S0006-3495(93)81091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- O'Brien E. T., Salmon E. D., Walker R. A., Erickson H. P. Effects of magnesium on the dynamic instability of individual microtubules. Biochemistry. 1990 Jul 17;29(28):6648–6656. doi: 10.1021/bi00480a014. [DOI] [PubMed] [Google Scholar]
- Trinczek B., Biernat J., Baumann K., Mandelkow E. M., Mandelkow E. Domains of tau protein, differential phosphorylation, and dynamic instability of microtubules. Mol Biol Cell. 1995 Dec;6(12):1887–1902. doi: 10.1091/mbc.6.12.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinczek B., Marx A., Mandelkow E. M., Murphy D. B., Mandelkow E. Dynamics of microtubules from erythrocyte marginal bands. Mol Biol Cell. 1993 Mar;4(3):323–335. doi: 10.1091/mbc.4.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D. Severing of stable microtubules by a mitotically activated protein in Xenopus egg extracts. Cell. 1991 Feb 22;64(4):827–839. doi: 10.1016/0092-8674(91)90511-v. [DOI] [PubMed] [Google Scholar]
- Verde F., Dogterom M., Stelzer E., Karsenti E., Leibler S. Control of microtubule dynamics and length by cyclin A- and cyclin B-dependent kinases in Xenopus egg extracts. J Cell Biol. 1992 Sep;118(5):1097–1108. doi: 10.1083/jcb.118.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F., Labbé J. C., Dorée M., Karsenti E. Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature. 1990 Jan 18;343(6255):233–238. doi: 10.1038/343233a0. [DOI] [PubMed] [Google Scholar]
- Voter W. A., O'Brien E. T., Erickson H. P. Dilution-induced disassembly of microtubules: relation to dynamic instability and the GTP cap. Cell Motil Cytoskeleton. 1991;18(1):55–62. doi: 10.1002/cm.970180106. [DOI] [PubMed] [Google Scholar]
- Walker R. A., O'Brien E. T., Pryer N. K., Soboeiro M. F., Voter W. A., Erickson H. P., Salmon E. D. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol. 1988 Oct;107(4):1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A., Pryer N. K., Salmon E. D. Dilution of individual microtubules observed in real time in vitro: evidence that cap size is small and independent of elongation rate. J Cell Biol. 1991 Jul;114(1):73–81. doi: 10.1083/jcb.114.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976 Nov;108(1):139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]


