Abstract
Background and Aims
Ethnobotanical studies in Mexico have documented that Mesoamerican peoples practise systems of in situ management of wild and weedy vegetation directed to control availability of useful plants. In situ management includes let standing, encouraging growing and protection of individual plants of useful species during clearance of vegetation, which in some cases may involve artificial selection. The aim of this study was to review, complement and re-analyse information from three case studies which examined patterns of morphological, physiological and genetic effects of artificial selection in plant populations under in situ management in the region.
Methods
Information on wild and in situ managed populations of the herbaceous weedy plants Anoda cristata and Crotalaria pumila, the tree Leucaena esculenta subsp. esculenta and the columnar cacti Escontria chiotilla, Polaskia chichipe and Stenocereus stellatus from Central Mexico was re-analysed. Analyses compared morphology and frequency of morphological variants, germination patterns, and population genetics parameters between wild and managed in situ populations of the species studied. Species of columnar cacti are under different management intensities and their populations, including cultivated stands of P. chichipe and S. stellatus, were also compared between species.
Key Results
Significant differences in morphology, germination patterns and genetic variation documented between wild, in situ managed and cultivated populations of the species studied are associated with higher frequencies of phenotypes favoured by humans in managed populations. Genetic diversity in managed populations of E. chiotilla and P. chichipe is slightly lower than in wild populations but in managed populations of S. stellatus variation was higher than in the wild. However, genetic distance between populations was generally small and influenced more by geographic distance than by management.
Conclusions
Artificial selection operating on in situ managed populations of the species analysed is causing incipient domestication. This process could be acting on any of the 600–700 plant species documented to be under in situ management in Mesoamerica. In situ domestication of plants could be relevant to understand early processes of domestication and current conditions of in situ conservation of plant genetic resources.
Key words: Anoda cristata, Crotalaria pumila, domestication, Escontria chiotilla, in situ management, Leucaena esculenta, Mesoamerica, Polaskia chichipe, Stenocereus stellatus
INTRODUCTION
Mesoamerica, the cultural area between southern Mexico and northern Costa Rica (Matos-Moctezuma, 1994) is one of the areas of the New World where agriculture was first practised (MacNeish, 1967; Harlan, 1975; Flannery, 1986) and one of the main centres of domestication of plants in the world (Vavilov, 1951; Harlan, 1975; Hawkes, 1983). These facts appear to be related to the high diversity of plants and cultures existing in the region. In the Mexican territory only, Rzedowski (1993), Toledo and Ordóñez (1993) and Villaseñor (2003) have estimated the occurrence of 20 000–30 000 species of vascular plants, and according to Toledo (2000) that territory is at present inhabited by peoples of 56 indigenous ethnic groups whose ancestors have occupied the area for 12 000–14 00 years (MacNeish, 1992).
Domestication is a continuous ongoing evolutionary process, acting on incipient and semi-domesticated plants as well as on fully domesticated plants. Currently, Mesoamerican peoples utilize 5000–7000 plant species (Casas et al., 1994; Caballero et al., 1998) and are domesticating > 200 native plant species that coexist with populations of wild relatives occurring in natural ecosystems. Some of them include plant species of worldwide economic importance and advanced degrees of domestication such as maize, Phaseolus beans, chilli peppers, squashes, cocoa, cotton and amaranths, among others. But they also include plant species economically important at a regional level with both intermediate and advanced levels of domestication such as species of Agave, Opuntia, Leucaena, columnar cacti, Chenopodium and Amaranthus. Also, domestication is acting incipiently on plant species of local importance such as the traditional Mesoamerican greens ‘quelites’ of the genera Amaranthus, Chenopodium, Porophyllum, Portulaca, Crotalaria, Anoda, and numerous ornamental species.
Along with the cultivation of plants under a high diversity of agricultural techniques (Rojas, 1991), indigenous peoples in Mexico practise different silvicultural systems involving in situ management of vegetation (Casas et al., 1996, 1997a). These systems, according to Caballero et al. (1998) might involve the management of 600–700 native plant species under some of the following practices.
Systematic gathering. Gathering consists of harvesting useful products from wild and weedy plant populations, and nearly 93 % of the useful plant species recorded in ethnobotanical studies is obtained through this method (Caballero et al., 1998). Traditional gathering in Mexican peasant economies more commonly determines a low impact on vegetation and does not involve real manipulation of plant populations. However, gathering may include incipient forms of systematic management such as selective harvesting of particular phenotypes, rotation of gathering areas, temporary restrictions to exploitation of particular resources, among others, which may have more important consequences on plant communities (see Casas et al., 1996).
-
Let standing. This type of interaction includes practices directed to maintain within human-made environments useful plants that occurred in those areas before the environments were transformed by humans. This management type has been documented in perennial plants such as Opuntia spp. (Colunga-García Marín et al., 1986, Reyes-Agüero, 2005), Leucaena spp. (Casas and Caballero, 1996; Zárate, 1999, Zárate et al., 2005), Prosopis laevigata, Pithecellobium dulce (Casas et al., 1996), columnar cacti (Casas et al., 1997b, 1999a, b; Cruz and Casas, 2002; Arellano and Casas 2003; Carmona and Casas; 2005), Agave spp. (Colunga-García Marín et al., 1996), palms (Caballero, 1994; Martínez-Ballesté et al., 2005) and the sapotaceous tree Sideroxylon palmeri (González-Soberanis and Casas, 2004). Also, this management type has been documented in weedy ‘quelites’ species such as Amaranthus hybridus, Chenopodium spp., Crotalaria pumila, Porophyllum spp. and Portulaca oleracea, among others, and in other weeds with edible fruits such as Jaltomata spp., Solanum nigrum, Physalis philadelphica and Lycopersicon lycopersicum (Davis and Bye, 1982; Caballero and Mapes, 1985; Williams, 1985; Mera, 1987; Vázquez, 1991; Casas et al., 1996).
Barrera et al. (1977), Wiseman (1978), Gómez-Pompa et al. (1987) and Gómez-Pompa (1991) have documented that for centuries the Maya have practised let standing of useful native species such as Manilkara zapota, Pouteria sapota, Annona spp., Brosimum alicastrum, Sabal spp., Casimiroa edulis and Acrocomia mexicana, among others, in sites cleared for cultivation. These authors described artificial jungles (the ‘pet kot’) made by the Maya, which have atypical abundance of individuals of useful species and that are probably the result of this management type.
-
Encouraging growing. This management type includes strategies directed at increasing density of populations of useful species within a plant community. It may be carried out through burning and taming of vegetation which favour particular plant species, or through sowing seeds or planting vegetative propagules of favoured plants within wild or weedy areas. An example of this management type is the management of the palm Brahea dulcis by the Mixtec of Guerrero (Casas et al., 1994, 1996). This palm propagates vegetatively and is resistant to fire. People remove trees and burn the remaining vegetation in order to eliminate competitors and to enhance the growth of palm populations. A similar principle is used by the Mixtec to promote grass growing for cattle.
It is common in Mesoamerican cultures to encourage useful plants to grow within fallow agricultural fields. Examples of this management type have been documented by Casas et al. (1994, 1997a) among the Mixtec, Nahua and Popoloca of Central Mexico, by Lundell (1937), Puleston (1982), Illsley (1984) and Gómez-Pompa (1991) among the Maya, by Nigh and Nations (1983) among the Lacandon, by Alcorn (1983, 1984) among the Huastec, and by Medellín (1988) among the Totonac. This form of management has apparently influenced the process of vegetation regeneration and therefore it has probably also contributed to the formation of artificial jungles and other artificial vegetation communities. Also, it is common practice to intentionally scatter seeds of useful weedy plants within agricultural fields to increase their abundance. Examples have been documented with Amaranthus hybridus, Anoda cristata, Crotalaria pumila, Physalis philadelphica and Porophyllum ruderale by Casas et al. (1996) and Mapes et al. (1996).
Protection. This includes the deliberate elimination of competitors and predators of useful plants, as well as their pruning, protection against frosts, and addition of fertilizers, to ensure the availability of wild and weedy plants of special value. For instance, Bye (1985) found that during gathering of wild onions, the Tarahumara disperse bulbils of the plants gathered and remove roots of perennial plants near the onions in order to ensure the further availability of onions and to reduce competition, respectively, increasing in this way the numbers of onions in the populations gathered. Casas et al. (1996) found that the Mixtec and the Nahua of the Balsas river basin occasionally prune branches and control pests of individuals with favourable phenotypes of tree species such as Pithecellobium dulce, Psidium spp., Leucaena esculenta subsp. esculenta, Spondias mombin and Byrsonima crassifolia, among others, in both wild and in situ managed populations. Also, these peoples fertilize and protect against frost and pests weedy plants such as Physalis philadelphica, Lycopersicon lycopersicum and Capsicum annuum, which are allowed to stand and occasionally encouraged to grow in agricultural fields.
Some of the studies mentioned above give information suggesting that there are signs that artificial selection could be occurring under in situ management. The intention of selection can be observed during gathering, when people distinguish plants with favourable features and harvest the best products. But more meaningfully, selection acts when people practise let standing, encourage growing and protect individuals with favourable phenotypes. We have hypothesized that morphological, physiological and genetic consequences of artificial selection would be observable in populations on which selection under in situ management has been practised for a long time. Variants favourable to humans are expected to be more abundant in managed in situ populations than in the wild and even more abundant in cultivated populations since cultivation is a more intensive form of manipulation of plants.
This study reanalysed three case studies of plant species under in situ management, some of them also being cultivated by peoples of Central Mexico, to examine the consequences of artificial selection under in situ conditions. In the case of the edible weeds Anoda cristata and Crotalaria pumila, research was directed to evaluate differential abundance of favourable and unfavourable phenotypes within sites under a gradient of management intensity. In the case of Leucaena esculenta subsp. esculenta, morphological differences between wild and managed in situ populations were analysed. And finally, in the case of columnar cacti species, variation of morphological, physiological and genetic aspects was compared between wild, in situ managed and, in some cases, cultivated populations. The species studied are under different management intensities, Escontria chiotilla being under the lowest intensity, Polaskia chichipe under an intermediate degree of intensity, and Stenocereus stellatus under the highest management intensity. Therefore variation was also compared between species, hypothesizing that the degree of divergence between wild, managed in situ and cultivated populations would be proportional to the intensity of management.
MATERIALS AND METHODS
Study systems
The present analysis comprised three study systems, one of which included the herbaceous plants Anoda cristata (Malvaceae) and Crotalaria pumila (Fabaceae), growing wild in disturbed areas of tropical deciduous forest and as weeds in agricultural areas in the La Montaña de Guerrero region (Fig. 1) (Casas et al., 1996, 1997a). These species have perennial woody subterranean parts which sprout during the rainy season, their aerial parts being lost during the dry season. Vegetative propagation in A. cristata plants occurs when pieces of their roots are dispersed in agricultural fields. Young leaves of both plant species are consumed as ‘quelites’, and are among the more appreciated greens in the region – they are sold in the regional markets and the seeds are encouraged to grow in irrigated areas during the dry season (Casas et al., 1996). In both species people distinguish the favourable phenotypes called ‘hembra’ (or ‘female’) variants and the unfavourable phenotypes called ‘macho’ (or ‘male’) variants. Flowers of both variant types are hermaphrodite and the names are not related to their sexuality. ‘Hembra’ variants have broad, glabrous and tender leaves with a better taste than ‘macho’ variants which have smaller, hairy (in the case of A. cristata) and fibrous leaves with a bitter flavour. People gather for consumption only ‘hembra’ variants and during weeding of agricultural fields, people eliminate individuals of the ‘macho’ variants whereas they let individuals of the ‘hembra’ variants stand and encourage them to grow (Casas et al., 1996).
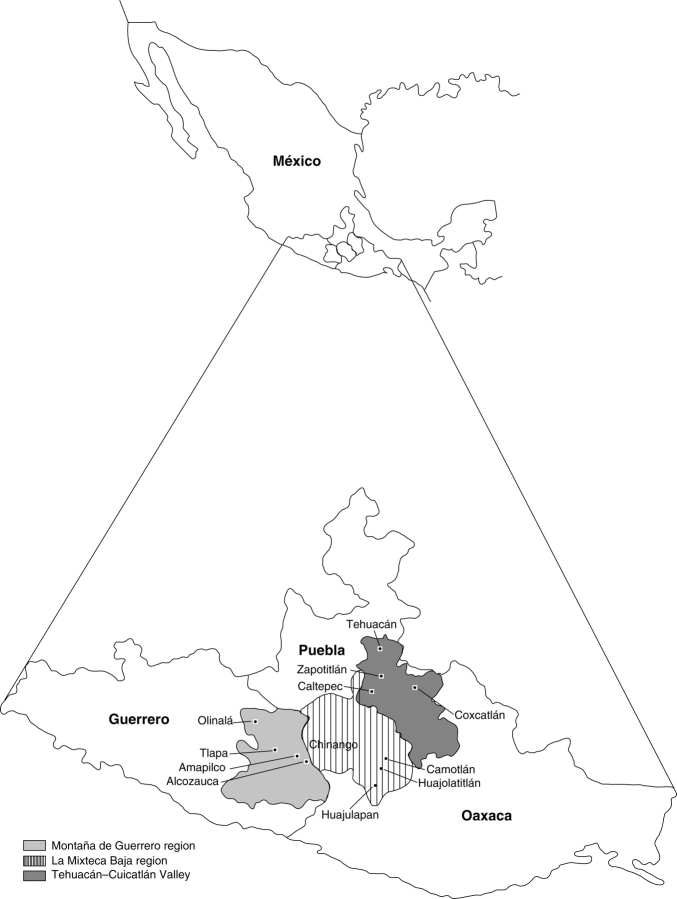
Fig. 1.
Map of the study area giving the location of Montaña de Guerrero, La Mixteca Baja and the Tehuacán-Cuicatlán Valley in central Mexico.
Leucaena esculenta subsp. esculenta (Fabaceae) was also studied in La Montaña de Guerrero region. This species is a self-incompatible perennial plant which is a constituent of tropical deciduous forests. Its buds of leaves and flowers, young pods and, especially, its green and mature seeds are widely appreciated as food by local people, and they have a high commercial value in local and regional markets. This species is cultivated by seed in home gardens (Casas and Caballero, 1996). People distinguish three types of guaje according to their qualities: (1) ‘guaje de vasca’ (vomitive guaje) which is toxic because of its high levels of secondary chemical compounds; (2) ‘guaje amargo’ (bitter guaje) which is slightly toxic but edible after being roasted, presumably with lower levels of secondary chemical compounds than the ‘guaje de vasca’; and (3) ‘guaje dulce’ (sweet guaje) that is edible raw, presumably because it has even lower levels of secondary chemical compounds than the other variants. People eliminate ‘guaje de vasca’ trees to prevent sickness in children, and prefer sweet guajes. Also preferred are guajes with larger pods and seeds. Following these criteria, let standing is practised and individual trees are encouraged to grow in situ and are protected and trees are cultivated in home gardens (Casas and Caballero, 1996).
The columnar cacti Escontria chiotilla, Polaskia chichipe and Stenocereus stellatus occur in the Tehuacán-Cuicatlán Valley and La Mixteca Baja regions (Fig. 1). These are arborescent plants, 2–6 m high, with spherical or ellipsoid fruits which are spiny (except E. chiotilla which has scaly fruits). Fruit peel is generally red when fruits are mature, but in some cultivated variants of S. stellatus it is green (Casas et al. 1997b, 1999b). Fruit pulp is predominantly red in the wild but it may be white, pink, purple, yellow or orange in cultivated variants of S. stellatus, species in which nearly 40 % of cultivated individuals may have fruit pulp which is not red according to Casas et al. (1999b). Fruits of the three species are edible and have commercial value in both local and regional markets. People prefer larger fruits with sweeter pulp and special colours, with fewer spines in the thinner peel, and use these criteria to practice artificial selection during in situ management and cultivation. Sexual reproduction of these species is self-incompatible, but in P. chichipe self-pollination occurs, having a higher frequency in managed populations (Casas et al., 1999c; Cruz and Casas, 2002; Otero-Arnaiz et al., 2003; Oaxaca-Villa et al., 2006). Flowers of S. stellatus have nocturnal anthesis and are pollinated by bats, whereas flowers of P. chichipe and E. chiotilla have diurnal anthesis and are pollinated by bees. Seed dispersal is carried out by birds, bats and humans. Stenocereus stellatus is propagated vegetatively and occurs naturally when branches fall down. People take advantage of this property for cultivating the plant (Casas et al., 1997b). Vegetative propagation does not occur naturally in P. chichipe but people occasionally manage to propagate branches of this species in their agricultural fields and home gardens. Management intensity is therefore lower than in S. stellatus. Vegetative propagation does not occur naturally or artificially in E. chiotilla and this species is not cultivated and, therefore it is under lower management intensity than P. chichipe.
Study area
La Montaña de Guerrero region is located in the north-east of the state of Guerrero, Central Mexico, within the Balsas river region (Fig. 1). This area is characterized by a complex mountainous landscape ranging from 600 m to nearly 3000 m a.s.l., the annual mean temperature being 22 °C and the annual rainfall being, on average, 760 mm (Casas et al., 1994). Vegetation includes thorn scrub and tropical dry forests in the lower dry areas (from 600 m to nearly 1400 m), oak forests in temperate areas from 1400 m to 1900 m, and pine forests, cloud forests and Abies forest in the higher wet temperate areas. In this region live the Mixtec, Nahua, Tlapanec and Amuzgo peoples, but the present study comprised the municipalities of Alcozauca, Tlapa, Olinalá and Temalacacingo, inhabited by the Mixtec and Nahua peoples. The Mixteca Baja region covers a similar area, mainly in the states of Puebla and Oaxaca (Fig. 1) inhabited by the Mixtec and Nahua peoples. Elevations range from 700 m to 2600 m. Climate and vegetation are similar to those described for the neighbouring Montaña de Guerrero region, and are predominately thorn-scrub and tropical dry forests.
The Tehuacán-Cuicatlán Valley is located in the south-east of the state of Puebla and the north-east of Oaxaca (Fig. 1), within the Papaloapan river basin. It is a region approx. 10 000 km2 in extent with a gradient of elevations from 600 m to 2800 m. It consists of a system of internal valleys surrounded by mountains. The climate is predominantly semi-arid with an average rainfall of 400 mm and an annual mean temperature of 21 °C (Dávila et al., 2002). The dry valleys and mountains are covered by a great variety of associations of thorn scrub and tropical dry forests, where columnar cacti are the dominant species. Temperate mountainous areas are also covered with a high variety of oak and pine forests (Valiente-Banuet et al., 2000).
Populations studied
Densities of populations of Anoda cristata and Crotalaria pumila sampled in Alcozauca, Guerrero in a previous study (Casas et al., 1997a) were reanalysed. Populations were sampled at five sites in each of the following habitats: (1) gaps in tropical deciduous forest; (2) fallow corn fields cultivated previously under seasonal regime; (3) corn fields under seasonal agriculture; (4) corn fields under irrigated agriculture. These habitats were considered to represent a gradient from low to high management intensity. The number of individuals of ‘macho’ and ‘hembra’ variants of each species was counted in 50 m2 squares. Three squares were sampled per site and the numbers of individual plants per variant type were averaged and extrapolated to estimate the number of individuals of each variant type per hectare. To determine whether the environmental type affected the abundance of ‘macho’ and ‘hembra’ variants, sampling data for each species were reanalysed through generalized linear models applying the GENMOD procedure (SAS, 2000). The model used variant type, habitat and the interaction term as categorical independent variables. The dependent variable was density of individuals per hectare. A Poisson distribution was used for the analyses, using a logarithmic link function.
The case study of Leucaena esculenta subsp. esculenta was partially based on the morphological and population genetics studies by Casas and Caballero (1996) and Zárate et al. (2005), respectively, in populations from Alcozauca, Guerrero, but unpublished information of samples of populations from Amapilca, Guerrero was included. Morphological variation of samples of 20 individuals of two wild and two in situ managed populations (80 individuals from four populations in total) was analysed. Wild populations were part of tropical deciduous forests with elevations ranging from 1300 m to 1650 m in the population of Alcozauca (near the village of San José Laguna), and 1250 m to 1500 m in the Amapilca population. Densities of guaje trees were 15 and 18 individuals per hectare in Alcozauca and Amapilca, respectively. The in situ managed populations were originally wild and they are composed of individuals that have been selectively spared after many cycles of forest clearance involved in the shifting cultivation of maize. Elevation of the in situ managed population of Alcozauca ranges from 1250 m to 1700 m and the guaje population density was 0·8 individuals per hectare, whereas the managed in situ population of Amapilca ranged from 1220 m to 1600 m and the density was 1·2 individuals of guaje trees per hectare. Samples of ten pods per tree and their seeds were analysed. Dimensions of pods and seeds and the number of ovules, seeds and seeds predated by bruchids were measured and counted, respectively. Principal component analysis (PCA) was used to analyse the pattern of morphological similarity among individuals. The NTSYS program version 2·0 (Rohlf, 1993) was used. Multivariate morphological differences were tested between populations through discriminant function analysis (DFA) by using SYSTAT Version 11 (SYSTAT, 2004). One-way ANOVAs were used to test differences in morphological features between populations.
The case study of columnar cacti was based on information generated by Arellano and Casas (2003) and Tinoco et al. (2005) for E. chiotilla, by Carmona and Casas (2005) and Otero-Arnaiz et al. (2003, 2005a, b) for P. chichipe, and by Casas et al. (1999b, 2006) for S. stellatus. Those studies analysed patterns of variation in samples of 20–50 individuals of at least three wild (from thorn scrub and tropical dry forests), three in situ managed (areas recurrently cleared for agriculture) and three cultivated (home gardens in villages) populations. A total of 29, 30 and 17 morphological characters of fruits, flowers and vegetative parts were analysed in Escontria chiotilla, Polaskia chichipe and S. stellatus, respectively. Populations were those previously analysed by the authors mentioned, but new samples of individuals of populations of S. stellatus from La Mixteca Baja region were included. General morphological similarity of wild, in situ managed and cultivated individual plants was analysed per species through PCA (using NTSYS 2·0), and patterns of divergence between these groups of individuals were compared between species. One-way ANOVAs were performed per morphological character between all wild and managed in situ individuals studied to test general differences related to management type. Flower buds or branch tissue were collected in the individuals studied for genetic analysis. All species were studied through isozyme analysis (for details of methods, see Lucio, 2005; Tinoco et al., 2005; Casas et al., 2006) and P. chichipe was also studied through microsatellites (for details of methods, see Otero-Arnaiz et al. 2004, 2005a, b). Germination percentage and rate were analysed between wild, managed in situ and cultivated populations of the species studied under similar conditions of temperature (constant 25 °C), photoperiod (12 h of white fluorescent light) and humidity (1 % agar in distilled water), controlled in a growth chamber.
RESULTS
Case study 1: the herbaceous ‘quelites’
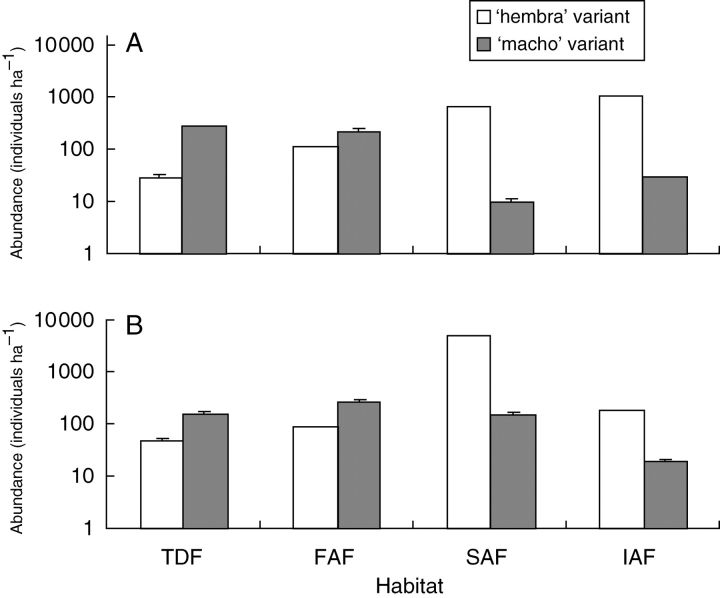
Figure 2 shows that according to analyses from the generalized linear model, significant differences in abundance of ‘hembra’ and ‘macho’ variants of both species were found within and between all habitats sampled. Individuals of the ‘macho’ variants of both species were significantly more abundant than individuals of the ‘hembra’ variant in the perturbed wild vegetation, as well as in fallow agricultural fields, where selective elimination of ‘macho’ variants and let standing of ‘hembra’ variants does not occur. In contrast, in the communities of weedy plants of cultivated fields individuals of the ‘hembra’ variants were much more abundant than individuals of the ‘macho’ variants of both species.
Fig. 2.
Least square means (LSM) obtained from generalized linear model (GENMOD procedure SAS, 200) for total number of individual plants per hectare (in logarithmic scale) of the variants ‘hembra’ (white columns) and ‘macho’ (shaded columns) of Anoda cristata and Crotalaria pumila in different habitats (TD, tropical dry forest; FAF, fallow agricultural fields; SAF, seasonal agricultural fields; IAF, irrigated agricultural fields) in La Montaña de Guerrero, Central Mexico. The error bar represents standard errors; some of them are not evident because they were small. For Anoda cristata, in the analysis for habitat F3,32 = 22·81, P < 0·0001, for variant type F1,32 = 198·43, P < 0·0001, and for the interaction between habitat and variant type F3,32 = 724·23, P < 0·0001. For Crotalaria pumila in the analysis for habitat F3,32 = 33·51, P < 0·0001, for variant type F1,32 = 12·04, P < 0·0015, and for the interaction between habitat and variant type F3,32 = 60·54, P < 0·0001.
Case study 2: the guaje tree
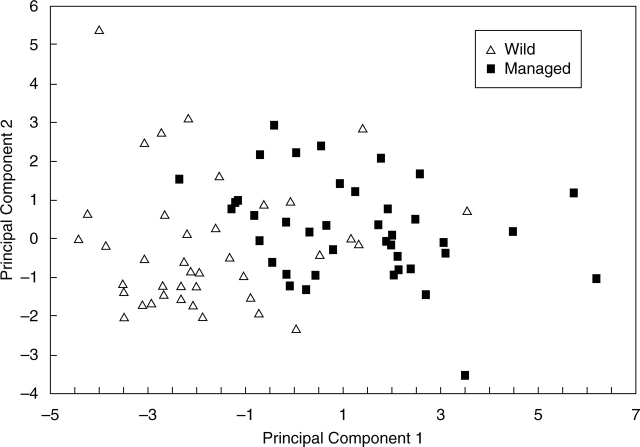
Table 1 indicates that dimensions of seeds, seed chambers and pods, as well as the number of predated seeds clearly vary between populations according to management type. Seeds and pods were significantly larger in managed in situ populations which also had significantly more predated seeds. The number of locules was not significantly different and differences in seed number (higher in the managed in situ population of Amapilca) appear to be related to pollination success to form seeds. According to PCA (Fig. 3), most of the managed in situ and wild individuals are grouped among themselves mainly by the first principal component. Eigenvectors shown in Table 2 indicate that variables related to seed and pod dimensions and number of predated seeds are the ones which are most important to explain the distribution of the individuals within the space of the first principal component, individuals of the managed in situ populations (with positive values) having larger seeds and pods, their seeds being more susceptible to bruchid attack. But ‘wild’ and ‘managed in situ’ groups are not discrete. Some wild individuals are morphologically similar to the ‘managed in situ’ group and vice versa. Table 3 indicates that there are significant differences between all populations, and that the expected wild and managed in situ groups classified by DFA have more overlaps with the actual wild and managed in situ groups, respectively, indicating a clear morphological identity of populations according to management.
Table 1.
Mean ± s.e. of morphological characters of pods and seeds of Leucaena esculenta subsp. esculenta in individuals of wild and managed in situ populations in Alcozauca (populations Wild 1 and Managed in situ 1) and Amapilca (populations Wild 2 and Managed in situ 2) in la Montaña de Guerrero, Mexico
| Population |
|||||
|---|---|---|---|---|---|
| Character | Wild 1 | Wild 2 | Managed 1 | Managed 2 | P |
| Seed length (cm) | 0·795 ± 0·018a | 0·755 ± 0·018a | 0·894 ± 0·018b | 0·896 ± 0·018b | < 0·0001 |
| Seed width (cm) | 0·595 ± 0·018a | 0·564 ± 0·018a | 0·728 ± 0·018b | 0·767 ± 0·018b | < 0·0001 |
| Seed thickness (cm) | 0·215 ± 0·005b | 0·188 ± 0·005a | 0·212 ± 0·005b | 0·212 ± 0·018b | 0·0008 |
| Seed chamber length (cm) | 0·646 ± 0·026a | 0·687 ± 0·026a | 0·782 ± 0·026b | 0·888 ± 0·026c | < 0·0001 |
| Seed chamber width (cm) | 1·164 ± 0·043b | 1·021 ± 0·043a | 1·302 ± 0·043c | 1·329 ± 0·043c | < 0·0001 |
| Pod length (cm) | 12·876 ± 0·610a | 12·879 ± 0·610a | 14·891 ± 0·610b | 15·284 ± 0·610b | 0·0062 |
| Pod width (cm) | 1·642 ± 0·054a | 1·538 ± 0·054a | 1·835 ± 0·054b | 1·918 ± 0·054b | < 0·0001 |
| Pod thickness (cm) | 0·424 ± 0·012c | 0·349 ± 0·012a | 0·396 ± 0·012bc | 0·389 ± 0·012b | 0·0003 |
| Pod peduncle length (cm) | 1·022 ± 0·046a | 1·064 ± 0·046ab | 1·230 ± 0·046c | 1·192 ± 0·046bc | 0·0045 |
| Locules number | 14·92 ± 0·501ab | 14·668 ± 0·501ab | 14·543 ± 0·501a | 15·99 ± 0·501b | 0·1674 |
| Septum thickness (cm) | 0·147 ± 0·009bc | 0·130 ± 0·009ab | 0·160 ± 0·009c | 0·110 ± 0·009a | 0·0022 |
| Pod margin thickness (cm) | 0·205 ± 0·007b | 0·286 ± 0·007d | 0·229 ± 0·007c | 0·179 ± 0·007a | < 0·0001 |
| Seed number | 12·385 ± 0·524a | 12·680 ± 0·524a | 12·213 ± 0·524a | 14·416 ± 0·524b | 0·0144 |
| Aborted seeds number | 2·515 ± 0·238b | 1·958 ± 0·238ab | 2·356 ± 0·238b | 1·574 ± 0·238a | 0·0297 |
| Predated seeds number | 3·933 ± 0·809a | 1·752 ± 0·809a | 6·724 ± 0·809b | 11·22 ± 0·809c | < 0·0001 |
Different letters indicate significant differences between means; n = 20 individuals per populations.
Fig. 3.
Classification of individuals of Leucaena esculenta subsp. esculenta from wild and managed in situ populations from Alcozauca and Amapilca, Guerrero, Central Mexico. The classification pattern resulted from PCA of morphological variation within the space of the first and second principal components.
Table 2.
Eigenvectors of the principal component analysis of variation patterns of morphological characters in wild and managed in situ populations of Leucaena esculenta subsp. esculenta in La Montaña de Guerrero, Mexico
| Character | PC1 | PC2 | PC3 |
|---|---|---|---|
| Seed length | 0·3516 | 0·2047 | 0·1273 |
| Seed width | 0·3621 | 0·1446 | −0·0516 |
| Seed thickness | 0·1551 | 0·4961 | 0·1127 |
| Seed chamber length | 0·3225 | −0·0406 | −0·1651 |
| Seed chamber width | 0·3271 | 0·0933 | 0·1507 |
| Pod length | 0·3342 | −0·2321 | 0·1188 |
| Pod width | 0·3570 | 0·0448 | 0·1994 |
| Pod thickness | 0·0848 | 0·3488 | 0·1023 |
| Pod peduncle length | 0·1523 | −0·3179 | 0·0516 |
| Locules number | 0·2491 | −0·3290 | 0·1872 |
| Septum thickness | −0·0009 | −0·0045 | 0·5085 |
| Pod margin thickness | −0·1510 | −0·2798 | 0·4590 |
| Seed number | 0·2547 | −0·4036 | −0·0365 |
| Aborted seeds number | −0·0594 | 0·2220 | 0·4636 |
| Predated seeds number | 0·2958 | 0·0586 | −0·3619 |
Table 3.
Jackknifed classification matrix resulting from DFA of morphological variation of wild (groups 1 and 2) and managed in situ (groups 3 and 4) populations of Leucaena esculenta subsp. esculenta from Alcozauca and Amapilca, Guerrero, central Mexico
| Predicted group |
|||||
|---|---|---|---|---|---|
| Actual group | 1 | 2 | 3 | 4 | % correct |
| 1 (20) | 13 | 2 | 4 | 1 | 65 |
| 2 (20) | 2 | 18 | 0 | 0 | 90 |
| 3 (20) | 1 | 0 | 15 | 4 | 75 |
| 4 (20) | 1 | 0 | 2 | 17 | 85 |
| Total | 17 | 20 | 21 | 22 | 79 |
Wilks' lambda = 0·0482 (d.f. = 15, 3, 76); F = 7·2576 (d.f. = 45, 184), P < 0·0001.
Case study 3: the columnar cacti
Figure 4 shows a series of plots classifying individuals from populations of the columnar cacti studied under different management regimes. No discrete groups were found, but in all cases individuals conform to a continuum of variation throughout the first principal component. Most individuals in the plot are closer to each other according to the management type under which their populations are subjected. Most wild individuals had negative values, whereas most individuals from managed in situ and cultivated populations had positive values in the first principal components, indicating the trends of variation of features with higher weight in this principal component. Table 4 indicates that, in general, fruit dimensions are the most relevant characters varying between populations according to their management regime, and it is these that influence the classification patterns most. In the case of Escontria chiotilla (Fig. 4A) it is notable that most individuals of wild and managed in situ populations overlap in the central area of the plot, indicating high similarity among themselves. In contrast, the differentiation of individuals of populations according to their management regime is more clear in Polaskia chichipe (Fig. 4B) and even more in the more intensively managed Stenocereus stellatus (Fig. 4C).
Fig. 4.
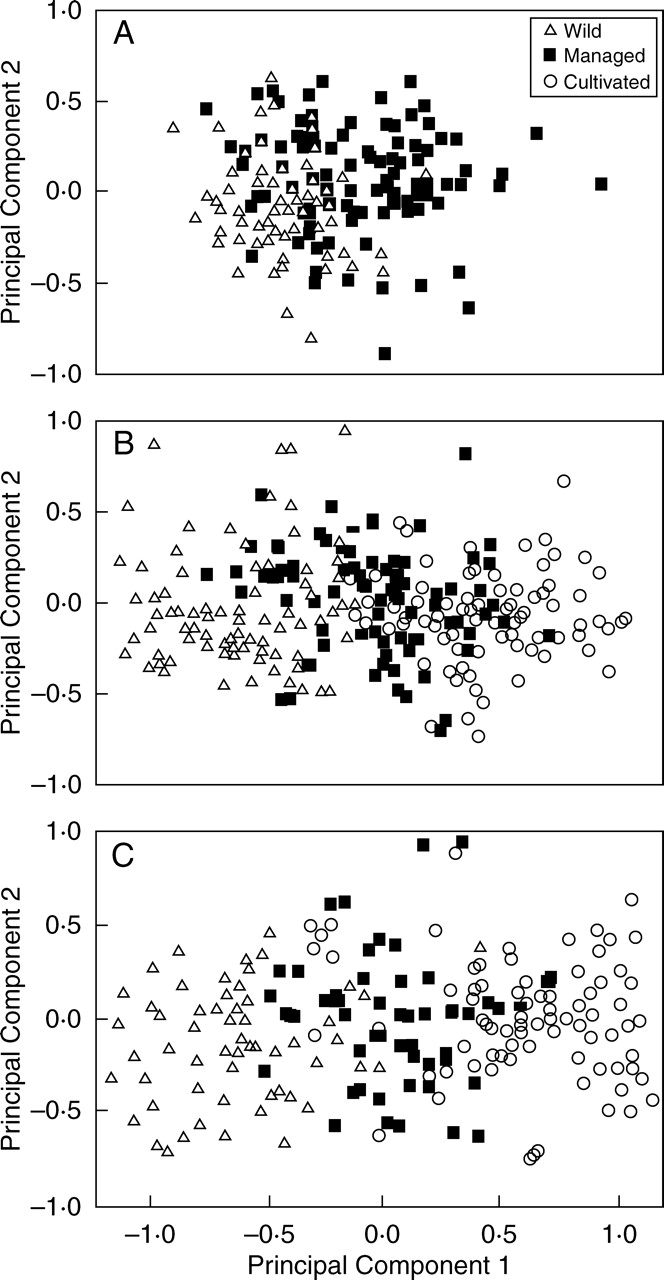
Classification of individuals from wild, managed in situ, and cultivated populations of (A) Escontria chiotilla (based on Arellano and Casas, 2003), (B) Polaskia chichipe (based on Carmona and Casas, 2005) and (C) Stenocereus stellatus (based on Casas et al., 1999b) of Central Mexico. The classification resulted from PCA of morphological variation within the space of the first two principal components.
Table 4.
Variation of morphological characters in wild and managed in situ populations of Escontria chiotilla, Polaskia chichipe and Stenocereus stellatus from the Tehuacán Valley and La Mixteca Baja regions, central Mexico
|
E. chiotilla |
P. chichipe |
S. stellatus |
||||
|---|---|---|---|---|---|---|
| Character | Wild | Managed in situ | Wild | Managed in situ | Wild | Managed in situ |
| Fruit length | 22·622 ± 0·305A | 27·225 ± 0·389B | 17·017 ± 0·243a | 21·294 ± 0·209b | – | – |
| Fruit diameter | 21·186 ± 0·296A | 25·414 ± 0·356B | 17·976 ± 0·249a | 21·453 ± 0·22b | – | – |
| Fruit size | – | – | – | – | 30·338 ± 1·6711 | 40·947 ± 1·6622 |
| Fruit weight | 6·444 ± 0·223A | 11·102 ± 0·438B | 2·943 ± 0·118a | 5·957 ± 0·141b | – | – |
| Proportion of pulp in fruits | 52·366 ± 1·1691 | 58·723 ± 1·1632 | ||||
| Thickness of fruit peel | 0·231 ± 0·008A | 0·260 ± 0·005B | 0·140 ± 0·005a | 0·147 ± 0·005a | 0·384 ± 0·0082 | 0·313 ± 0·0081 |
| Number of areoles on peel | – | – | 18·078 ± 0·296a | 18·091 ± 0·334a | 31·403 ± 0·5902 | 27·291 ± 0·5861 |
| Density of areoles on peel | – | – | 1·816 ± 0·072a | 1·867 ± 0·057a | 3·060 ± 0·0882 | 2·201 ± 0·0881 |
| Weight of fruit peel | 4·105 ± 0·133A | 6·130 ± 0·193B | 1·826 ± 0·087a | 1·867 ± 0·066b | – | – |
| Weight of fruit pulp | 2·328 ± 0·114A | 4·841 ± 0·269B | 1·266 ± 0·062a | 3·134 ± 0·086b | – | – |
| Total weight of seeds | 0·243 ± 0·012A | 0·340 ± 0·013B | 0·219 ± 0·005a | 0·279 ± 0·005b | 0·948 ± 0·0481 | 1·316 ± 0·0472 |
| Total number of seeds | 407·632 ± 20·673A | 532·718 ± 15·601B | 311·09 ± 7·082a | 359·724 ± 7·463b | 963·182 ± 32·6501 | 1219·800 ± 32·4682 |
| Mean weight per seed | 0·624 ± 0·015A | 0·780 ± 0·013B | – | – | 0·948 ± 0·0211 | 1·131 ± 0·0212 |
| Length of pericarpel | 14·669 ± 0·192A | 15·281 ± 0·144B | 13·042 ± 0·130a | 13·598 ± 0·154b | – | – |
| Diameter of pericarpel | 11·070 ± 0·080A | 11·188 ± 0·114A | 10·603 ± 0·113a | 10·786 ± 0·070b | – | – |
| Perianth length | 23·767 ± 0·292A | 24·385 ± 0·219A | 17·286 ± 0·235a | 17·342 ± 0·22a | – | – |
| Ovary length | 4·197 ± 0·083A | 4·463 ± 0·085B | 2·830 ± 0·060a | 2·721 ± 0·041a | – | – |
| Ovary diameter | 3·784 ± 0·060A | 3·742 ± 0·047A | 3·767 ± 0·059a | 3·675 ± 0·094a | – | – |
| Style length | 18·287 ± 0·215A | 18·683 ± 0·167A | 16·852 ± 0·233a | 18·377 ± 0·178a | – | – |
| Number of stigma lobes | 7·213 ± 0·097A | 7·283 ± 0·079A | 8·622 ± 0·082a | 8·510 ± 0·067a | – | – |
| Length of stigma lobes | 6·009 ± 0·187A | 5·879 ± 0·094A | 5·035 ± 0·072a | 5·206 ± 0·070a | – | – |
| Length of nectar chamber | 2·819 ± 0·065B | 2·569 ± 0·046A | 2·838 ± 0·070a | 2·835 ± 0·061a | – | – |
| Diameter of nectar chamber | 3·794 ± 0·051A | 3·748 ± 0·047A | 3·651 ± 0·066a | 3·628 ± 0·045a | – | – |
| Anther length | 1·573 ± 0·024A | 1·596 ± 0·016A | 1·947 ± 0·023a | 1·861 ± 0·021a | – | – |
| Anther width | 0·753 ± 0·011B | 0·697 ± 0·009A | 1·125 ± 0·110a | 1·011 ± 0·008a | – | – |
| Plant height | 4·334 ± 0·093A | 4·187 ± 0·062A | 3·434 ± 0·798b | 3·123 ± 0·064a | – | – |
| Stem diameter | 16·020 ± 0·533A | 19·028 ± 0·533B | 11·839 ± 0·165a | 12·005 ± 0·181a | – | – |
| Number of ribs per branch | 7·230 ± 0·046A | 7·242 ± 0·039A | 9·645 ± 0·065a | 9·786 ± 0·068a | 9·583 ± 0·1181 | 10·393 ± 0·1172 |
| Rib width | 3·360 ± 0·056A | 3·789 ± 0·060B | 2·384 ± 0·048a | 2·270 ± 0·050a | 3·070 ± 0·0661 | 3·246 ± 0·0661 |
| Rib depth | 2·624 ± 0·033A | 2·757 ± 0·034B | 2·017 ± 0·025a | 2·288 ± 0·028a | 2·550 ± 0·0401 | 2·606 ± 0·0391 |
| Number of spines per areole | 13·254 ± 0·230A | 13·089 ± 0·146A | 8·766 ± 0·754a | 8·818 ± 0·077a | 12·732 ± 0·2391 | 14·801 ± 0·2382 |
| Spine size | 2·699 ± 0·097A | 2·806 ± 0·084A | 1·261 ± 0·032a | 1·378 ± 0·037a | 2·106 ± 0·0011 | 2·608 ± 0·0012 |
| Distance between areoles | 1·385 ± 0·020A | 1·426 ± 0·017A | 0·891 ± 0·020a | 0·875 ± 0·020a | 2·512 ± 0·0412 | 2·301 ± 0·0401 |
| Number of branches | – | – | – | – | 15·472 ± 1·6331 | 20·344 ± 1·6242 |
| Length of the highest branch | – | – | – | – | 3·576 ± 0·0972 | 3·143 ± 0·0961 |
| Diameter of the highest branch | – | – | – | – | 12·611 ± 0·1771 | 13·921 ± 0·1762 |
Different upper- and lower-case letters and numbers indicate significant differences with P = 0·05 between populations of E. chiotilla, P. chichipe and S. stellatus, respectively
Divergence in germination behaviour follows a similar pattern. Figure 5A indicates that seeds from individuals of wild and managed in situ populations of E. chiotilla showed no differences in either germination percentage or rate, whereas these are significantly different between seeds of wild, managed in situ and cultivated individuals of P. chichipe, and even more different between individuals of S. stellatus managed under these regimes (Fig. 5B, C).
Fig. 5.
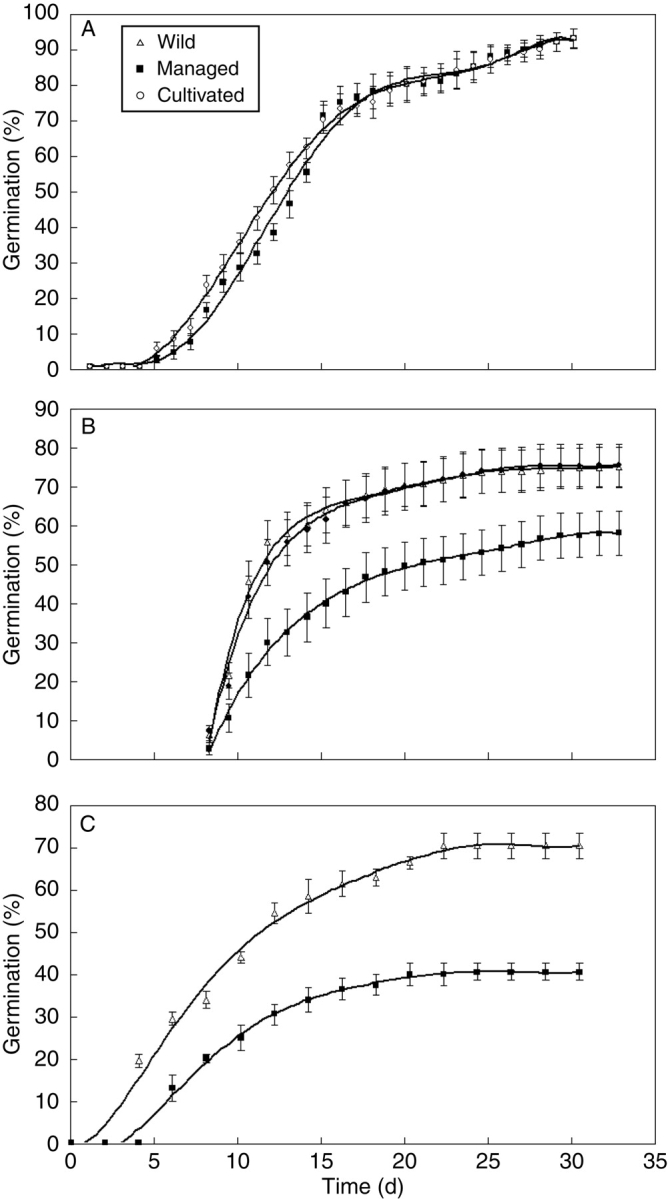
Germination percentage of seeds from wild, managed in situ, and cultivated populations of (A) Escontria chiotilla, (B) Polaskia chichipe (based on Otero-Arnaiz et al., 2003), and (C) Stenocereus stellatus (based on Rojas-Aréchiga et al., 2001) from the Tehuacán-Cuicatlán Valley and La Mixteca Baja region, Central Mexico.
Table 5 indicates that genetic variation decreases slightly in managed in situ and cultivated populations of E. chiotilla and P. chichipe in relation to wild populations of those species but, in the case of S. stellatus, genetic variation is higher in managed in situ and cultivated populations than in wild populations. Table 5 also indicates that the proportion of genetic variation is in general higher within populations than between populations, and that gene flow is high in all cases studied. Figure 6 indicates that genetic distance between the populations of each species studied is generally small, being the lowest among populations of E. chiotilla (Fig. 6A), and the highest among populations of S. stellatus (Fig. 6C). Genetic distance was not clearly related to management type. In P. chichipe and S. stellatus, according to Otero-Arnaiz et al. (2005b) and Casas et al. (2006), respectively (Fig. 6B, C), wild and managed in situ populations are indistinctly similar among themselves according to the distance separating them. In the case of E. chiotilla, wild and managed in situ populations are more similar among themselves (Fig. 6A), but these similarities according to Tinoco et al. (2005) are also associated with geographic distance since wild and managed in situ populations are closer among themselves.
Table 5.
Mean ± s.e. of He in wild and managed in situ populations of Escontria chiotilla (13 loci by isozyme analysis, according to Tinoco et al., 2005), Polaskia chichipe [five loci by microsatellites, according to Otero-Arnaiz et al. (2005a) and 15 loci by isozyme analysis according to Lucio (2005) and Stenocereus stellatus [16 loci by isozyme analysis, according to Casas et al. (2006) and J. Cruse et al. (University of Georgia, USA, unpubl. res.)] in the Tehuacán Valley, Central Mexico
|
E. chiotilla |
P. chichipe (microsatellites) |
P. chichipe (isozyme analysis) |
S. stellatus |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population type | Hee | FST | NmFST | He | FST | NmFST | He | FST | NmFST | He | FST | NmFST |
| Wild | 0·134 ± 0·043 | 0·075 | 7·326 | 0·683 ± 0·043 | 0·009 | 6·515 | 0·504 ± 0·044 | 0·067 | 6·515 | 0·253 ± 0·016 | 0·094 | 6·321 |
| Managed in situ | 0·110 ± 0·003 | 0·061 | 10·620 | 0·621 ± 0·054 | 0·022 | 4·488 | 0·505 ± 0·045 | 0·113 | 4·488 | 0·270 ± 0·006 | 0·167 | 11·016 |
| Cultivated | – | – | – | 0·660 ± 0·039 | 0·016 | 5·358 | 0·476 ± 0·054 | 0·106 | 5·740 | 0·289 ± 0·015 | 0·137 | 10·237 |
Fig. 6.
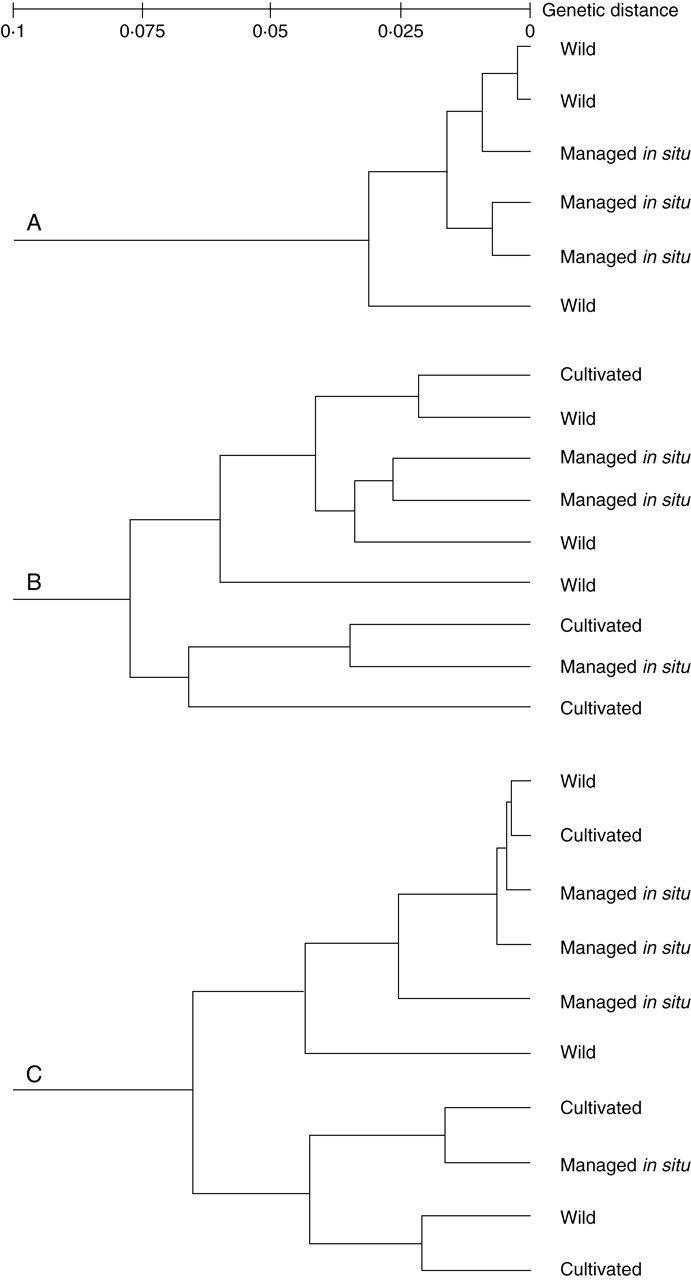
Genetic distance (Nei, 1973) of wild, managed in situ and cultivated populations of (A) Escontria chiotilla (based on Tinoco et al., 2005), (B) Polaskia chichipe (Otero-Arnaiz et al. 2005a; Lucio, 2005) and (C) Stenocereus stellatus (based on J. Cruse et al., University of Georgia, USA, unpubl. res.) from Central Mexico.
DISCUSSION
All cases analysed in this study showed that artificial selection associated with in situ management has had significant consequences in morphological, physiological and genetic aspects of plant populations. In all cases the phenotypes favourable to humans were more abundant in managed in situ populations than in the wild and even more abundant in cultivated populations, and this would contribute to the explanation of why average values of the features analysed significantly differed from the average values of wild populations. But influences of both environment and genes on phenotypes have not yet been evaluated, and this uncertainty makes it necessary to be cautious when trying to explain the nature of the divergence patterns. Long-term common garden experiments are now in progress with S. stellatus, and these are directed to test the hypothesis that features favoured by artificial selection are inherited. However, with the information available some important considerations are possible in this respect as discussed below.
Case study 1: the herbaceous ‘quelites’
In La Montaña de Guerrero, people distinguish between ‘hembra’ and ‘macho’ variants of Anoda cristata and Crotalaria pumila, preferring to use and encouraging the growth of individual plants of the ‘hembra’ variants. ‘Hembra’ and ‘macho’ variants of both species were recorded coexisting within all plots sampled in this study and, therefore, morphological differences are apparently determined genetically rather than environmentally. Abundance of the desirable ‘hembra’ variants of the two species is significantly favoured in areas under higher management intensity, whereas ‘macho’ variants are more successful in environments receiving no human management such as gaps in tropical dry forest. In fallow agricultural fields with similar environmental conditions to seasonal agricultural fields, ‘macho’ variants are also more abundant than ‘hembra’ variants, suggesting that it is human procurement of ‘hembra’ variants and removal of ‘macho’ variants, in addition to environmental conditions, that are the principal causes of abundance differences between these variants. Favouring numbers of individual plants of ‘hembra’ variants and removing those of ‘macho’ variants is a form of artificial selection operating under in situ management of these useful weeds. The consequences are highly significant, increasing the abundance of the favoured morphological variants while decreasing numbers of the unfavoured variants.
Case study 2: the guaje tree
Also in La Montaña de Guerrero, it was found in the present study with Leucaena esculenta subsp. esculenta that, after analysing two new populations, the results were consistent with those reported by Casas and Caballero (1996). In this species, the effect of selective let standing of desirable phenotypes in managed in situ populations also increased significantly the numbers of favourable morphological variants, as indicated by the higher average of pod and seed size in managed in situ populations. At for the moment there are no data to evaluate how much the differences found are caused by environmental differences in wild and managed in situ areas. However, as can be seen in the PCA plot of Fig. 3, a number of wild and managed in situ individuals overlapped their morphology, as is especially notable in the middle of the plot. Such overlap indicates that some wild and managed in situ individuals are morphologically similar, even when provenance of those similar phenotypes is from different sites. The overlaps also indicate that phenotypes with larger and shorter pods and seeds may coexist within both types of populations. These observations suggest that morphological variation is not only influenced by environment but also by genes. Figure 3 and Tables 1–3 indicate that phenotypes with larger pods and seeds are more abundant in managed in situ than in wild populations and vice versa, which suggests that differences in frequencies of phenotypes are apparently caused more by human management than by environmental differences. In addition to this observation, seeds of individuals of managed in situ populations are more vulnerable to bruchid attack, which may be due to differences in abundance of bruchids in both types of populations, but also it is probably related to the artificial selection favouring ‘sweeter’ phenotypes.
In the same populations from Alcozauca, Zárate et al. (2005) found that the managed in situ population had higher genetic variation (P = 87·5 %, Ap = 2·8 ± 0·3, He = 0·335 ± 0·043, Ho = 0·227 ± 0·028) than the wild population (P = 75·0 %, Ap = 2·4 ± 0·3, He = 0·264 ± 0·056, Ho = 0·203 ± 0·052), with higher biparental inbreeding occurring in the wild population. These authors also found that the two populations formed groups genetically well differentiated, and considered that differentiation is probably due to local inbreeding and limited gene flow between populations. These authors discussed that such differentiation was possibly the result of ecotypic differentiation, or a combination of drift and selection. The present morphological data give evidence that artificial selection is an ongoing process and that it is probably a principal factor influencing the genetic differentiation reported by Zárate et al. (2005).
Case study 3: the columnar cacti
In the case of the columnar cacti analysed, patterns of morphological variation and germination behaviour are strongly related to management intensity. Figure 4 shows that morphological divergence between wild and managed in situ populations is clearer as long as management intensity is higher. This pattern suggests that artificial selection favouring abundance of desirable phenotypes causes an increase in average values of morphological characters and the intensity in which this selection occurs determines the degree of divergence of managed in situ populations with respect to wild populations. As in the cases of ‘quelites’ and L. esculenta, desirable and undesirable phenotypes coexist in both wild and managed in situ populations but their frequencies change according to human intervention of environments, causing differences in average values between unmanaged and managed populations. Management intensity accentuates such differences, suggesting that morphological patterns are significantly influenced by human management. The experiments analysing germination behaviour were conducted under similar environmental conditions and significant differences were found in germination patterns of seeds from wild, managed in situ, and cultivated individuals of Polaskia chichipe and Stenocereus stellatus, and also it was found that these differences were stronger in the more intensely managed S. stellatus, whereas no differences were found in the less intensely managed E. chiotilla. The explanation for differences in germination patterns has been discussed by Rojas-Aréchiga et al. (2001) and Otero-Arnaiz et al. (2003) who considered it to be an indirect consequence of selection in favour of larger fruits with larger seeds. But more experiments are needed to arrive at a conclusion; particularly important are experiments to test the response of wild seeds in environments under in situ management and cultivation, and the reciprocal treatments. Similar experiments of reciprocal translocation would be important to test success of seedling establishment in wild, in situ managed and cultivated environments.
In contrast, genetic distance between populations of the three species studied is more related to geographic distance rather than to management type (Otero-Arnaiz et al., 1995a, b; Tinoco et al., 2005; Casas et al., 2006). This can be explained by the high rates of gene flow between the coexisting wild, managed in situ and cultivated populations. Although artificial selection is directed to favour some morphological features, neutral markers did not detect this process. It was expected that genetic variation would decrease according to management intensity between wild, managed in situ and cultivated populations within each species, and between species. This pattern can be appreciated in populations of E. chiotilla and P. chichipe, in which a slight reduction in genetic variation was recorded. But this is not the case of S. stellatus in which managed in situ and cultivated populations averaged higher genetic diversity than wild populations, showing that human management may be a determinant in maintaining and even increasing levels of genetic diversity. Casas et al. (2006) have discussed that this pattern is a consequence of continual replacement of plant materials in managed in situ populations and home gardens, including the introduction of plant material from other areas to home gardens, as well as the high gene flow between populations determined by bats and birds participating in pollination and seed dispersal. According to these authors, managed in situ and cultivated populations are important reservoirs of genetic diversity to be considered for strategies of in situ conservation.
Artificial selection under in situ management in the cases analysed is generally directed to increase numbers of desirable phenotypes of useful plants by let standing, encouraging growing and caring for them while relieving from care or even removing undesirable phenotypes. Plants favoured were part of the wild and weedy vegetation and they are able to survive and reproduce independently of human actions. But because of continual alteration of both phenotypic and genotypic frequencies, artificial selection is influencing evolution of plant populations, and because these processes are intentionally regulated by human actions, they should be considered as domestication processes. In the cases of perennial plants analysed, managed in situ populations are sources of materials for home gardens and other systems of ex situ management and, therefore, domestication under in situ management is closely related to domestication under ex situ management (Casas and Caballero, 1996; Casas et al., 1999b, 2006).
Zohary and Spiegel-Roy (1975) discussed the shifting from sexual reproduction to vegetative propagation as a requisite for domestication of fruit trees in the Middle East. This is especially true for those plant species with long life cycles and outcrossing breeding systems in which domestication may be slow under a model of cycles of planting, harvesting and selection since any mother tree may segregate numerous traits (Torres, 1989; Zohary and Hopf, 1993). However, in Mesoamerica several species of outcrossing trees without vegetative propagation were domesticated. In situ management and artificial selection have been hypothesized as mechanisms that may facilitate domestication of this type of plant (Casas et al., 1997a; Zárate et al., 2005). Selective in situ management is directed to maintain (or even increase) maternal genotypes with favourable morphological features and this activity in theory may increase the probability of crosses among such favourable genotypes and the occurrence of favourable phenotypes in progenies. The results of such processes may be influenced by a number of factors such as selection intensity, the characteristics of the breeding system, the pollination biology and the behaviour of pollinators and the size of the area influenced by in situ management, among others. Therefore, ecological and population genetics studies of such management systems are particularly relevant to test such hypotheses and to document how these processes could have operated in the past or how they are currently operating.
The present case studies included species of long-lived perennial outcrossing plants with vegetative propagation (S. stellatus) and without vegetative propagation (L. esculenta subsp. esculenta, P. chichipe and E. chiotilla), as well as herbaceous plant species with (A. cristata) and without (C. pumila) vegetative propagation, and this situation suggests that in situ management and artificial selection may occur in a broad spectrum of plant species. In other words, the panorama of the present study suggests that similar processes may be occurring in other species of the 600–700 species under in situ management in Mesoamerica. An increasing number of case studies covering different situations of human cultural importance, selection intensity, life cycle, breeding systems and pollination biology would be relevant to analyse patterns of these processes.
Currently, in situ management and artificial selection are practised by people in association with agriculture, and these processes therefore could have resulted in the experience of people as agriculturalists. But also, these practices could be reminiscent of old practices that, as gathering, have survived for thousands of years. To solve this question appears to be also an interesting challenge for botanical, genetic and archaeological research in order to clarify how processes that led to agriculture in Mesoamerica could have occurred.
ACKNOWLEDGEMENTS
The authors thank Fondo Sectorial SEMARNAT-CONACYT (research project 2002-0544) and the DGAPA, UNAM (research project IN220005), Mexico, and the Royal Botanic Garden, Kew (project ‘Integral study of columnar cacti of the Tehuacán-Cuicatlán Biosphere Reserve’) for financial support, as well as Nidia Pérez for assistance in laboratory work, Heberto Ferreira and Alberto Valencia for computer assistance, and Dr Mauricio Quesada for advise in statistical analyses. Funding to pay the Open Access publication charges for this article was provided by the OECD.
LITERATURE CITED
- Alcorn JB. Huastec noncrop resource management: implications for prehispanic rain forest management. Human Ecology. 1981;9:395–417. [Google Scholar]
- Alcorn JB. El te'lom huasteco: presente, pasado y futuro de un sistema de silvicultura indígena. Biótica. 1983;8:315–331. [Google Scholar]
- Alcorn JB. Huastec Mayan ethnobotany. Austin, TX: University of Texas Press; 1984. [Google Scholar]
- Arellano E, Casas A. Morphological variation and domestication of Escontria chiotilla (Cactaceae) under silvicultural management in the Tehuacán Valley, Central Mexico. Genetic Resources and Crop Evolution. 2003;50:439–453. [Google Scholar]
- Barrera A, Gómez-Pompa A, Vázquez-Yañes C. El manejo de las selvas por los Mayas sus implicaciones silvícolas y agrícolas. Biótica. 1977;2:47–61. [Google Scholar]
- Bye RA. Botanical perspectives of ethnobotany of the Greater Southwest. Economic Botany. 1985;4:375–386. [Google Scholar]
- Caballero J. Use and management of Sabal palms among the Maya of Yucatán. Berkeley, CA: University of California; 1994. PhD Dissertation. [Google Scholar]
- Caballero J, Mapes C. Gathering and subsistence patterns among the Purhepecha Indians of Mexico. Journal of Ethnobiology. 1985;5:31–47. [Google Scholar]
- Caballero J, Casas A, Cortés L, Mapes C. Patrones en el conocimiento, uso y manejo de plantas en pueblos indígenas de México. Revista de Estudios Atacameños. 1998;16:181–196. [Google Scholar]
- Carmona A, Casas A. Management, domestication and phenotypic patterns of Polaskia chichipe (Cactaceae) in the Tehuacán Valley, Central Mexico. Journal of Arid Environments. 2005;60:115–132. [Google Scholar]
- Casas A, Caballero J. Traditional management and morphological variation in Leucaena esculenta (Moc. et Sessé ex A.DC.) Benth. (Leguminosae: Mimosoideae) in the Mixtec region of Guerrero, Mexico. Economic Botany. 1996;50:167–181. [Google Scholar]
- Casas A, Viveros JL, Caballero J. Etnobotánica mixteca: sociedad, cultura y recursos naturales en la Montaña de Guerrero. México: Instituto Nacional Indigenista-Consejo Nacional para la Cultura y las Artes; 1994. [Google Scholar]
- Casas A, Vázquez MC, Viveros JL, Caballero J. Plant management among the Nahua and the Mixtec from the Balsas River Basin: and ethnobotanical approach to the study of plant domestication. Human Ecology. 1996;24:455–478. [Google Scholar]
- Casas A, Caballero J, Mapes C, Zárate S. Manejo de la vegetación, domesticación de plantas y origen de la agricultura en Mesoamérica. Boletín de la Sociedad Botánica de México. (a) 1997;61:31–47. [Google Scholar]
- Casas A, Pickersgill B, Caballero J, Valiente-Banuet A. Ethnobotany and domestication in xoconochtli, Stenocereus stellatus (Cactaceae), in the Tehuacán Valley and La Mixteca Baja, Mexico. Economic Botany. (b) 1997;51:279–292. [Google Scholar]
- Casas A, Caballero J, Valiente-Banuet A. Use, management and domestication of columnar cacti in south-central Mexico: a historical perspective. Journal of Ethnobiology. (a) 1999;19:71–95. [Google Scholar]
- Casas A, Caballero J, Valiente-Banuet A, Soriano JA, Dávila P. Morphological variation and the process of domestication of Stenocereus stellatus (Cactaceae) in Central Mexico. American Journal of Botany. (b) 1999;86:522–533. [PubMed] [Google Scholar]
- Casas A, Valiente-Banuet A, Rojas-Martínez A, Dávila P. Reproductive biology and the process of domestication of the columnar cactus Stenocereus stellatus in Central Mexico. American Journal of Botany. (c) 1999;86:534–542. [PubMed] [Google Scholar]
- Casas A, Cruse J, Morales E, Otero-Arnaiz A, Valiente-Banuet A. Maintenance of phenotypic and genotypic diversity of Stenocereus stellatus (Cactaceae) by indigenous peoples in Central Mexico. Biodiversity and Conservation. 2006;15:879–898. [Google Scholar]
- Colunga-García Marín P, Hernández-Xolocotzi E, Castillo A. Variación morfológica, manejo agrícola y grados de domesticación de Opuntia spp. en el Bajío Guanajuatense. Agrociencia. 1986;65:7–49. [Google Scholar]
- Colunga-García Marín P, Estrada-Loera E, May-Pat F. Patterns of morphological variation, diversity, and domestication of wild and cultivated populations of Agave in Yucatán, Mexico. American Journal of Botany. 1996;83:1069–1082. [Google Scholar]
- Cruz M, Casas A. Reproductive biology and morphological variation of Polaskia chende (Cactaceae) under domestication in Central Mexico. Journal of Arid Environments. 2002;51:561–576. [Google Scholar]
- Dávila P, Arizmendi MC, Valiente-Banuet A, Villaseñor JL, Casas A, Lira R. Biological diversity in the Tehuacán-Cuicatlán Valley, Mexico. Biodiversity and Conservation. 2002;11:421–442. [Google Scholar]
- Davis T, Bye R. Ethnobotany and progressive domestication of Jaltomata (Solanaceae) in Mexico and Central America. Economic Botany. 1982;36:225–241. [Google Scholar]
- Flannery KV. Guilá Naquitz. New York, NY: Academic Press; 1986. [Google Scholar]
- Gómez-Pompa A. On Mayan silviculture. Mexican Studies/Estudios Mexicanos. 1987;3:1–17. [Google Scholar]
- Gómez-Pompa A. Learning from traditional ecological knowledge: insights from Mayan silviculture. In: Gómez-Pompa A, Whitmore TC, Hadley M, editors. Rain forest regeneration and management. Paris: UNESCO/The Parthenon Publishing Group; 1991. pp. 335–341. [Google Scholar]
- Gómez-Pompa A, Flores E, Sosa V. The ‘pet-kot’: a man-made tropical forest of the Maya. Interciencia. 1987;12:10–15. [Google Scholar]
- González-Soberanis MC, Casas A. Traditional management and domestication of tempesquistle, Sideroxylon palmeri (Sapotaceae) in the Tehuacán Valley, Central Mexico. Journal of Arid Environments. 2004;59:245–258. [Google Scholar]
- Harlan JR. Madison, WI: American Society of Agronomy; 1975. Crops and man. Foundation for Modern Cropscience Series. [Google Scholar]
- Hawkes JG. The diversity of crop plants. London: Harvard University Press; 1983. [Google Scholar]
- Illsley GC. Vegetación y producción de la milpa bajo roza-tumba-quema en el ejido de Yaxcabá, Yucatán, México. Michoacán, Mexico: 1984. BSc Dissertation, Escuela de Biología, Universidad Michoacana de San Nicolás de Hidalgo Morelia. [Google Scholar]
- Lucio JD. Genética de poblaciones y proceso de domesticación de Polaskia chichipe en el Valle de Tehuacán, Puebla. Morelia, Michoacán, Mexico: Universidad Michoacana de San Nicolás de Hidalgo; 2005. BSc Dissertation. [Google Scholar]
- Lundell CL. The vegetation of Peten. Washington, DC: Carnegie Institution of Washington; 1937. Publication no. 436. [Google Scholar]
- MacNeish RS. A summary of subsistence. In: Byers DS, editor. The prehistory of the Tehuacan Valley. Vol. 1. Austin, TX: University of Texas Press; 1967. pp. 290–309. Environment and subsistence. [Google Scholar]
- MacNeish RS. The origins of agriculture and settled life. Norman and London: University of Oklahoma Press; 1992. [Google Scholar]
- Mapes C, Caballero J, Espitia E, Bye R. Morphophysiological variation in some Mexican species of vegetable Amaranthus: evolutionary tendencies under domestication. Genetic Resources and Crop Evolution. 1996;43:283–290. [Google Scholar]
- Martínez-Ballesté A, Martorell C, Martínez-Ramos M, Caballero J. Applying retrospective demographic models to assess sustainable use: the Maya management of Xa'an palms. Ecology and Society. 2005;10(2):17. [Google Scholar]
- Matos-Moctezuma E. Manzanilla L, López-Luján L, editors. Mesoamérica. Historia antigua de México. 1994;I:49–73. El México antiguo, sus áreas culturales, los orígenes y el horizonte Preclásico. México: Consejo Nacional para la Cultura y las Artes, Instituto Nacional de Antropología e Historia, Universidad Nacional Autónoma de México, Porrúa. [Google Scholar]
- Medellín MSG. Arboricultura y silvicultura tradicional en una comunidad totonaca de la costa. Veracruz, México: Instituto Nacional de Investigaciones sobre Recursos Bióticos Xalapa; 1988. MSc Dissertation. [Google Scholar]
- Mera LM. Chapingo, México: Colegio de Postgraduados; 1987. Estudio comparativo del proceso de cultivo de la arvense Physalis chenopodifolia Lamarck, y Physalis philadelphica var. philadelphica cultivar Rendidora. MSc Dissertation. [Google Scholar]
- Nei M. Analysis of gene diversity in subdivided populations. Proceedings of the National Academy of Science. 1973;70:3321–3323. doi: 10.1073/pnas.70.12.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigh RB, Nations JD. La agrosilvicultura tropical de los lacandones de Chiapas. Configuraciones de la diversidad. México: CADAL and CESTEM; 1983. [Google Scholar]
- Oaxaca-Villa B, Casas A, Valiente-Banuet A. Reproductive biology in wild and silvicultural managed populations of Escontria chiotilla (Cactaceae) in the Tehuacán Valley, Central Mexico. Genetic Resources and Crop Evolution. 2006;53:277–287. [Google Scholar]
- Otero-Arnaiz A, Casas A, Bartolo MC, Pérez-Negrón E, Valiente-Banuet A. Evolution of Polaskia chichipe (Cactaceae) under domestication in the Tehuacán Valley, Central Mexico. Reproductive biology. American Journal of Botany. 2003;90:593–602. doi: 10.3732/ajb.90.4.593. [DOI] [PubMed] [Google Scholar]
- Otero-Arnaiz A, Cruse-Sanders J, Casas A, Hamrick JL. Isolation and characterization of microsatellites in the columnar cactus Polaskia chichipe (Cactaceae) Molecular Ecology Notes. 2004;4:265–267. [Google Scholar]
- Otero-Arnaiz A, Casas A, Hamrick JL. Direct and indirect estimates of gene flow among wild and managed populations of Polaskia chichipe, an endemic columnar cactus in Central Mexico. Molecular Ecology. (a) 2005;14:3313–4322. doi: 10.1111/j.1365-294X.2005.02762.x. [DOI] [PubMed] [Google Scholar]
- Otero-Arnaiz A, Casas A, Hamrick JL, Cruse J. Molecular Ecology. (b) 2005;14:1603–1611. doi: 10.1111/j.1365-294X.2005.02494.x. [DOI] [PubMed] [Google Scholar]
- Puleston DE. The role of ramon in Maya subsistence. In: Flannery KV, editor. Maya subsistence. Studies in memory of Dennis E. Puleston. New York, NY: Academic Press; 1982. pp. 353–366. [Google Scholar]
- Reyes-Agüero JA. Variación morfológica de Opuntia (Cactaceae) y su relación con la domesticación en la Altiplanicie Meridional de México. Mexico: Universidad Nacional Autónoma de México; 2005. PhD Dissertation, Facultad de Ciencias. [Google Scholar]
- Rohlf J. Numerical taxonomy system and multivariate analysis system for IBMpc microcomputer. New York, NY: Applied Biostatistics; 1993. [Google Scholar]
- Rojas T. La agricultura en tierras mexicanas desde sus orígenes hasta nuestros días. México, D.F: Consejo Nacional para la Cultura y las Artes/Grijalbo; 1991. [Google Scholar]
- Rojas-Aréchiga M, Casas A, Vázquez-Yanes C. Seed germination of wild and cultivated Stenocereus stellatus (Cactaceae) from the Tehuacán-Cuicatlán Valley, Central Mexico. Journal of Arid Environments. 2001;49:279–287. [Google Scholar]
- Rzedowski J. Diversity and origins of the phanerogamic flora of Mexico. In: Ramamoorthy TP, Bye RA, Lot A, Fa J, editors. Biological diversity of Mexico. New York, NY: Oxford University Press; 1993. pp. 129–144. [Google Scholar]
- SAS. SAS user's guide: statistics. Release 8·02. Cary, NC: SAS Institute; 2000. [Google Scholar]
- SYSTAT. SYSTAT Version 11. Richmond, CA: SYSTAT Software Inc; 2004. [Google Scholar]
- Torres AW. Isozyme analysis of tree fruits. In: Solitis DE, Soltis PS, editors. Isozymes in plant biology. Vol. 4. Portland, OR: Dioscorides Press; 1989. pp. 192–205. Advances in Plant Sciences Series. [Google Scholar]
- Tinoco A, Casas A, Luna R, Oyama K. Population genetics of wild and silvicultural managed populations of Escontria chiotilla in the Tehuacán Valley, Central Mexico. Genetic Resources and Crop Evolution. 2005;52:525–538. [Google Scholar]
- Toledo VM. Biodiversity and indigenous peoples. In: Levin SA, editor. Encyclopedia of biodiversity. Vol. 3. San Diego, CA: Academic Press; 2000. pp. 451–463. [Google Scholar]
- Toledo VM, Ordóñez MJ. The biodiversity of Mexico: a review of terrestrial habitats. In: Ramamoorthy TP, Bye RA, Lot A, Fa J, editors. Biological diversity of Mexico. New York, NY: Oxford University Press; 1993. pp. 757–777. [Google Scholar]
- Valiente-Banuet A, Casas A, Alcántara A, Dávila P, Flores N, Arizmendi MC, et al. La vegetación del Valle de Tehuacán Cuicatlán. Boletín de la Sociedad Botánica de México. 2000;67:25–74. [Google Scholar]
- Vavilov NI. The origin, variation, immunity and breeding of cultivated plants. Chronica Botanica. 1951;13:1–16. [Google Scholar]
- Vázquez MC. Tendencias en el proceso de domesticación del papaloquelite (Porophyllum ruderale (Jacq.) Cass. subsp. macrocephalum (DC.) R.R. Johnson. Asteraceae) México: Universidad Nacional Autónoma de México; 1991. MSc Dissertation, Facultad de Ciencias. [Google Scholar]
- Villaseñor JL. Diversidad y distribución de las Magnoliophyta de México. Interciencia. 2003;28(3):160–167. [Google Scholar]
- Williams DE. Tres arvenses solanáceas comestibles y su proceso de domesticación en el estado de Tlaxcala, México. Chapingo, México: Colegio de Postgraduados; 1985. MSc Dissertation. [Google Scholar]
- Wiseman FM. Agricultural and historical ecology of the Maya lowlands. In: Harrison PD, Turner BL II, editors. Pre-hispanic maya agriculture. Albuquerque, NM: University of New Mexico Press; 1978. pp. 63–116. [Google Scholar]
- Zárate S. Ethnobotany and domestication process of Leucaena in Mexico. Journal of Ethnobiology. 1999;19:1–23. [Google Scholar]
- Zárate S, Pérez-Nasser N Casas A. Genetics of wild and managed populations of Leucaena esculenta subsp. esculenta (Fabaceae: Mimosoideae) in La Montaña of Guerrero, Mexico. Genetic Resources and Crop Evolution. 2005;52:941–957. [Google Scholar]
- Zohary D, Hopf M. Domestication of plants in the Old World. Oxford: Clarendon Press; 1993. [Google Scholar]
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319–327. doi: 10.1126/science.187.4174.319. [DOI] [PubMed] [Google Scholar]