Abstract
Background
The history of domestication of artichoke and leafy cardoon is not yet fully understood and when and where it occurred remains unknown. Evidence supports the hypothesis that wild cardoon is the wild progenitor of both these crops. Selection for large, non-spiny heads resulted in artichoke and selection for non-spiny, large stalked tender leaves resulted in leafy cardoon. The two crops differ in their reproductive system: artichoke is mostly vegetatively propagated and perennial, while leafy cardoon is seed propagated and mostly grown as an annual plant. Here, new trends in artichoke cultivation are analysed, while the consequences of these tendencies on the conservation of artichoke genetic resources are highlighted.
Scope
The historical and artistic records, together with recent literature on genetics and biosystematics, are examined with the aim of achieving a better understanding of the present-day knowledge on the domestication of these two crops.
Conclusions
Historical, linguistic and artistic records are consistent with genetic and biosystematic data and indicate that the domestication of artichoke and cardoon diverged at different times and in different places. Apparently, artichoke was domesticated in Roman times, possibly in Sicily, and spread by the Arabs during early Middle Ages. The cardoon was probably domesticated in the western Mediterranean in a later period.
Key words: Cynara cardunculus, domestication, artichoke, cardoon, wild progenitor, genetic resources
INTRODUCTION
Cynara cardunculus L. is a diploid (2n = 2x = 34), mostly cross-pollinated species belonging to the Asteraceae family, native to the Mediterranean basin. The wild perennial taxon [var. sylvestris (Lamk) Fiori] has been recognized as the ancestor of both the globe artichoke [var. sativa Moris, var. scolymus (L.) Fiori, ssp. scolymus (L.) Hegi] and the leafy or cultivated cardoon (var. altilis DC) (Rottenberg and Zohary, 1996). Globe artichoke represents an important component of the agricultural economy of southern Europe, and it is grown for its large immature inflorescences, called capitula or heads (Bianco, 1990); its commercial production is mainly based on perennial cultivation of vegetatively propagated clones. Artichokes also have nonfood uses as their leaves are a source of antioxidant compounds, such as luteolin and dicaffeoylquinic acids (cynarin) (Gebhardt, 1997; Di Venere et al., 2005) and the roots contain inulin, an oligosaccharide known to have a positive effect on human intestinal flora, thus on health (Raccuia and Melilli, 2004). Cultivated cardoon is grown for its fleshy stems and leaf stalks and has some regional importance in Italy, Spain and southern France (Dellacecca, 1990); its propagation is carried out by seeds.
Previous classifications considered the cultivated artichoke as a separate species: C. scolymus L. However, recent studies based on cladistic analysis of morphological data (Wiklund, 1992) and hybridization and isozyme analyses (Basnizki and Zohary, 1994; Rottenberg and Zohary, 1996; Rottenberg et al., 1996) support Fiori's classification (Fiori, 1904) which included cultivated artichoke, leafy cardoon and wild cardoon, in the single species C. cardunculus L.
The domestication of these crops is not yet fully understood and when and where it occurred are still unknown. The two crops are reported to have resulted from human selection pressure for either large, non-spiny heads or non-spiny, large stalked tender leaves (Basnizki and Zohary, 1994). Therefore the two cultivated forms appear to be the result of concurrent directional selection for distinct traits, and not disruptive selection (Sonnante et al., 2004).
The origin of the artichoke is often associated with Arabs, who dominated the southern Mediterranean during the Middle Ages. Arabs likely had an important role in the diffusion of this crop, as for other plants like eggplants and spinach, since they had a particular interest in horticultural and garden crops (Idrisi, 2005).
In the present contribution we will analyse the literature to try and clarify the domestication process that led to artichoke and cardoon.
HISTORICAL, LINGUISTIC, AND ARTISTIC RECORDS
Whether artichoke was known to the ancient classical world is still an open question. Both Greek and Roman writers reported the consumption of this species, but classical literature records can be misleading. For instance, in ancient Greek the word scolymos relates to ‘spiny’ and this word might also refer to thistles other than C. cardunculus. This vagueness has to be taken into account when reading, for instance, Pliny the Elder (ad 23–79, in Naturalis historia) whose comments have been interpreted to indicate cultivated artichoke in south Italy and south Spain. In fact, De Candolle (1890) suggested that cultivated artichoke was unknown in classical times; Montelucci (1962) states that Theophrastus (371–287 bc) reported cultivation of artichokes in Sicily but not in Greece; an unidentified species of Cynara is shown in a mosaic in the Bardo Museum in Tunis belonging to the Imperial period (3rd century ad); Columella (1st century ad) in De re rustica reports on ‘cinara’ cultivation in Italy, but defines the plant ‘hispida’ (= spiny) and states that ‘pinea vertice pungit’ (= its head apex pierces). Based on the writings of Pliny and Columella, Foury (1989) deduced that the cultivation of artichoke started around the 1st century ad; however, it is likely that around the first century of the modern era the domestication of artichoke was ongoing, but not yet accomplished.
Despite the positive role the Arabs had in the diffusion of artichoke, only the current names for this plant in Italian, Spanish and Portuguese (‘Carciofo’, ‘Alcachofa’, and ‘Alcachofra’, respectively) derive from the Arabic ‘al harshuff’. Interestingly, in English, French and German, as well as in northern European languages and Russian, the name of this plant comes from the late Latin/old Italian ‘Alcocalum’, ‘Articocalus’, ‘Articiocco’ or ‘Articoca’ of uncertain origin, but possibly related to the Latin ‘coculum’ (= cardoon; Lonitzer, 1551–1555), while in Greek the plant is known as ‘Aγγιναρα’ (= Agginara), which relates to old Greek ‘Kυoν’ (= Kyon, dog), possibly for spines recalling dog teeth. This strongly suggests that Italy was the bridge for the diffusion of artichoke in Europe.
The first certain records of artichoke commerce refer to Filippo Strozzi trading artichokes from Sicily to Florence in the early 15th century (Bianco, 1990). Artichokes are also present in Renaissance paintings by Vincenzo Campi (1536–1591; L'ortolana, c. 1580; http://www.wga.hu/art/c/campi/vincenzo/1fruit.jpg) or Giuseppe Arcimboldo (1527–1593; L'estate, 1563; http://mk29.image.pbase.com/u37/karibaer/upload/34781868.DSC_5386.jpg; Vertumnus, 1590; http://www.spamula.net/blog/i24/arcimboldo13.jpg).
Old literature regarding the cultivated leafy cardoon is lacking, but this crop is present in painting at the beginning of the 17th century: in Caravaggio's Natura morta con fiori, frutta e verdure (uncertain dating, after 1600; http://www.wga.hu/art/c/caravagg/12/89d_stil.jpg) and in Juan Sánchez Cotán's still life paintings (c. 1602; http://www.epdlp.com/fotos/sanchezcotan1.jpg and http://www.epdlp.com/fotos/sanchezcotan4.jpg). The fact that cultivated cardoons appear in Spanish and Italian paintings almost at the same time might relate to the fact that Spain dominated Italy starting from the mid-16th century (ending at the beginning of the 18th and mid-19th century in northern and southern Italy, respectively). It is interesting that the cultivated cardoon name in all European languages derives from the Latin ‘carduus’, which mostly relates to spininess.
PHYLOGENETIC AND EVOLUTIONARY STUDIES
The search for the ancestry of Cynara crops has followed many approaches. Recently, using a cladistic method based on morphological characters and on a large set of specimens, Wiklund (1992) confirmed the inclusion of cultivated artichoke, leafy cardoon and wild cardoon, in a single species: C. cardunculus L. Her results also indicated that C. auranitica, C. syriaca and C. baetica were close relatives of C. cardunculus.
Hybridization experiments demonstrated that wild cardoon and cultivated species are genetically cohesive since they are completely interfertile and, therefore, they belong to the same gene pool (Basnizki and Zohary, 1994; Rottenberg and Zohary, 1996; Rottenberg and Zohary, 2005). Other wild Cynara species, in particular C. algarbiensis and C. syriaca, show only limited capacity to set seeds and produce viable hybrids when crossed to the cultigen, while other wild allies show almost complete genetic isolation (Rottenberg and Zohary, 1996).
Studies based on variation of isozymes and molecular markers such as RAPDs and AFLPs (Rottenberg et al., 1996; Sonnante et al., 2002, 2004; Lanteri et al., 2004; Raccuia et al., 2004b) have confirmed that both crops evolved from the wild cardoon gene pool, which can therefore be considered the progenitor of both of them. Based on AFLP markers it was demonstrated that all C. cardunculus samples share a high genetic similarity compared with the other Cynara wild species, and, at the same time, artichoke germplasm is well separated from both wild and cultivated cardoon samples (Sonnante et al., 2004).
Among the other wild species, C. syriaca was initially considered a possible donor of genes to the cultivated artichoke (Zohary and Basnizki, 1975); however, other evidence (Rottenberg and Zohary, 1996; Rottenberg et al., 1996), including a recent analysis based on AFLP and other DNA markers (Sonnante et al., 2004, 2007) do not support this hypothesis.
To clarify the ancestry and domestication of Cynara crops, recent studies made use of rDNA spacer sequences, since they are generally considered a good marker of evolution (Small et al., 2004). A first study by Robba et al. (2005) used the internal transcribed spacer sequences of the ribosomal regions to analyse the phyletic relationships among Cynara species. The results show close agreement with the phylogeny proposed by Wiklund (1992), but this analysis revealed more about the phyletic relationships among the species of the genus Cynara than the evolution and origin of the complex species C. cardunculus.
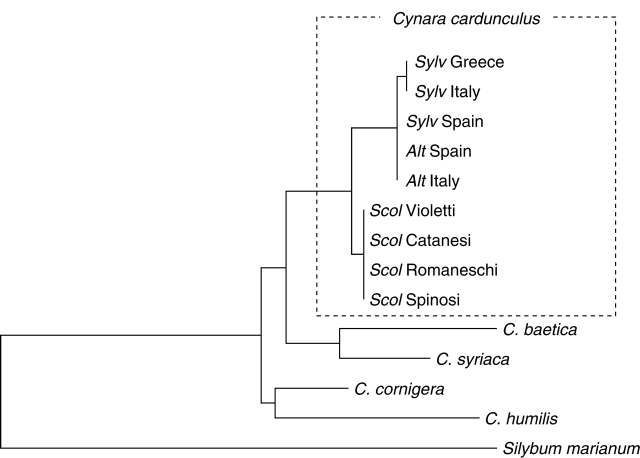
To analyse in detail the phyletic relationships within C. cardunculus and among some other Cynara species internal transcribed spacer and external transcribed spacer sequences, together with plastidial spacers were analysed (Sonnante et al., 2007) (Fig. 1). This study revealed that the whole genus is quite recent, possibly arising during the last 20 millennia, and that the domestication of artichoke and of cardoon are two distinctive events, separated in time and in space, which led to the two crops diverging for reproduction system and end use. The domestication of artichoke took place around the beginning of the first millennium (Foury, 1989; Pignone and Sonnante, 2004) while domestication of cardoon took place in the first half of the second millennium. Moreover, Pignone and Sonnante (2004) hypothesize that the artichoke was possibly domesticated in Sicily, while cardoon originated in the western range of the Mediterranean, probably within Spain and France (Sonnante et al., 2007).
Fig. 1.
Relationships among Cynara species and within C. cardunculus revealed by rDNA spacers parsimony analysis, redrawn from Sonnante et al. (2007). Abbreviations for the Cynara cardunculus clade: Sylv = var. sylvestris; Alt = var. altilis; Scol = var. scolymus, followed by geographic origin (Sylv and Alt) or varietal group (Scol).
VARIATION IN WILD CARDOONS
Wild cardoon is widely distributed across the Mediterranean basin, from as far east as Cyprus and the Black Sea and in the west to Gibraltar, Atlantic Spain, Portugal and the Canary Islands (Wiklund, 1992). Variation in morphological traits has been reported within this range. Foury (1989) recognizes three types based on head morphology: Sicilian, Tunisian and Catalan. The Catalan type has few spines. Wiklund (1992) distinguishes two subspecies on the basis of bracts characters and geographical distribution, namely ssp. flavescens in the west and ssp. cardunculus in the eastern Mediterranean, the two subspecies occur in Sicily. The Institute of Plant Genetics, CNR, in Italy, has been collecting samples of wild cardoon from the Mediterranean and reported variation for capitula traits and plant morphology (Pignone and Sonnante, 2004). Differences between wild samples from Spain compared with Italian and Greek materials have been observed for leaf size and spininess, head shape and size, and spine length and number; preliminary data from analyses of these material using molecular markers support genetic differentiation in the western wild gene pool (Sonnante et al., 2006b). Studies of wild cardoon populations collected in different areas of Sicily showed variability for salinity and water stress resistance during seed germination (Raccuia et al., 2004a). A correlation between genetic variation and geographical origin among seven populations of wild cardoon from Sicily and Sardinia has been reported (Portis et al., 2005). A similar correlation was observed for Sicilian wild cardoon populations (Raccuia et al., 2004b).
When a collection of wild cardoons becomes available that is more representative of the whole range of distribution, improved appreciation of the level of variation present in the gene pool of this taxon will result and the identity of the genetic stocks from which artichoke and leafy cardoon were domesticated may be established. rDNA spacers data and simple sequence repeat analysis seem to indicate that the leafy cardoon is genetically closer to wild germplasm of Spain rather than wild germplasm of Italy or Greece, thus supporting the view that the leafy cardoon was domesticated in the western part of the Mediterranean (Sonnante et al., 2006a, 2007).
HETEROZYGOSITY AND GIGANTISM
The process of plant domestication has often favoured plants able to express, to a higher degree, traits associated with production; this implies that different end uses of each crop have oriented the domestication of that plant (Gepts, 2002). When the plant part used is not the seed, often human selection has favoured the maintenance of high levels of heterozygosity (Zohary and Hopf, 2001; Hancock, 2004) or the affirmation of specific QTLs. In a vegetatively propagated crop like artichoke, this has meant the clonal multiplication of plants showing at the same time desired Mendelian (e.g. absence of spines) and complex (e.g. big capitula) traits (Porceddu et al., 1976); these latter traits might have high levels of heterosis (Hammer, 1988; Balloux et al., 2003). Recently, it has been demonstrated that in fields of cassava landraces (Manihot esculenta, another vegetatively propagated crop), high heterozygosity persists despite farmers regularly incorporating ‘volunteer’ plants from sexually produced seeds into their clonal stocks; these plants generally show a low level of heterosis, but few heterotic plants are present (Pujol et al., 2005). It has been observed that negative selection of the less heterotic plants helps simultaneously to maintain high levels of individual heterozygosity and high genotypic diversity within those landraces.
A similar mechanism has possibly led to the great diversification of artichoke and the maintenance of high levels of heterozygosity. The high level of heterozygosity in artichoke has been demonstrated by Basnizki and Zohary (1994) who reported that selfing clonally propagated artichoke varieties leads to a high level of morphological segregation in the offspring, accompanied by considerable inbreeding depression. Progeny of artichoke × wild cardoon crosses generally show a high degree of variation for many quantitative and qualitative characters (Portis et al., 2006; G. Sonnante, D. Pignone and K. Hammer, pers. obs.) thus confirming that modern cultivars of artichoke retain a high degree of heterozygosity. Investigations based on molecular markers such as simple sequence repeats confirm this (Acquadro et al., 2005; Sonnante et al., 2006a).
A great deal of variation is observed within artichoke germplasm for agronomic characters, mostly regarding the capitula (colour, shape and weight of capitula, lower number of capitula per plant as compared with wild cardoon, presence of spines on bracts, flowering time as early = reflowering vs. late, etc.), while the vegetative part of the plant shows a lower level of variation (Dellacecca et al., 1976). Vegetatively propagated crops are easily and quickly domesticated (Gepts, 2002); this also can account for the high degree of variation present in artichoke germplasm. Four main varietial groups are distinguished in artichoke (‘Spinosi’, ‘Violetti’, ‘Catanesi’ and ‘Romaneschi’) although much local germplasm does not fall in any of these categories (Porceddu et al., 1976). Out of 115 distinct landraces collected from seven Mediterranean and two American countries, as many as 80 were of Italian origin, thus testifying for the great variation present in Italian artichoke germplasm (Dellacecca et al., 1976).
Conversely, the level of variation observed in leafy cardoon is quite limited and only few landraces of this crop are known. These cardoon landraces differ slightly for minor characters relevant to domestication, such as the dimension of leaf stalk, which represents the edible part of this crop. Moreover, during domestication, the average number of capitula per plant has slightly increased compared with wild cardoon (Dellacecca, 1990; Portis et al., 2005).
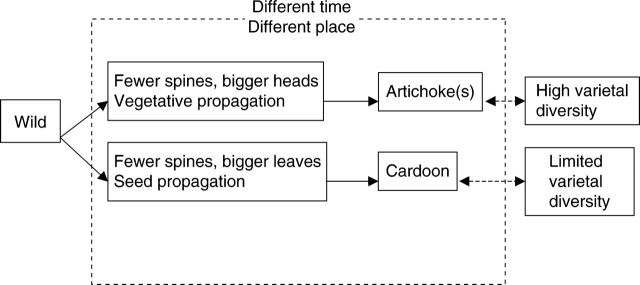
Within this framework, the gigantism observed for head traits in artichoke and leaf traits on leafy cardoon might be due to the action of a limited number of QTLs, respectively, but in artichoke fixed heterosis cannot be underestimated (Table 1 and Fig. 2), as data from molecular markers confirm (Sonnante et al., 2006a).
Table 1.
Gigantic traits and genetic features of artichoke and leafy cardoon
| Artichoke | Leafy cardoon | |
|---|---|---|
| Distinctive trait | Huge heads | Huge leaves |
| Reproduction | Vegetative | Seed |
| Flowering time | Autumn + spring | Spring only |
| QTLs | For head size and shape | For leaf shape and size |
| Heterosis | Heterosis maintained by vegetative reproduction | Little evidence |
Fig. 2.
Diverging domestication patterns in artichoke and leafy cardoon.
TOWARDS A NEW CROP: SEED PROPAGATED ARTICHOKES
Clonal propagation of artichoke, as seen before, has some advantages, particularly the maintenance of uniformity and heterosis. But, at the same time, it poses some important problems, the most significant being the difficulty of field rotation and the build-up of pathogens. Clonal reproduction makes it difficult to produce pathogen-free propagation material, thus making certification complicated. For these reasons, but mostly to obtain pathogen-free material, in vitro micropropagation techniques have been developed which allowed the large-scale production of micropropagated clones of globe artichoke, taking advantage of new hormones and mycorrhizal inocula (Ancora et al., 1981; Pécaut et al., 1983; Ancora, 1986; Tavazza et al., 2004; Ruta et al., 2005). The use of micropropagation to obtain pathogen-free plants of selected varieties may also help to preserve the genetic diversity of artichoke germplasm, especially for those types where virus infection is pandemic. The main disadvantage is the loss of precocity for the re-flowering types (Pécaut and Martin, 1992).
A different and more practical approach has been the recent development of seed-planted, hybrid varieties of globe artichoke, which transformed this vegetable from a labour-intensive, perennial crop, maintained by vegetative propagation into a crop better fitting the needs of modern agriculture. Dependable male sterile mutants were discovered and successfully used to change the life cycle of the plants. Despite initial problems relating to the loss of heterosis, lower crop uniformity and inbreeding depression (Basnizki and Zohary, 1994), seed-propagated artichokes are gaining in economical importance (López Anido et al., 1998; Calabrese and Bianco, 2000). Producing competitive seed-propagated varieties for fresh consumption is still a future goal, but the present seed-propagated varieties appear to perform well for industrial production, such as for canning.
After 2000 years, farmers are looking at modifying the artichoke from a garden crop to a field crop (Hammer, 2003); the potential of seed-propagated artichoke appears to be high, especially in a period of global climatic change in which perennial cultivations face important problems, like the increase of salinity in irrigation water due to the rising aridity in lower latitudes, where the production of artichoke is economically important (Bianchimano et al., 2005).
The price to be paid for these advantages will be a reduction in the genetic base of artichoke. Currently artichoke germplasm cultivated on a small scale is differentiated into many local varieties that have some economic potential. These varieties differ not only in head characters, but also for production physiology and other possible useful traits. Should one or few seed-propagated varieties of similar value become available in the future, the destiny of all this germplasm appears threatened (Hammer, 1984). In the past, this occurred when policies relating to seed certification accelerated the loss of wheat germplasm in some traditionally genetically rich areas of Italy, like Sicily and Sardinia (Pignone et al., 1997). This loss of genetic diversity is serious because artichoke is not only a food crop, but also a source of pharmaceutically useful compounds, and a potentially good energy crop (Foti et al., 1999; Raccuia and Melilli, 2007).
The efforts in producing a seed-propagated artichoke crop demonstrate a basic truth: for no plants is domestication an accomplished process.
ACKNOWLEDGEMENTS
This paper is publication no. 79 of the Institute of Plant Genetics, CNR. Funding to pay the Open Access publication charges for this article was provided by the OECD.
LITERATURE CITED
- Acquadro A, Portis E, Lee D, Donini P, Lanteri S. Development and characterization of microsatellite markers Cynara cardunculus L. Genome. 2005;48:217–225. doi: 10.1139/g04-111. [DOI] [PubMed] [Google Scholar]
- Ancora G. Globe artichoke (Cynara scolymus L.) In: Bajaj JPS, editor. Biotechnology in agriculture and forestry. Vol. 2. Berlin: Springer-Verlag; 1986. pp. 471–486. [Google Scholar]
- Ancora G, Belli Donini ML, Cuozzo L. Globe artichoke plants obtained from shoot apices through rapid in vitro micropropagation. Scientia Horticulturae. 1981;14:207–213. [Google Scholar]
- Balloux F, Lehmann L, de Meeûs T. The population genetics of clonal and partially clonal diploids. Genetics. 2003;164:1635–1644. doi: 10.1093/genetics/164.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basnizki J, Zohary D. Breeding of seed-planted artichoke. Plant Breed Reviews. 1994;12:253–269. [Google Scholar]
- Bianchimano V, Cantore V, Bianco VV, Boari F. Response of artichoke to salinity. Acta Horicolturae. 2005;681:143–150. [Google Scholar]
- Bianco VV. Carciofo (Cynara scolymus L.) In: Bianco VV, Pimpini F, editors. Orticoltura. Bologna: Patron; 1990. pp. 209–251. In [in Italian] [Google Scholar]
- Calabrese N, Bianco VV. Effect of gibberellic acid on yield and quality of seed grown artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori) Acta Horicolturae. 2000;514:25–32. [Google Scholar]
- De Candolle A. Origin of cultivated plants. New York, NY: Appleton and Company; 1890. pp. 233–236. [Google Scholar]
- Dellacecca V. Cardo (Cynara cardunculus L.) In: Bianco VV, Pimpini F, editors. Orticoltura. Bologna: Patron; 1990. pp. 252–258. In [in Italian] [Google Scholar]
- Dellacecca V, Magnifico V, Marzi V, Porceddu E, Scarascia Mugnozza GT. Contributo alla conoscenza delle varietà di carciofo coltivate nel mondo. Turin: Minerva Medica; Proceedings II Congresso Internazionale di Studi sul Carciofo; 1976. pp. 119–316. [Google Scholar]
- Di Venere D, Linsalata V, Calabrese N, Cardinali A, Sergio L. Biochemical characterization of wild and cultivated cardoon accessions. Acta Horicolturae. 2005;681:523–528. [Google Scholar]
- Fiori A. Fiori A, Beguinot A. Flora analitica d'Italia. 1904;III:380. In. [Google Scholar]
- Foti S, Mauromicale G, Raccuia SA, Fallico B, Fanella F, Maccarone E. Possible alternative utilization of Cynara spp. I. Biomass, grain yield and chemical composition of grain. Industrial Crops and Products. 1999;10:219–228. [Google Scholar]
- Foury C. Ressources génétiques et diversification de l'artichaut (Cynara scolymus L.) Acta Horticolturae. 1989;242:155–166. [Google Scholar]
- Gebhardt R. Antioxidative and protective properties of extracts from leaves of artichoke (Cynara scolymus L.) against hydroperoxide induced oxidative stress in cultured rat hepatocites. Toxicology and Applied Pharmacology. 1997;144:279–286. doi: 10.1006/taap.1997.8130. [DOI] [PubMed] [Google Scholar]
- Gepts P. A comparison between crop domestication, classical plant breeding, and genetic engeneering. Crop Science. 2002;42:1780–1790. [Google Scholar]
- Hammer K. The domestication syndrome. Die Kulturpflanze. 1984;32:11–34. [in German with English summary] [Google Scholar]
- Hammer K. Preadaptations and the domestication of crops and weeds. Biologische Zentralblatt. 1988;107:631–636. [in German with English summary] [Google Scholar]
- Hammer K. Evolution of cultivated plants and biodiversity. Nova Acta Leopoldina NF. 2003;87:133–146. [in German with English summary] [Google Scholar]
- Hancock JF. Plant evolution and the origin of crop species. Wallingford, UK: CABI Publishing; 2004. [Google Scholar]
- Idrisi Z. The Muslim agricultural revolution and its influence on Europe. Manchester, UK: Foundation for Science, Technology and Civilization FSTC); 2005. [Google Scholar]
- Lanteri S, Saba E, Cadinu M, Mallica GM, Baghino L, Portis E. Amplified fragment length polymorphism for genetic diversity assessment in globe artichoke. Theoretical and Applied Genetics. 2004;108:1534–1544. doi: 10.1007/s00122-003-1576-6. [DOI] [PubMed] [Google Scholar]
- Lonitzer A. Naturalis historiae opus novum. 1551–1555 Frankfurt am Main. Online version at http://www.uni-mannheim.de .
- López Anido FS, Firpo IT, García SM, Cointry EL. Estimation of genetic parameters for yield traits in globe artichoke (Cynara scolymus L.) Euphytica. 1998;103:61–66. [Google Scholar]
- Montelucci G. Un'escursione a Montetosto, presso Cerveteri. Annali di Botanica – Roma. 1962;27:323–330. [in Italian] [Google Scholar]
- Pécaut P, Martin F. Non-conformity of in vitro propagated plants of early Mediterranean varieties of globe artichoke (Cynara scolymus L.) Acta Horicolturae. 1992;300:363–366. [Google Scholar]
- Pécaut P, Dumas de Vaulx R, Lot H. Virus-free clones of globe artichoke (Cynara scolymus) obtained after in vitro propagation. Acta Horicolturae. 1983;131:303–310. [Google Scholar]
- Pignone D, Sonnante G. Wild artichokes of south Italy: did the story begin here? Genetic Resources and Crop Evolution. 2004;51:577–580. [Google Scholar]
- Pignone D, Hammer K, Gladis T, Perrino P. Collecting in southern Sardinia (Italy), 1995. Plant Genetic Resources Newsletter. 1997;109:7–10. [Google Scholar]
- Porceddu E, Dellacecca V, Bianco VV. Atti del II Congresso Internazionale di Studi sul Carciofo. Turin: Minerva Medica; 1976. Classificazione numerica di cultivar di carciofo; pp. 1105–1119. [in Italian] [Google Scholar]
- Portis E, Barchi L, Acquadro A, Macua JI, Lanteri S. Genetic diversity assessment in cultivated cardoon by AFLP (amplified fragment length polymorphism) and microsatellite markers. Plant Breeding. 2005;124:299–304. [Google Scholar]
- Portis E, Acquadro A, Lanteri S, Mauro R, Mauromicale G. Linkage mapping of Cynara cardunculus L; Fifth Plant Genomics European Meeting; 11–14 October 2006; Venice, Italy: 2006. Poster Abstract 221. [Google Scholar]
- Pujol B, David P, McKey D. Microevolution in agricultural environments: how a traditional Amerindian farming practice favours heterozygosity in cassava (Manihot esculenta Crantz, Euphorbiaceae) Ecology Letters. 2005;8:138–147. [Google Scholar]
- Raccuia SA, Melilli MG. Cynara cardunculus L., a potential source of inulin in the Mediterranean environment: screening of genetic variability. Australian Journal of Agricultural Research. 2004;55:693–698. [Google Scholar]
- Raccuia SA, Melilli MG. Biomass and grain oil yields in Cynara cardunculus L. genotypes grown in a Mediterranean environment. Field Crops Research. 2007;101:187–197. [Google Scholar]
- Raccuia SA, Cavallaro V, Melilli MG. Intraspecific variability in Cynara cardunculus L. var. sylvestris Lam. Sicilian populations: seed germination under salt and moisture stresses. Journal of Arid Environment. (a) 2004;56:107–116. [Google Scholar]
- Raccuia SA, Mainolfi A, Mandolino G, Melilli MG. Genetic diversity in Cynara cardunculus revealed by AFLP markers: comparison between cultivars and wild types from Sicily. Plant Breeding. (b) 2004;123:280–284. [Google Scholar]
- Robba L, Carine MA, Russell SJ, Raimondo FM. The monophyly and evolution of Cynara L. (Asteraceae) sensu lato: evidence from the internal transcribed spacer region of nrDNA. Plant Systematics and Evolution. 2005;253:53–64. [Google Scholar]
- Rottenberg A, Zohary D. The wild ancestry of the cultivated artichoke. Genetic Resources and Crop Evolution. 1996;43:53–58. [Google Scholar]
- Rottenberg A, Zohary D. Wild genetic resources of cultivated artichoke. Acta Horticulturae. 2005;681:307–311. [Google Scholar]
- Rottenberg A, Zohary D, Nevo E. Isozyme relationships between cultivated artichoke and the wild relatives. Genetic Resources and Crop Evolution. 1996;43:59–62. [Google Scholar]
- Ruta C, Tagarelli A, Morone Fortunato I. Mycorrhization on micropropagated artichoke. Acta Horicolturae. 2005;681:407–412. [Google Scholar]
- Small RL, Cronn RC, Wendel JF. Use of nuclear genes for phylogeny reconstruction in plants. Australian Systematic Botany. 2004;17:145–170. [Google Scholar]
- Sonnante G, De Paolis A, Lattanzio V, Perrino P. Genetic variation in wild and cultivated artichoke revealed by RAPD markers. Genetic Resources and Crop Evolution. 2002;49:247–252. [Google Scholar]
- Sonnante G, De Paolis A, Pignone D. Relationships among artichoke cultivars and some related wild taxa based on AFLP markers. Plant Genetic Resources: Characterization and Utilization. 2004;1:125–133. [Google Scholar]
- Sonnante G, De Paolis A, Carluccio AV, Pignone D. Characterization of newly isolated microsatellite markers from artichoke. Proceedings of the 50th Italian Society of Agricultural Genetics Annual Congress; 10–14 September, 2006; Ischia, Italy. 2006. Poster Abstract A.44. [Google Scholar]
- Sonnante G, Sannino L, Morgese A, Sonnante G, Pignone D. III Convegno Nazionale Piante Mediterranee. b. Bari, Italy: 2006. Variabilità genetica in popolazioni di carciofo selvatico (Cynara cardunculus var. sylvestris) mediante marcatori molecolari. September to 1 October, 2006 Poster Abstract 92 [In Italian] [Google Scholar]
- Sonnante G, Carluccio AV, Vilatersana R, Pignone D. On the origin of artichoke and cardoon from the Cynara gene pool as revealed by rDNA sequence variation. Genetic Resources and Crop Evolution. 2007;54:483–495. [Google Scholar]
- Tavazza R, Papacchioli V, Ancora G. An improved medium for in vitro propagation of globe artichoke (Cynara scolymus L.) cv. Acta Horicolturae. 2004;660:91–97. [Google Scholar]
- Wiklund A. The genus Cynara L. (Asteraceae-Cardueae) Botanical Journal of the Linnean Society. 1992;109:75–123. [Google Scholar]
- Zohary D, Basnizki J. The cultivated artichoke Cynara scolymus: its probable wild ancestors. Economic Botany. 1975;29:233–235. [Google Scholar]
- Zohary D, Hopf M. Domestication of plants in the old world. Oxford, UK: Oxford University Press; 2001. [Google Scholar]